Insulin-Mimetic Activity of Herbal Extracts Identified with Large-Scale Total Internal Reflection Fluorescence Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. In-House Extract Preparation
2.3. Total Internal Reflection Fluorescence Microscopy (TIRFM)
2.4. Modified Hens Egg Test (Gluc-HET)
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Data Analysis
3. Results
3.1. Investigation of GLUT4 Translocation Activity of 772 Plant Extracts in HeLa-GLUT4-myc-GFP Cells
3.2. Validation of Extracts Stimulating GLUT4 Translocation with Newly Prepared Extracts from the Same Source
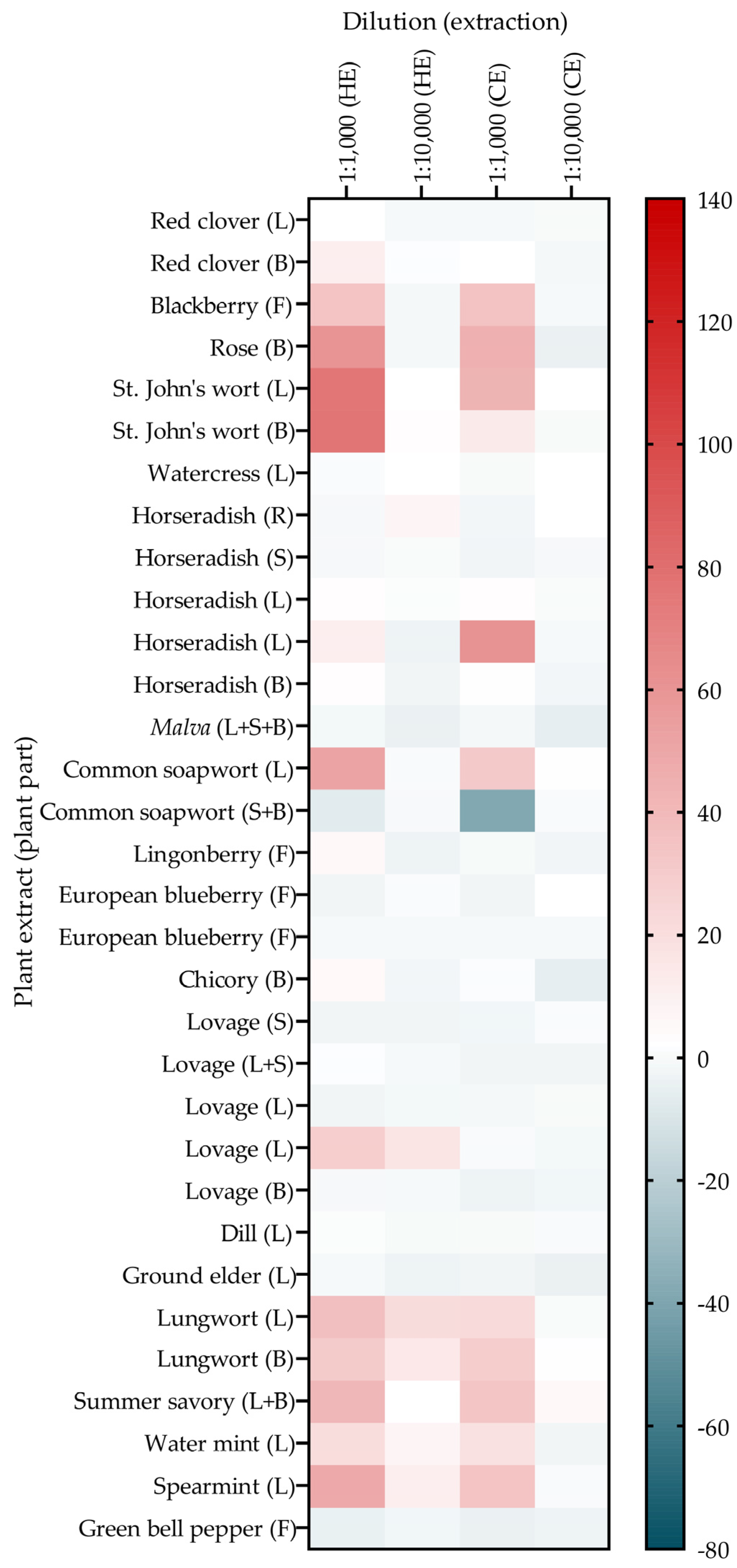
3.3. Determination of Active Plant Parts Using in-House Prepared Extracts from Leaves, Blossoms, Stems, or Roots from Regionally Available and GLUT4 Translocation-Active PECKISH Plants
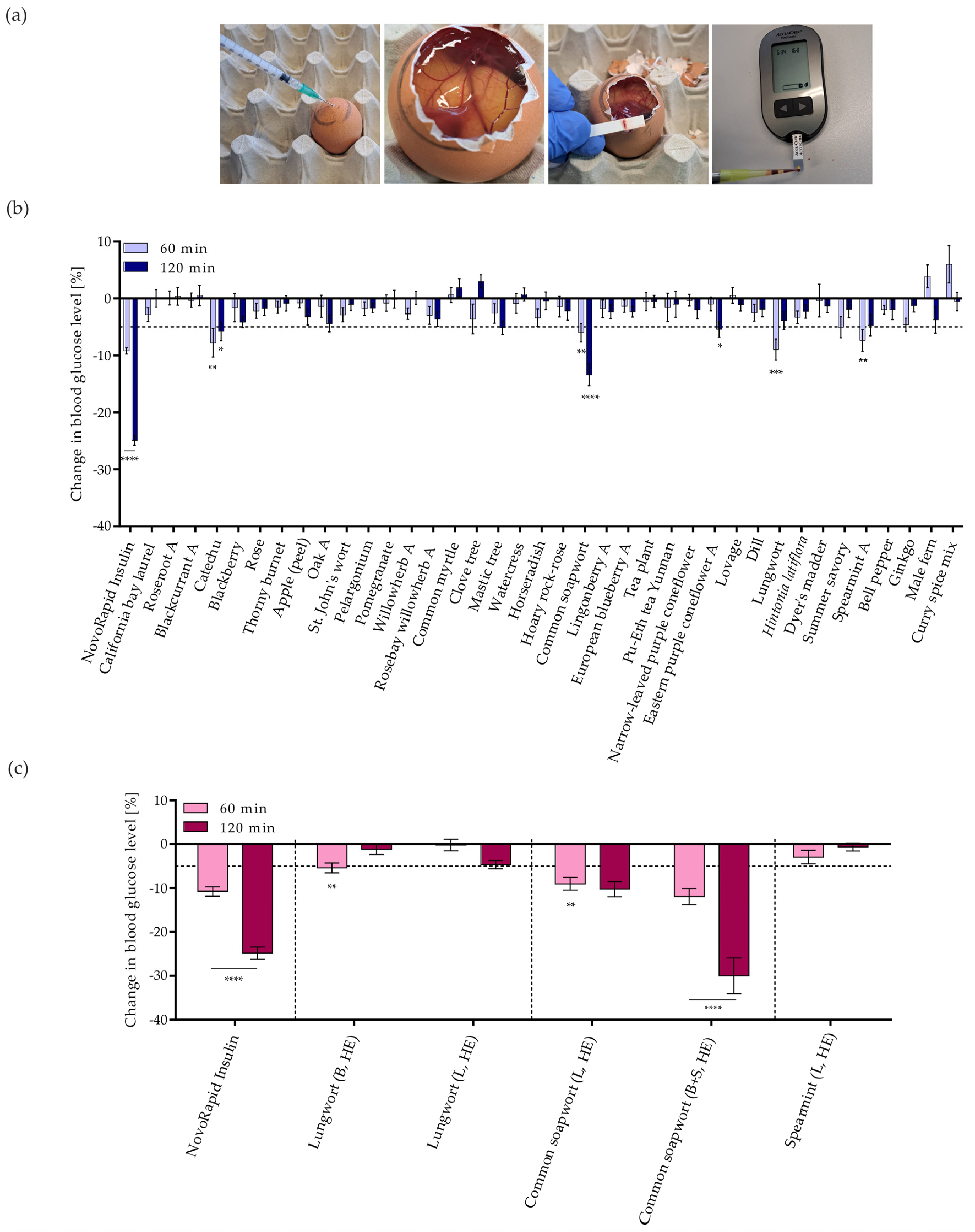
3.4. Investigation of Changes in Blood Glucose Levels in Chicken Embryos of GLUT4 Translocation-Active Plant Extracts (In Ovo)
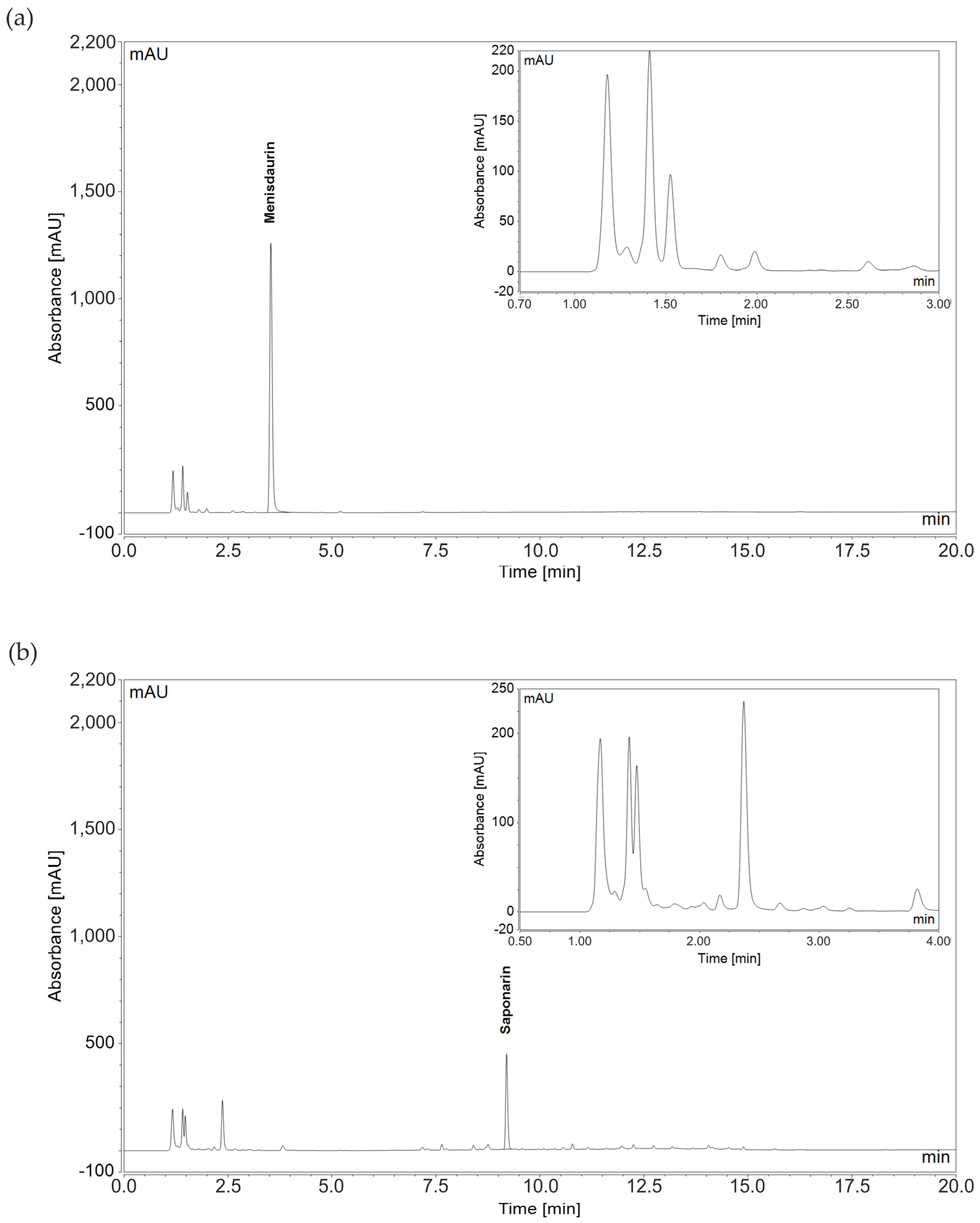
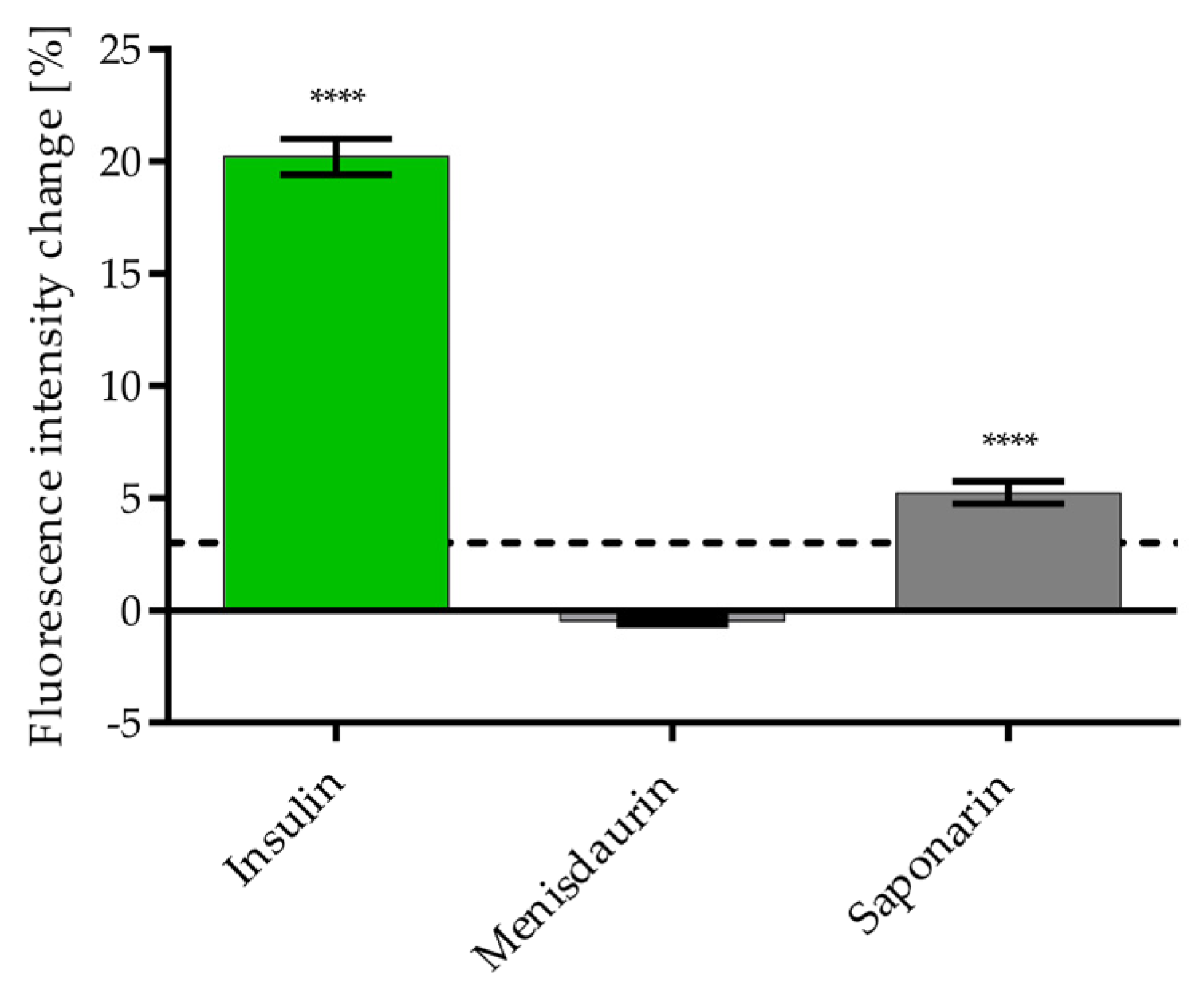
3.5. Identification of Phytochemicals in Lungwort and Common Soapwort Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Roden, M. Diabetes mellitus—Definition, Klassifikation und Diagnose. Wien. Klin. Wochenschr. 2016, 128 (Suppl 2), 37–40. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Federici, M.; Lauro, D.; Sbraccia, P.; Lauro, R. Molecular mechanism of insulin resistance in type 2 diabetes mellitus: Role of the insulin receptor variant forms. Diabetes Metab. Res. Rev. 2001, 17, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.G.; Hoekstra, J.B.; DeVries, J.H. Insulin Therapy for Type 2 Diabetes. Diabetes Care 2009, 32, S253–S259. [Google Scholar] [CrossRef]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; D’Errico, V.; Fallucca, S.; Menini, S.; Pugliese, G. Physical exercise as therapy for type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2014, 30 (Suppl. 1), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [CrossRef]
- Bain, S.; Druyts, E.; Balijepalli, C.; Baxter, C.A.; Currie, C.J.; Das, R.; Donnelly, R.; Khunti, K.; Langerman, H.; Leigh, P.; et al. Cardiovascular events and all-cause mortality associated with sulphonylureas compared with other antihyperglycaemic drugs: A Bayesian meta-analysis of survival data. Diabetes Obes. Metab. 2017, 19, 329–335. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef]
- Saadi, T.; Waterman, M.; Yassin, H.; Baruch, Y. Metformin-induced mixed hepatocellular and cholestatic hepatic injury: Case report and literature review. Int. J. Gen. Med. 2013, 6, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Govers, R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Metab. 2014, 40, 400–410. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Selvakumar, P.; Russell, R.R.; Cushman, S.W.; Holman, G.D.; Young, L.H. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E834–E841. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Da Silva Xavier, G.; Leclerc, I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem. J. 2003, 375, 1–16. [Google Scholar] [CrossRef]
- Malide, D.; Ramm, G.; Cushman, S.W.; Slot, J.W. Immunoelectron microscopic evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J. Cell Sci. 2000, 113, 4203–4210. [Google Scholar] [CrossRef]
- Ryder, J.W.; Fahlman, R.; Wallberg-Henriksson, H.; Alessi, D.R.; Krook, A.; Zierath, J.R. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J. Biol. Chem. 2000, 275, 1457–1462. [Google Scholar] [CrossRef]
- Xi, X.; Han, J.; Zhang, J.Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J. Biol. Chem. 2001, 276, 41029–41034. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Maianu, L.; Zhu, J.H.; Brechtel-Hook, G.; Wallace, P.; Baron, A.D. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Investig. 1998, 101, 2377–2386. [Google Scholar] [CrossRef]
- Chamarthi, S.K.; Sharma, H.C.; Sahrawat, K.L.; Narasu, L.M.; Dhillon, M.K. Physico-chemical mechanisms of resistance to shoot fly, Atherigona soccata in sorghum, Sorghum bicolor. J. Appl. Entomol. 2011, 135, 446–455. [Google Scholar] [CrossRef]
- Barile, E.; Bonanomi, G.; Antignani, V.; Zolfaghari, B.; Sajjadi, S.E.; Scala, F.; Lanzotti, V. Saponins from Allium minutiflorum with antifungal activity. Phytochemistry 2007, 68, 596–603. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Day, C. Metformin: Its botanical background. Pract. Diabetes Int. 2004, 21, 115–117. [Google Scholar] [CrossRef]
- Ghadage, D.M.; Kshirsagar, P.R.; Pai, S.R.; Chavan, J.J. Extraction efficiency, phytochemical profiles and antioxidative properties of different parts of Saptarangi (Salacia chinensis L.)—An important underutilized plant. Biochem. Biophys. Rep. 2017, 12, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Da Machado, A.P.F.; Sumere, B.R.; Mekaru, C.; Martinez, J.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of polyphenols and antioxidants from pomegranate peel using ultrasound: Influence of temperature, frequency and operation mode. Int. J. Food Sci. Technol. 2019, 54, 2792–2801. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Hartati, I. Microwave Assisted Extraction of Dioscorin from Gadung (Dioscorea Hispida Dennst) Tuber Flour. Procedia Chem. 2015, 14, 47–55. [Google Scholar] [CrossRef]
- Muanda, F.; Koné, D.; Dicko, A.; Soulimani, R.; Younos, C. Phytochemical composition and antioxidant capacity of three malian medicinal plant parts. Evid. Based Complement. Alternat. Med. 2011, 2011, 674320. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical Profile and Antioxidant Activity of Aerial and Underground Parts of Salvia bulleyana Diels. Plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef]
- Georgescu, C.; Frum, A.; Virchea, L.-I.; Sumacheva, A.; Shamtsyan, M.; Gligor, F.-G.; Olah, N.K.; Mathe, E.; Mironescu, M. Geographic Variability of Berry Phytochemicals with Antioxidant and Antimicrobial Properties. Molecules 2022, 27, 4986. [Google Scholar] [CrossRef]
- Krzyżanowska-Kowalczyk, J.; Pecio, Ł.; Mołdoch, J.; Ludwiczuk, A.; Kowalczyk, M. Novel Phenolic Constituents of Pulmonaria officinalis L. LC-MS/MS Comparison of Spring and Autumn Metabolite Profiles. Molecules 2018, 23, 2277. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Mwamatope, B.; Tembo, D.; Kampira, E.; Maliwichi-Nyirenda, C.; Ndolo, V. Seasonal Variation of Phytochemicals in Four Selected Medicinal Plants. Pharmacogn. Res. 2021, 13, 218–226. [Google Scholar] [CrossRef]
- Teoh, S.L.; Das, S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochem. Rev. 2018, 17, 1111–1128. [Google Scholar] [CrossRef]
- Onur, S.O.; Stöckmann, H.; Zenthoefer, M.; Piker, L.; Döring, F. The Plant Extract Collection Kiel in Schleswig-Holstein (PECKISH) Is an Open Access Screening Library. J. Food Res. 2013, 2, 101. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Neuhauser, C.; Aumiller, T.; Stallinger, A.; Iken, M.; Weghuber, J. Identification of Insulin-Mimetic Plant Extracts: From an In Vitro High-Content Screen to Blood Glucose Reduction in Live Animals. Molecules 2021, 26, 4346. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Lanzerstorfer, P.; Neuhauser, C.; Weber, F.; Stübl, F.; Weber, P.; Wagner, M.; Plochberger, B.; Wieser, S.; Schneckenburger, H.; et al. Fluorescence Microscopy-Based Quantitation of GLUT4 Translocation: High Throughput or High Content? Int. J. Mol. Sci. 2020, 21, 7964. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Haselgrübler, R.; Lanzerstorfer, P.; Plochberger, B.; Borgmann, D.; Jacak, J.; Winkler, S.M.; Schröder, K.; Höglinger, O.; Weghuber, J. Biomolecular Characterization of Putative Antidiabetic Herbal Extracts. PLoS ONE 2016, 11, e0148109. [Google Scholar] [CrossRef] [PubMed]
- Lanzerstorfer, P.; Stadlbauer, V.; Chtcheglova, L.A.; Haselgrübler, R.; Borgmann, D.; Wruss, J.; Hinterdorfer, P.; Schröder, K.; Winkler, S.M.; Höglinger, O.; et al. Identification of novel insulin mimetic drugs by quantitative total internal reflection fluorescence (TIRF) microscopy. Br. J. Pharmacol. 2014, 171, 5237–5251. [Google Scholar] [CrossRef]
- Haselgrübler, R.; Stübl, F.; Essl, K.; Iken, M.; Schröder, K.; Weghuber, J. Gluc-HET, a complementary chick embryo model for the characterization of antidiabetic compounds. PLoS ONE 2017, 12, e0182788. [Google Scholar] [CrossRef]
- Haselgrübler, R.; Stübl, F.; Stadlbauer, V.; Lanzerstorfer, P.; Weghuber, J. An In Ovo Model for Testing Insulin-mimetic Compounds. J. Vis. Exp. 2018, 134, e57237. [Google Scholar] [CrossRef]
- Müller, U.; Stübl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Höglinger, O.; et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium guajava) Extracts. Mol. Nutr. Food Res. 2018, 62, e1701012. [Google Scholar] [CrossRef]
- Chamberlain, S.A.; Szöcs, E. taxize: Taxonomic search and retrieval in R. F1000Research 2013, 2, 191. [Google Scholar] [CrossRef] [PubMed]
- Foster, Z.S.L.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Haselgrübler, R.; Stadlbauer, V.; Stübl, F.; Schwarzinger, B.; Rudzionyte, I.; Himmelsbach, M.; Iken, M.; Weghuber, J. Insulin Mimetic Properties of Extracts Prepared from Bellis perennis. Molecules 2018, 23, 2605. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.-D.; Lee, J.H.; Jia, Y.; Wu, C.; Lee, S.-J. Saponarin activates AMPK in a calcium-dependent manner and suppresses gluconeogenesis and increases glucose uptake via phosphorylation of CRTC2 and HDAC5. Bioorg. Med. Chem. Lett. 2015, 25, 5237–5242. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- de Vienne, D.M. Lifemap: Exploring the Entire Tree of Life. PLoS Biol. 2016, 14, e2001624. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C.F. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Yuan, H. Quantitative study of electrostatic and steric effects on physicochemical property and biological activity. J. Mol. Graph. Model. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Ghica, A.; Drumea, V.; Moroșan, A.; Mihaiescu, D.E.; Costea, L.; Luță, E.A.; Mihai, D.P.; Balaci, D.T.; Fița, A.C.; Olaru, O.T.; et al. Phytochemical Screening and Antioxidant Potential of Selected Extracts from Betula alba var. pendula Roth., Glycyrrhiza glabra L., and Avena sativa L. Plants 2023, 12, 2510. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xiao, J.; Xu, B. An insight into anti-diabetic properties of dietary phytochemicals. Phytochem. Rev. 2017, 16, 535–553. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.-K.; Park, Y.B.; Jeon, S.-M.; Choi, M.-S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Jiang, H.; Nomura, T.; Hironao, K.-Y.; Yamashita, Y.; Ashida, H. Kaempferol Promotes Glucose Uptake in Myotubes through a JAK2-Dependent Pathway. J. Agric. Food Chem. 2020, 68, 13720–13729. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Shih, H.-Y.; Chia, Y.-C.; Lee, C.-H.; Ashida, H.; Lai, Y.-K.; Weng, C.-F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Balasubramanian, K.; Rajalakshmi, M.; Eliza, J.; Selvaraj, J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine 2010, 17, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.N.V.; Anjana, T.; Banerji, A.; Gopalakrishnapillai, A. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett. 2010, 584, 531–536. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, H.; Cheong, M.S.; Zhong, R.; Garcia-Oliveira, P.; Prieto, M.A.; Cheng, K.-W.; Wang, M.; Cao, H.; Nie, S.; et al. Anti-diabetic potential of apigenin, luteolin, and baicalein via partially activating PI3K/Akt/Glut-4 signaling pathways in insulin-resistant HepG2 cells. Food Sci. Hum. Wellness 2023, 12, 1991–2000. [Google Scholar] [CrossRef]
- Castro, A.J.G.; Frederico, M.J.S.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; Da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta 2015, 1850, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hawrył, M.A.; Waksmundzka-Hajnos, M. Micro 2D-TLC of Selected Plant Extracts in Screening of Their Composition and Antioxidative Properties. Chromatographia 2013, 76, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Ponczek, M.B.; Pecio, Ł.; Nowak, P.; Kolodziejczyk-Czepas, J. Pulmonaria obscura and Pulmonaria officinalis Extracts as Mitigators of Peroxynitrite-Induced Oxidative Stress and Cyclooxygenase-2 Inhibitors-In Vitro and In Silico Studies. Molecules 2021, 26, 631. [Google Scholar] [CrossRef] [PubMed]
- Meeus, S.; Janssens, S.; Helsen, K.; Jacquemyn, H. Evolutionary trends in the distylous genus Pulmonaria (Boraginaceae): Evidence of ancient hybridization and current interspecific gene flow. Mol. Phylogenetics Evol. 2016, 98, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.L.; Ivancheva, S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J. Ethnopharmacol. 2003, 87, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Tita, I.; Mogosanu, G.D.; Tita, M.G. Ethnobotanical inventory of medicinal plants from the South-West of Romania. Farmacia 2009, 57, 141–156. [Google Scholar]
- Neagu, E.; Radu, G.L.; Albu, C.; Paun, G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 2018, 25, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. National Health Products Ingredients Database. Available online: https://webprod.hc-sc.gc.ca/nhpid-bdipsn/searchIngred (accessed on 17 June 2024).
- Chen, L.; Zhang, Q.; Yi, Z.; Chen, Y.; Xiao, W.; Su, D.; Shi, W. Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors. Foods 2022, 11, 2946. [Google Scholar] [CrossRef] [PubMed]
- Österreichisches Lebensmittelbuch Online. Empfehlung Österreichische Liste essbarer Wildpflanzen und Blüten. Available online: https://www.lebensmittelbuch.at/beschluesse/leitlinien-richtlinien-empfehlungen-usw-der-codexkommission/essbare-wildpflanzen-und-blueten/empfehlung-oesterreichische-liste-essbarer-wildpflanzen-und-blueten.html (accessed on 25 April 2023).
- Dobler, S.; Haberer, W.; Witte, L.; Hartmann, T. Selective Sequestration of Pyrrolizidine Alkaloids from Diverse Host Plants by Longitarsus Flea Beetles. J. Chem. Ecol. 2000, 26, 1281–1298. [Google Scholar] [CrossRef]
- Malinowska, P. Effect of flavonoids content on antioxidant activity of commercial cosmetic plant extracts. Herba Pol. 2013, 59, 63–75. [Google Scholar] [CrossRef]
- Chauhan, S.; Jaiswal, V.; Cho, Y.-I.; Lee, H.-J. Biological Activities and Phytochemicals of Lungworts (Genus Pulmonaria) Focusing on Pulmonaria officinalis. Appl. Sci. 2022, 12, 6678. [Google Scholar] [CrossRef]
- POWO. Plants of the world online.: Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:156627-1#sources (accessed on 24 April 2023).
- Zaynab, M.; Sharif, Y.; Abbas, S.; Afzal, M.Z.; Qasim, M.; Khalofah, A.; Ansari, M.J.; Khan, K.A.; Tao, L.; Li, S. Saponin toxicity as key player in plant defense against pathogens. Toxicon 2021, 193, 21–27. [Google Scholar] [CrossRef]
- Osbourn, A. Saponins and plant defence—A soap story. Trends Plant Sci. 1996, 1, 4–9. [Google Scholar] [CrossRef]
- Budan, A.; Bellenot, D.; Freuze, I.; Gillmann, L.; Chicoteau, P.; Richomme, P.; Guilet, D. Potential of extracts from Saponaria officinalis and Calendula officinalis to modulate in vitro rumen fermentation with respect to their content in saponins. Biosci. Biotechnol. Biochem. 2014, 78, 288–295. [Google Scholar] [CrossRef]
- Ferreras, J.M.; Barbieri, L.; Girbés, T.; Battelli, M.G.; Rojo, M.A.; Arias, F.J.; Rocher, M.A.; Soriano, F.; Mendéz, E.; Stirpe, F. Distribution and properties of major ribosome-inactivating proteins (28 S rRNA N-glycosidases) of the plant Saponaria officinalis L. (Caryophyllaceae). Biochim. Biophys. Acta 1993, 1216, 31–42. [Google Scholar] [CrossRef]
- Chandra, S.; Rawat, D.S.; Bhatt, A. Phytochemistry and pharmacological activities of Saponaria officinalis L.: A review. Not. Sci. Biol. 2021, 13, 10809. [Google Scholar] [CrossRef]
- Girbés, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional antihelmintic, antiparasitic and repellent uses of plants in Central Italy. J. Ethnopharmacol. 1999, 68, 183–192. [Google Scholar] [CrossRef]
- Loi, M.C.; Poli, F.; Sacchetti, G.; Selenu, M.B.; Ballero, M. Ethnopharmacology of ogliastra (villagrande strisaili, sardinia, Italy). Fitoterapia 2004, 75, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Ligor, M.; Kiełbasa, A.; Ratiu, I.-A.; Buszewski, B. Separation and Quantification of Selected Sapogenins Extracted from Nettle, White Dead-Nettle, Common Soapwort and Washnut. Molecules 2021, 26, 7705. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Arora, R.; Mahajan, J.; Mahey, S.; Arora, S. Polyphenols from Cutch Tree (Acacia catechu Willd.): Normalize In Vitro Oxidative Stress and Exerts Antiproliferative Activity. Braz. Arch. Biol. Technol. 2018, 61, e17160728. [Google Scholar] [CrossRef]
- Adhikari, B.; Aryal, B.; Bhattarai, B.R. A Comprehensive Review on the Chemical Composition and Pharmacological Activities of Acacia catechu (L.f.) Willd. J. Chem. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Aryal, B.; Adhikari, B.; Aryal, N.; Bhattarai, B.R.; Khadayat, K.; Parajuli, N. LC-HRMS Profiling and Antidiabetic, Antioxidant, and Antibacterial Activities of Acacia catechu (L.f.) Willd. Biomed Res. Int. 2021, 2021, 7588711. [Google Scholar] [CrossRef]
- Monga, J.; Chauhan, C.S.; Sharma, M. Human breast adenocarcinoma cytotoxicity and modulation of 7,12-dimethylbenzaanthracene-induced mammary carcinoma in Balb/c mice by Acacia catechu (L.f.) Wild heartwood. Integr. Cancer Ther. 2013, 12, 347–362. [Google Scholar] [CrossRef]
- Panya, A.; Yongpitakwattana, P.; Budchart, P.; Sawasdee, N.; Krobthong, S.; Paemanee, A.; Roytrakul, S.; Rattanabunyong, S.; Choowongkomon, K.; Yenchitsomanus, P.-T. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia catechu. Chem. Biol. Drug Des. 2019, 93, 100–109. [Google Scholar] [CrossRef]
- Negi, B.S.; Dave, B.P. In Vitro Antimicrobial Activity of Acacia catechu and Its Phytochemical Analysis. Indian J. Microbiol. 2010, 50, 369–374. [Google Scholar] [CrossRef]
- Yimam, M.; Brownell, L.; Hodges, M.; Jia, Q. Analgesic effects of a standardized bioflavonoid composition from Scutellaria baicalensis and Acacia catechu. J. Diet. Suppl. 2012, 9, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Sunil, M.A.; Sunitha, V.S.; Radhakrishnan, E.K.; Jyothis, M. Immunomodulatory activities of Acacia catechu, a traditional thirst quencher of South India. J. Ayurveda Integr. Med. 2019, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rahmatullah, M.; Hossain, M.; Mahmud, A.; Sultana, N.; Rahman, S.M.; Islam, M.R.; Khatoon, M.S.; Jahan, S.; Islam, F. Antihyperglycemic and antinociceptive activity evaluation of ‘khoyer’ prepared from boiling the wood of Acacia catechu in water. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gunindro, N.; Devi, K.P.; Singh, T.I. Effect of Acacia catechu on intestinal absorption of glucose in rats. J. Chem. Pharm. Res. 2013, 5, 78–81. [Google Scholar]
- Bhatia, V.; Srivastava, S.P.; Srivastava, R.; Mishra, A.; Narender, T.; Maurya, R.; Srivastava, A.K. Antihyperglycaemic and aldose reductase inhibitory potential of Acacia catechu hard wood and Tectona grandis leaves. Med. Chem. Res. 2011, 20, 1724–1731. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Gupta, U. Evaluation of Hypoglycemic and Antihyperglycemic Effects of Acacia tortilis Seed Extract in Normal and Diabetic Rats. Int. J. PharmTech Res. 2013, 5, 330–336. [Google Scholar]
- Shen, D.; Wu, Q.; Wang, M.; Yang, Y.; Lavoie, E.J.; Simon, J.E. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization-mass spectrometry. J. Agric. Food Chem. 2006, 54, 3219–3224. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Antioxidant, Anti-inflammatory, and Chemoprotective Properties of Acacia catechu Heartwood Extracts. Phytother. Res. 2015, 29, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- El Menyiy, N.; Mrabti, H.N.; El Omari, N.; Bakili, A.E.; Bakrim, S.; Mekkaoui, M.; Balahbib, A.; Amiri-Ardekani, E.; Ullah, R.; Alqahtani, A.S.; et al. Medicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Mentha spicata. Evid.-Based Complement. Altern. Med. 2022, 2022, 7990508. [Google Scholar] [CrossRef]
- Bayani, M.; Ahmadi-hamedani, M.; Jebelli Javan, A. Study of Hypoglycemic, Hypocholesterolemic and Antioxidant Activities of Iranian Mentha Spicata Leaves Aqueous Extract in Diabetic Rats. Iran. J. Pharm. Res. 2017, 16, 75–82. [Google Scholar]


| Extract Name | Concentration of Compound | |
|---|---|---|
| Menisdaurin [mg/mL] | Saponarin [mg/mL] | |
| Lungwort (B, CE) | 1.3638 | n.q. |
| Lungwort (B, HE) | 0.2172 | n.q. |
| Lungwort (L, CE) | 0.2958 | n.q. |
| Lungwort (L, HE) | 0.985 | n.q. |
| Common soapwort (L, CE) | n.q. | 1.0264 |
| Common soapwort (L, HE) | n.q. | 0.4682 |
| Common soapwort (B+S, CE) | n.q. | 1.1202 |
| Common soapwort (B+S, HE) | n.q. | 1.0664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuhauser, C.; Schwarzinger, B.; Schwarzinger, C.; Feichtinger, M.; Stadlbauer, V.; Arnaut, V.; Drotarova, I.; Blank-Landeshammer, B.; Weghuber, J. Insulin-Mimetic Activity of Herbal Extracts Identified with Large-Scale Total Internal Reflection Fluorescence Microscopy. Nutrients 2024, 16, 2182. https://doi.org/10.3390/nu16142182
Neuhauser C, Schwarzinger B, Schwarzinger C, Feichtinger M, Stadlbauer V, Arnaut V, Drotarova I, Blank-Landeshammer B, Weghuber J. Insulin-Mimetic Activity of Herbal Extracts Identified with Large-Scale Total Internal Reflection Fluorescence Microscopy. Nutrients. 2024; 16(14):2182. https://doi.org/10.3390/nu16142182
Chicago/Turabian StyleNeuhauser, Cathrina, Bettina Schwarzinger, Clemens Schwarzinger, Michaela Feichtinger, Verena Stadlbauer, Verena Arnaut, Ivana Drotarova, Bernhard Blank-Landeshammer, and Julian Weghuber. 2024. "Insulin-Mimetic Activity of Herbal Extracts Identified with Large-Scale Total Internal Reflection Fluorescence Microscopy" Nutrients 16, no. 14: 2182. https://doi.org/10.3390/nu16142182
APA StyleNeuhauser, C., Schwarzinger, B., Schwarzinger, C., Feichtinger, M., Stadlbauer, V., Arnaut, V., Drotarova, I., Blank-Landeshammer, B., & Weghuber, J. (2024). Insulin-Mimetic Activity of Herbal Extracts Identified with Large-Scale Total Internal Reflection Fluorescence Microscopy. Nutrients, 16(14), 2182. https://doi.org/10.3390/nu16142182






