The Protective Effect of Flavonoids in the Diet on Autophagy-Related Cardiac Impairment
Abstract
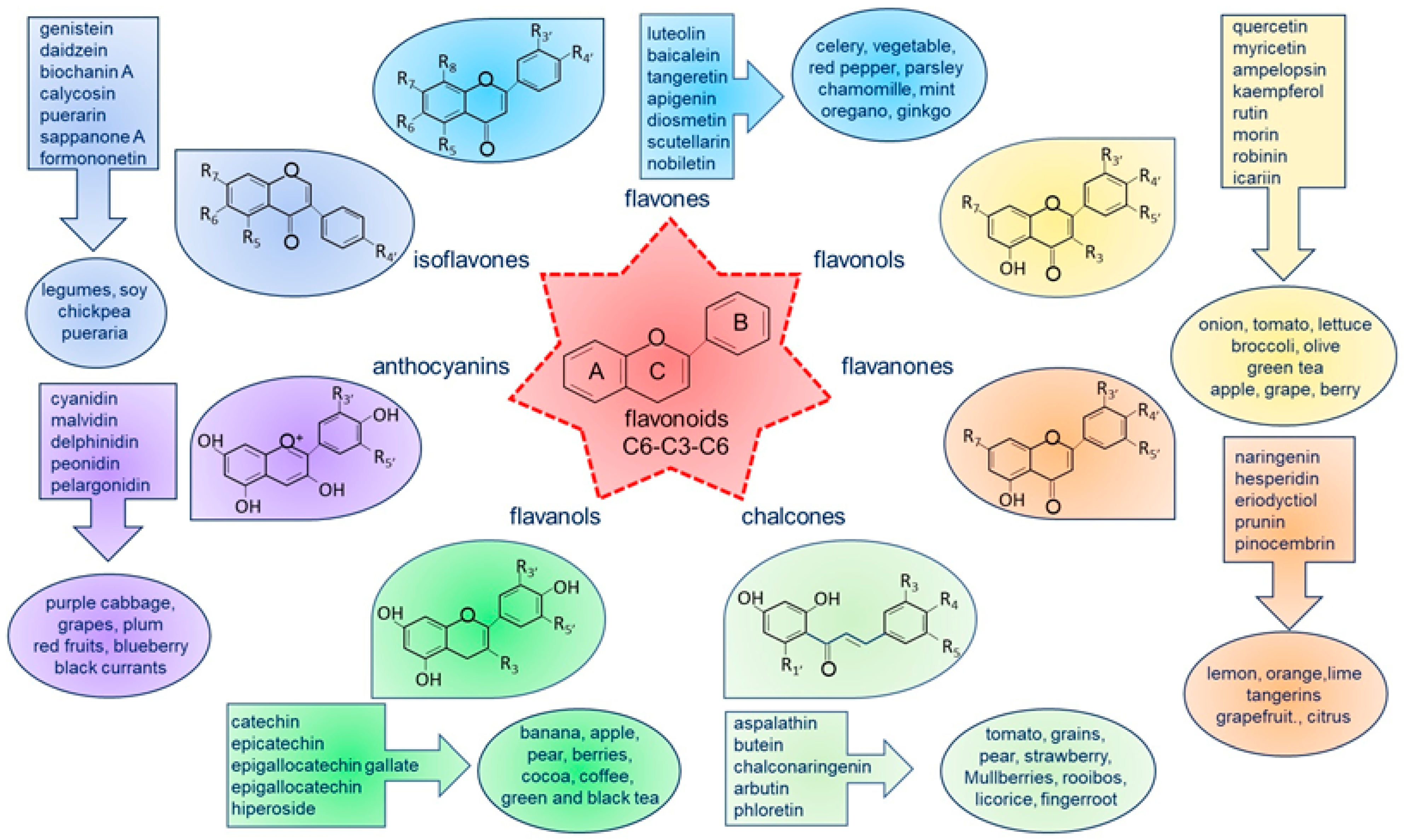
1. Introduction
2. Mitochondrial Homeostasis in the Heart
Selective Autophagy Helps in Maintaining Cellular Stability
3. Dietary Flavonoids Are Capable of Modulating Autophagy in Cardiomyocytes
3.1. The Effects of Flavonoids on Autophagy in Ischemia and I/R Injury
| Compound | Animal/ Cell Line | Model | Flavonoid Dose | Effect on the Heart/Cells | Auto-Phagy | Ref |
|---|---|---|---|---|---|---|
| Apigenin (flavone) | Mouse (C57BL6) | In vivo I/R (30 min/48 h) | 40 mg/kg iv at reperfusion | ↓ Infarct size | ↑ | [67] |
| Mouse cardiomyocytes | In vitro H/R | 100 μM incubated after H/R | ↑ Mitochondria function ↓ Apoptosis | ↑ | ||
| Luteolin (flavone) | Mouse (C57BL6) | In vivo MI (4-wk ischemia) | 10 µg/kg ip for 3 d prior MI | ↑ LV function ↓ Apoptosis Serum LDH, IL-1α, MPO CK, TNF-α | ↑ | [65] |
| Mouse neonatal cardiomyocytes | In vitro hypoxia (8 h) | 8 µM 48 h prior hypoxia | ↑ Mitochondria function | ↑ | ||
| Nobiletin (flavone) | Rat (Sprague-Dawley) | In vivo MI (3-wk ischemia) | 5 mg/kg/d ip 1 d after MI | ↓ Mortality ↑ LV function ↓ Infarct size, fibrosis | ↑ | [66] |
| H9c2 | in vitro ischemia OGD (12 h) | 20 µM 2 h prior OGD | ↓ Apoptosis | ↑ | ||
| Scutellarin (flavone) | Rat (Sprague-Dawley) | In vivo I/R (30 min/24 h) | 10–20 mg/kg ip 15 min prior ischemia | ↓ Infarct size, apoptosis ↑ LV function | ↑ | [68] |
| H9c2 | In vitro OGD/R (6 h/24 h) | 6.25–12.5 mg/mL 6 h prior OGD | ↓ Apoptosis, LDH release | ↑ | ||
| Formononetin (isoflavone) | Mouse | Ex vivo Langendorff no flow I/R (40 min/60 min) | 5 mM for 10 s at reperfusion, repeated 5 times over 60 min | ↑ LV function ↓ Infarct size, apoptosis | ↑ | [69] |
| H9c2 (aged cells) | In vitro H/R | 5 mM | ↓ Apoptosis | ↑ | ||
| Sappanone A (isoflavone) | Rat (Wistar) | Ex vivo Langendorff no flow I/R (30 min/90 min) | 100 μM in the first 15 min of reperfusion | ↑ Mitochondria function and quality control | ↑ | [70] |
| Epigallocatechin gallate (flavanol) | Rat (Sprague-Dawley) | In vivo I/R (30 min/2 h) | 10 mg/kg iv 10 min prior reperfusion | ↑ LV function ↓ infarct size, apoptosis, serum CK and LDH | ↓ | [73] |
| Epigallocatechin gallate (flavanol) | Rat (Sprague-Dawley) | In vivo I/R (30 min/12 h) | 10 mg/kg iv 30 min prior ischemia | ↓ Infarct size, serum cTnI | ↓ | [74] |
| H9c2 | In vitro H/R (6 h/12 h) | 25 μM incubated 4 h prior H/R | ↓ cTnI release | ↓ | ||
| Hiperoside (flavanol) | Mouse (Kunming) | In vivo MI (2-wk ischemia) | 9–36 mg/kg/d ig for 2 wk after MI | ↑ LV function ↓ Fibrosis ↓ Serum CK, cTnI, LDH | ↑ | [75] |
| Hesperidin (flavanone) | Rat (Sprague-Dawley) | In vivo I/R (30 min/4 h) | 200 mg/kg/d ig for 3 d prior I/R | ↓ Infarct size, serum CK, and cTnI | ↓ | [72] |
| Cyanidin-3-glucoside (anthocyanin) | Rat (Sprague-Dawley) | In vivo I/R (30 min/2 h) | 10–20 mg/kg/d ip for 7d prior I/R | ↓ Infarct size, tissue damage, and ferroptosis | ↓ | [71] |
| H9c2 | In vitro OGD/R (6 h/24 h) | 25–100 μM incubated during OGD/R | ↓ ROS, ferroptosis | ↓ |
3.2. The Effects of Flavonoids on Autophagy in Doxorubicin Cardiotoxicity
| Compound | Animal/ Cell Line | Model | Flavonoid Dose | Time of Analysis | Effect on the Heart/Cells | Auto-phagy | Ref |
|---|---|---|---|---|---|---|---|
| Apigenin (flavone) | Mouse (Kunming) | In vivo | 125–250 mg/kg/d ig with DOX for 16 d | 1 d after reaching DOX cumulative dose (24 mg/kg) | ↓ Apoptosis Serum AST, CK, LDH activity | ↓ | [81] |
| Luteolin (flavone) | Adult mouse cardiomyocytes | In vitro | 1–50 μM incubated with DOX | 24 h after DOX + Luteolin incubation | ↑ Contractile function ↓ Apoptosis and ROS | ↑ | [82] |
| Scutellarin (flavone) | Rat | In vivo | 10 mg/kg/d ip with DOX for 6 wk | 2 wk after reaching DOX cumulative dose (20 mg/kg) | ↑ LV function ↓ cTnT, fibrosis Apoptosis | ↓ | [83] |
| Calycosin (isoflavone) | Zebrafish adult | In vivo | 5 μmol/L in water for 4 wk after 4 wk from DOX injection | 8 wk after a single DOX injection | ↑ LV function ↓ Natriuretic peptides | ↑ | [84] |
| Daidzein (isoflavone) | Rat (Sprague-Dawley) | In vivo | 20 mg/kg/d sc prior weekly DOX | 6 wk after DOX initiation | ↑ LV function ↓ Apoptosis | ↓ | [86] |
| Puerarin (isoflavone) | Mouse | In vivo | 100 mg/kg with DOX | 3 wk to reach DOX cumulative dose (15 mg/kg) | ↑ LV function ↓ Inflammation, tissue damage | ↑ | [85] |
| H9c2 | In vitro | 80–320 μM incubated 24 h prior DOX | 48 h post DOX exposure | ↑ Viability, mitochondria function ↓ ROS, LDH release | ↑ | ||
| Ampelopsin (flavonol) | Mouse (C57BL6) | In vivo | 50–100 mg/kg ig 24 h prior and during DOX, daily over 12 d | 24 h after reaching the DOX cumulative dose (15 mg/kg) | ↑ LV function ↓ Fibrosis and apoptosis | ↑ | [76] |
| Icariin (flavonol) | H9c2 | In vitro | 1–5 μM incubated 3 h prior DOX | 24 h after DOX incubation | ↑ Viability ↓ Apoptosis, ROS | ↓ | [77] |
| Rosa roxburghii (flavanol + flavonol) | Rat neonatal cardiomyocytes + H9c2 | In vitro | 40–80 μg/mL incubated 12–36 h prior DOX | 12 h after DOX incubation | ↓ Morphological changes | ↓ | [78] |
| Rutin (flavonol) | Mouse (C57BL6) | In vivo | 100 mg/kg os from 6 d prior DOX to sacrifice | 8 wk after reaching DOX cumulative dose (21 mg/kg) | ↑ LV function ↓ Fibrosis and apoptosis | ↓ | [79] |
| Rat neonatal cardiomyocytes | In vitro | 10 μM (24 h pretreatment + 24 h with DOX) | 24 h after DOX exposure | ↓ Apoptosis | ↓ | ||
| Spinacetin (flavonol) | Rat (Sprague-Dawley) | In vivo + in vitro | 50–100 mg/kg ig concomitant to DOX for 14 d | 24 h after the last DOX injection | ↑ Survival rate ↓ Apoptosis, injury markers | ↑ | [80] |
| Aspalathin (chalcone) | H9c2 | In vitro | 0.2 μM incubated with DOX | After 5 d of DOX + Aspalathin exposure | ↓ Apoptosis | ↑ | [87] |
3.3. The Effects of Flavonoids on Hypertrophic Cardiomyopathy
| Compound | Animal/ Cell Line | Model | Flavonoid Dose | Effect on the Heart/Cells | Auto-Phagy | Ref |
|---|---|---|---|---|---|---|
| Baicalein (flavone) | Mouse (C57BL6) | In vivo (ISO-induced hypertrophy) | 25 mg/kg iv every 3 d during 15 d daily ISO treatment | ↑ LV function ↓ Hypertrophic markers | ↑ | [93] |
| Rat neonatal cardiomyocytes | In vitro (ISO-induced hypertrophy) | 30 μM 4 h prior 24 h ISO incubation | ↓ Cell surface, hypertrophic markers | ↑ | ||
| Diosmetin (flavone) | Rat neonatal cardiomyocytes | In vitro (PE-induced hypertrophy) | 10 -50 μM coincubated with PE for 12–24 h | ↓ Cell surface, hypertrophic markers | ↓ | [94] |
| Luteolin (flavone) | Rat neonatal cardiomyocytes | In vitro (LPS-induced hypertrophy) | 50–100 mg/mL coincubated with LPS for 8 h | ↑ Cell viability ↓ Cell surface, hypertrophic markers | ↓ | [95] |
| Hiperoside (flavanol) | Rat (Wistar) | In vivo (TAC-induced hypertrophy) | 200 mg/kg/d ig after TAC for 6 wk | ↑ LV function ↓ Hypertrophy, CSA, apoptosis | ↑ | [92] |
| H9C2 | In vitro (AngII-induced hypertrophy | 10 μM coincubated with AngII for 48 h | ↓ Apoptosis | ↑ | ||
| Puerarin (isoflavone) | Rat (Sprague-Dawley) | In vivo (TAC- induced hypertrophy) | 100 mg/kg/d sc at TAC for 6 wk | ↑ LV function ↓ Hypertrophic markers, CSA, apoptosis | ↑ | [91] |
| H9c2 | In vitro (ISO-induced hypertrophy) | 20 µM 24 h prior ISO followed by incubation with ISO for 6 h | ↓ Cell surface, hypertrophic markers, apoptosis | ↑ |
3.4. The Effects of Flavonoids on Autophagy in Other Cardiomyopathies
| Compound | Animal/ Cell Line | Model | Flavonoid Dose | Effect on the Heart/Cells | Auto-Phagy | Ref |
|---|---|---|---|---|---|---|
| Type II Diabetes | ||||||
| Ampelopsin (flavonol) | Rat (Wistar) | In vivo (STZ + diet cardiomyopathy) | 100 mg/kg/d 3 wk after diabetes induction for 15 wk | ↑ LV function ↓ Fibrosis, apoptosis | ↑ | [97] |
| Ampelopsin (flavonol) | Mouse (C57BL6) | In vivo (STZ cardiomyopathy) | 100 mg/kg/d ig 3 wk after diabetes induction for 14 wk | ↑ LV function ↓ Fibrosis, apoptosis, inflammation, oxidative markers | ↑ | [101] |
| Epigallocatechin gallate (flavanol) | Rat (Goto–Kakizaki, spontaneous model of diabetes) | In vivo (cardiomyopathy) | 100 mg/kg/d ig for 6 wk | ↑ Mitochondrial function ↓ Oxidative stress | ↓ | [96] |
| Epigallocatechin gallate (flavanol) | Rat (Sprague-Dawley) | In vivo (HFD + STZ cardiomyopathy) | 40–80 mg/kg ig for 8 wk after diabetes assessment | ↑ LV function ↓ Injury markers, fibrosis | ↑ | [98] |
| Luteolin (flavone) | Rat (Sprague-Dawley) | In vivo (STZ cardiomyopathy) | 50–200 mg/kg ig for 4 wk after 6 wk of diabetes assessment | ↑ LV function ↓ Fibrosis | ↓ | [100] |
| Scutellarin (flavone) | Rat (Sprague-Dawley) | In vivo (STZ + diet cardiomyopathy) | 100–200 mg/kg/d for 8 wk during diabetes onset | ↓ Hypertrophy, LDH, CK release, apoptosis | ↑ | [99] |
| Sepsis | ||||||
| Apigenin (flavone) | Mouse (C57BL6) | In vivo (endotoxin-induced cardiomyopathy) | 50 mg/kg ip 1 h post sepsis induction | ↑ LV function ↓ Apoptosis, cardiac damage, LDH, CK release | ↑ | [102] |
| Luteolin (flavone) | Mouse (C57BL6) | In vivo (endotoxin-induced cardiomyopathy) | 10 μg/kg ip 10 d prior to sepsis induction | ↑ LV function ↑ Mitochondrial function, ↓ Apoptosis, inflammatory markers | ↑ | [104] |
| Tangeretin (flavone) | Rat (Sprague-Dawley) | In vivo (endotoxin-induced cardiomyopathy) | 50–100 mg/kg os concomitant with sepsis induction for 24 h | ↓ Apoptosis, oxidative markers, cTnI, cMLC1 release, inflammatory infiltration | ↓ | [103] |
| Puerarin (isoflavone) | H9c2 | In vitro (endotoxin-induced cardiomyopathy) | 100 mg/l 24 h prior LPS incubation for 24 h | ↓ Apoptosis, ROS, mitochondrial injury | ↑ | [105] |
4. Discussion
4.1. Lessons Learned from Preclinical Studies and Potential Critical Issues for the Translational Perspective
4.2. Autophagy as a Cellular Target of Flavonoids in Cardiovascular Diseases
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMP | adenosine monophosphate |
| AMPK | AMP-activated kinase |
| AngII | angiotensin II |
| AST | aspartate aminotransferase |
| ATG | autophagy-related |
| ATP | adenosine triphosphate |
| BCL2L13 | BCL2 Like 13 |
| BNIP3 | BCL-2/adenovirus E1B 19kD protein-interacting protein 3 |
| CK | creatine kinase |
| cMLC1 | cardiac myosin-light chains 1 |
| CSA | cross-sectional area |
| cTnI | cardiac troponin I |
| cTnT | cardiac troponin T |
| CVD | cardiovascular disease |
| DOX | doxorubicin |
| EGG | epigallocatechin gallate |
| FUNDC1 | FUN14 Domain Containing 1 |
| GABA | gamma-aminobutyric acid |
| GABARAP | GABA type A receptor-associated protein |
| GK | Goto–Kakizaki |
| HFD | high-fat diet |
| H/R | hypoxia/reoxygenation |
| HSC70 | heat shock cognate 70 protein |
| IL-1α | interleukin 1α |
| I/R | ischemia/reperfusion |
| ig | intragastric |
| ip | intraperitoneal |
| ISO | isoproterenol |
| iv | intravenous |
| LAMP-2A | lysosomal-associated membrane protein-2A |
| LDH | lactate dehydrogenase |
| LIR | LC3-interacting region |
| LPS | lipopolysaccharide |
| LV | left ventricle |
| MAP1LC3/LC3 | microtubule-associated protein 1 light chain 3 |
| MI | myocardial infarction |
| MPO | myeloperoxidase |
| mTOR | mechanistic target of rapamycin |
| OGD | oxygen-glucose deprivation |
| OGD/R | oxygen-glucose deprivation/reoxygenation |
| OPTN | optineurin |
| os | oral |
| OXPHOS | oxidative phosphorylation |
| Parkin | Parkin RBR E3 ubiquitin-protein ligase |
| PE | phenylephrine |
| PINK1 | PTEN-induced kinase 1 |
| ROS | reactive oxygen species |
| sc | subcutaneous |
| STZ | streptozotocin |
| TAC | thoracic aorta constriction |
| TNF-α | Tumor necrosis factor-α |
| ULK-1 | Unc-51 kinase-like-1 |
References
- Global Nutrition Report. 2021 Global Nutrition Report: The State of Global Nutrition. 2021. Available online: https://globalnutritionreport.org/reports/2021-global-nutrition-report/ (accessed on 28 April 2024).
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health. A review of emerging biologic pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhou, X.; Yang, T.; Wang, L.; Feng, L.; Wang, Z.; Xu, J.; Jing, W.; Wang, T.; Su, H.; et al. The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets. Front. Cardiovasc. Med. 2023, 10, 1088575. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G.; Gerstenblith, G.; Bottomley, P.A. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc. Natl. Acad. Sci. USA 2005, 102, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Qi, Y.; Tsang, S.-Y. Mitochondrial biogenesis, mitochondrial dynamics, and mitophagy in the maturation of cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Schuck, S. Microautophagy–distinct molecular mechanisms handle cargoes of many sizes. J. Cell Sci. 2020, 133, jcs246322. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.; Sequeida, A.; Albornoz, A.; Budini, M. Chaperone mediated autophagy substrates and components in cancer. Front. Oncol. 2021, 10, 614677. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Lamark, T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 2020, 432, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Germain, K.; Kim, P.K. Pexophagy: A model for selective autophagy. Int. J. Mol. Sci. 2020, 21, 578. [Google Scholar] [CrossRef] [PubMed]
- Kravic, B.; Behrends, C.; Meyer, H. Regulation of lysosome integrity and lysophagy by the ubiquitin-conjugating enzyme UBE2QL1. Autophagy 2020, 16, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, P.; Huang, Y.; Li, Y.F.; Lu, J.; Li, M.; Kurihara, H.; Luo, Z.; Meng, T.; Onishi, M.; et al. Selective autophagy of intracellular organelles: Recent research advances. Theranostics 2021, 11, 222–256. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Martens, S.; Ferrari, L. Aggrephagy at a glance. J. Cell Sci. 2023, 136, jcs260888. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.; Kim, J.; Shaw, R.J.; Guan, K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Rogov, V.V. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 2019, 76, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Gubas, A.; Dikic, I. A guide to the regulation of selective autophagy receptors. FEBS J. 2022, 289, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Sadoshima, J. Mitochondrial autophagy in cardiomyopathy. Curr. Opin. Genet. Dev. 2016, 38, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Maejima, Y.; Zablocki, D.; Sadoshima, J. The role of autophagy in the heart. Annu. Rev. Physiol. 2018, 80, 31–38. [Google Scholar] [CrossRef]
- Ikeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015, 116, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Maiuri, M.C.; Kaushik, S.; Fernández, Á.F.; de Bruijn, J.; Castoldi, F.; Chen, Y.; Ito, J.; Mukai, R.; Murakawa, T.; et al. Comprehensive autophagy evaluation in cardiac disease models. Cardiovasc. Res. 2020, 116, 483–504. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Maruyama, R.; Tsujimoto, A.; Ogino, A.; Takeyama, T.; Kawaguchi, T.; Watanabe, T.; Fujiwara, T.; et al. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc. Res. 2011, 91, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kubli, D.A.; Zhang, X.; Lee, Y.; Hanna, R.A.; Quinsay, M.N.; Nguyen, C.K.; Jimenez, R.; Petrosyan, S.; Murphy, A.N.; Gustafsson, A.B. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J. Biol. Chem. 2013, 288, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jasper, H.; Toan, S.; Muid, D.; Chang, X.; Zhou, H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021, 45, 102049. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Sciarretta, S.; Galeotti, J.; Volpe, M.; Sadoshima, J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ. Res. 2011, 109, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Kong, Y.; Tan, W.; May, H.; Battiprolu, P.K.; Pedrozo, Z.; Wang, Z.V.; Morales, C.; Luo, X.; Cho, G.; et al. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 2014, 129, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakis, P.; Romay-Ogando, M.I.; Timolati, F.; Suter, T.M.; Zuppinger, C. Effects of doxorubicin cancer therapy on autophagy and the ubiquitin-proteasome system in long-term cultured adult rat cardiomyocytes. Cell Tissue Res. 2012, 350, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.L.; Chen, H.L.; Wu, D.; Chen, J.X.; Wang, X.X.; Li, R.L.; He, J.H.; Mo, L.; Cen, X.; et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem. Pharmacol. 2014, 88, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, K.; Kobayashi, S.; Timm, D.; Liang, Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. J. Pharmacol. Exp. Ther. 2012, 341, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Takemura, G.; Kanamori, H.; Takeyama, T.; Watanabe, T.; Morishita, K.; Ogino, A.; Tsujimoto, A.; Goto, K.; Maruyama, R.; et al. Prior starvation mitigates acute doxorubicin cardiotoxicity through restoration of autophagy in affected cardiomyocytes. Cardiovasc. Res. 2012, 96, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Sishi, B.J.; Loos, B.; van Rooyen, J.; Engelbrecht, A.M. Autophagy upregulation promotes survival and attenuates doxorubicin-induced cardiotoxicity. Biochem. Pharmacol. 2013, 85, 124–134. [Google Scholar] [CrossRef]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663–46680. [Google Scholar] [CrossRef] [PubMed]
- Dirks-Naylor, A.J. The role of autophagy in doxorubicin-induced cardiotoxicity. Life Sci. 2013, 93, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative stress in cell death and cardiovascular diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yan, D.; Li, S.; Liu, S.; Zeng, F.; Cheung, C.W.; Liu, H.; Irwin, M.G.; Huang, H.; Xia, Z. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. J. Cell. Mol. Med. 2020, 24, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; He, C.; Zou, M.H. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 2011, 7, 1254–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Woollard, J.R.; Ebrahimi, B.; Crane, J.A.; Jordan, K.L.; Lerman, A.; Wang, S.M.; Lerman, L.O. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Boppana, V.S.; Umapathi, M.; Frati, G.; Sadoshima, J. Boosting autophagy in the diabetic heart: A translational perspective. Cardiovasc. Diagn. Ther. 2015, 5, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ. Res. 2019, 124, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. A review of biologically active flavonoids as inducers of autophagy and apoptosis in neoplastic cells and as cytoprotective agents in non-neoplastic cells. Cell Biol. Int. 2022, 46, 1179–1195. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, biosynthesis and chemical ecology. In Flavonoids: From Biosynthesis to Human Health; IntechOpen: London, UK, 2017; Volume 13, pp. 78–94. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED Study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, M.; Estruch, R.; Shahbaz, M.; Casas, R. Relation of fruits and vegetables with major cardiometabolic risk factors, markers of oxidation, and inflammation. Nutrients 2019, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I.; et al. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. 2020, 11, 6777–6806. [Google Scholar] [CrossRef]
- Allawadhi, P.; Khurana, A.; Sayed, N.; Kumari, P.; Godugu, C. Isoproterenol-induced cardiac ischemia and fibrosis: Plant-based approaches for intervention. Phytother. Res. 2018, 32, 1908–1932. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, G.L.; Shylaja, H.; Keni, R.; Nandakumar, K.; Rajesh, S. A systematic review and meta-analysis on the cardio-protective activity of naringin based on pre-clinical evidences. Phytother. Res. 2022, 36, 1064–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Man, W.; Shen, M.; Zhang, M.; Lin, J.; Wang, T.; Duan, Y.; Li, C.; Zhang, R.; Gao, E.; et al. Luteolin alleviates post-infarction cardiac dysfunction by up-regulating autophagy through Mst1 inhibition. J. Cell. Mol. Med. 2016, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, D.; Qin, Y.; Liu, Z.; Zhang, G.; Zhu, X.; Zeng, L.; Liang, Z. Nobiletin attenuates adverse cardiac remodeling after acute myocardial infarction in rats via restoring autophagy flux. Biochem. Biophys. Res. Commun. 2017, 492, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Liu, Z.; Ma, Z.; An, D.; Xu, D. Apigenin attenuates myocardial infarction-induced cardiomyocyte injury by modulating Parkin-mediated mitochondrial autophagy. J. Biosci. 2020, 45, 75. [Google Scholar] [CrossRef]
- Xu, L.J.; Chen, R.C.; Ma, X.Y.; Zhu, Y.; Sun, G.B.; Sun, X.B. Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine 2020, 68, 153169. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.; Huang, X. Formononetin may protect aged hearts from ischemia/reperfusion damage by enhancing autophagic degradation. Mol. Med. Rep. 2018, 18, 4821–4830. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Wang, Y.; Ding, T.; Zhang, X.; Wu, N. Pharmacological postconditioning with sappanone A ameliorates myocardial ischemia reperfusion injury and mitochondrial dysfunction via AMPK-mediated mitochondrial quality control. Toxicol. Appl. Pharmacol. 2021, 427, 115668. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Lv, Z.Y.; Yin, M.J.; Chen, J.; Wang, J.; Wu, Q.N. The Protective effect of cyanidin-3-glucoside on myocardial ischemia-reperfusion injury through ferroptosis. Oxid. Med. Cell Longev. 2021, 2021, 8880141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, X.; Wang, J.; Xu, W.; Yi, H.; Ma, R.; Jiang, H. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. Int. J. Mol. Med. 2018, 42, 917–1924. [Google Scholar] [CrossRef] [PubMed]
- Xuan, F.; Jian, J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int. J. Mol. Med. 2016, 38, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, R.; Gan, X.; Yang, X.; Chen, L.; Jian, J. MicroRNA-384-5p/Beclin-1 as potential indicators for Epigallocatechin Gallate against cardiomyocytes ischemia reperfusion injury by inhibiting autophagy via PI3K/Akt pathway. Drug Des. Dev. Ther. 2019, 13, 3607–3623. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Rao, T.; Fang, Z.; Zhang, J. The role and mechanism of hyperoside against myocardial infarction in mice by regulating autophagy via NLRP1 inflammation pathway. J. Ethnopharmacol. 2021, 276, 114187. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Wang, B.; Chi, W.; Li, Z.; Zhang, M.; Shen, Y.; Liu, X.; Lu, Y.; Liu, Y. Dihydromyricetin protects against Doxorubicin-induced cardiotoxicity through activation of AMPK/mTOR pathway. Phytomedicine 2022, 99, 154027. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, M.; Carresi, C.; Nucera, S.; Ruga, S.; Maiuolo, J.; Macrì, R.; Scarano, F.; Bosco, F.; Mollace, R.; Cardamone, A.; et al. Icariin protects H9c2 rat cardiomyoblasts from Doxorubicin-induced cardiotoxicity: Role of caveolin-1 upregulation and enhanced autophagic response. Nutrients 2021, 13, 4070. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, Y.; Chen, H.; Cai, X. Protective effect of flavonoids from Rosa roxburghii Tratt on myocardial cells via autophagy. 3 Biotech 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, L. Spinacetin alleviates doxorubicin-induced cardiotoxicity by initiating protective autophagy through SIRT3/AMPK/mTOR pathways. Phytomedicine 2022, 101, 154098. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, H.; Zha, W.; Cui, W.; Xu, L.; Min, Q.; Wu, J. Apigenin attenuates adriamycin-induced cardiomyocyte apoptosis via the PI3K/AKT/mTOR pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 2590676. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin attenuates doxorubicin-induced cardiotoxicity through promoting mitochondrial autophagy. Front. Physiol. 2020, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, L.; Han, Y.; Yang, Q.; Li, X.; Xin, B.; Chi, M.; Wang, Y.; Guo, C. Scutellarin attenuates doxorubicin-induced cardiotoxicity by inhibiting myocardial fibrosis, apoptosis and autophagy in rats. Chem. Biodivers. 2023, 20, e202200450. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, L.; Gao, L.; Wang, Y.; Wang, W. Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomed. Pharmacother. 2021, 137, 111375. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, L.; Zhang, Z.; He, X.; Fan, Q.; Cheng, X.; Qiao, Y.; Huang, H.; Lai, S.; Wan, Q.; et al. Puerarin activates adaptive autophagy and protects the myocardium against doxorubicin-induced cardiotoxicity via the 14-3-3gamma/PKCepsilon pathway. Biomed. Pharmacother. 2022, 153, 113403. [Google Scholar] [CrossRef]
- Wu, J.; Li, K.; Liu, Y.; Feng, A.; Liu, C.; Adu-Amankwaah, J.; Ji, M.; Ma, Y.; Hao, Y.; Bu, H.; et al. Daidzein ameliorates doxorubicin-induced cardiac injury by inhibiting autophagy and apoptosis in rats. Food Funct. 2023, 14, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Shabalala, S.; Louw, J.; Kappo, A.P.; Muller, C.J.F. Aspalathin reverts doxorubicin-induced cardiotoxicity through in-creased autophagy and decreased expression of p53/mTOR/p62signaling. Molecules 2017, 22, 1589. [Google Scholar] [CrossRef] [PubMed]
- Songbo, M.; Lang, H.; Xinyong, C.; Bin, X.; Ping, Z.; Liang, S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, W.; Song, Z.P.; Chen, Y.G.; Zhang, D.D.; Wang, C.Q. Rapamycin inhibits cardiac hypertrophy by promoting autophagy via the MEK/ERK/Beclin-1 pathway. Front. Physiol. 2016, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, D.; Liu, Z.; Li, M.; Hong, H.; Liu, C.; Gao, S.; Li, H.; Cai, Y.; Chen, S.; et al. SIRT6 suppresses isoproterenol-induced cardiac hypertrophy through activation of autophagy. Transl. Res. 2016, 172, 96–112.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, Z.; Li, Y.; Ou, C.; Huang, Z.; Zhang, J.; Liu, P.; Luo, C.; Chen, M. Puerarin prevents cardiac hypertrophy induced by pressure overload through activation of autophagy. Biochem. Biophys. Res. Commun. 2015, 464, 908–915. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Lu, C.; Qu, F.; Jiang, X. Protective effect of hyperoside on heart failure rats via attenuating myocardial apoptosis and inducing autophagy. Biosci. Biotechnol. Biochem. 2020, 84, 714–724. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Liu, G.; Ding, W.; Chang, W.; Xu, T.; Ji, X.; Zheng, X.; Zhang, J.; Wang, J. Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol. Sin. 2021, 42, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, D.; Cen, X.F.; Qiu, H.L.; Ma, Y.L.; Liu, Y.; Huang, S.H.; Liu, L.B.; Xu, M.; Tang, Q.Z. Diosmetin protects against cardiac hypertrophy via p62/Keap1/Nrf2 signaling pathway. Oxid. Med. Cell Longev. 2022, 2022, 8367997. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Wang, J.; Zhang, D. Luteolin suppresses lipopolysaccharide-induced cardiomyocyte hypertrophy and autophagy in vitro. Mol. Med. Rep. 2019, 19, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, Y.; Feng, Z.; Long, J. (–)-Epigallocatechin-3-gallate attenuated myocardial mitochondrial dysfunction and autophagy in diabetic Goto–Kakizaki rats. Free Radic. Res. 2014, 48, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Lin, N.; Lu, W.; Sun, Z.; Lin, H.; Chi, J.; Guo, H. Dihydromyricetin prevents diabetic cardiomyopathy via miR-34a suppression by activating autophagy. Cardiovasc. Drugs Ther. 2020, 34, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Yang, R.; Mehmood, S.; Li, Y. Epigallocatechin-3-gallate attenuates myocardial fibrosis in diabetic rats by activating autophagy. Exp. Biol. Med. 2022, 247, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fan, X.; Li, S.; Li, Z.; Tian, M.; Li, S. Scutellarin improves type 2 diabetic cardiomyopathy by regulating cardiomyocyte autophagy and apoptosis. Dis. Markers 2022, 2022, 3058354. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Chen, M.Y.; Han, Y.P.; Liu, L.J.; Yan, J.L.; Qian, L.B. The protection of luteolin against diabetic cardiomyopathy in rats is related to reversing JNK-suppressed autophagy. Food Funct. 2023, 14, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lin, J.; Luo, J.; Han, D.; Fan, M.; Guo, T.; Tao, L.; Yuan, M.; Yi, F. Dihydromyricetin protects against diabetic cardiomyopathy in Streptozotocin-induced diabetic mice. Biomed. Res. Int. 2017, 2017, 3764370. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lang, F.; Zhang, H.; Xu, L.; Wang, Y.; Zhai, C.; Hao, E. Apigenin alleviates endotoxin-induced myocardial toxicity by modulating inflammation, oxidative stress, and autophagy. Oxid. Med. Cell Longev. 2017, 2017, 2302896. [Google Scholar] [CrossRef]
- Shiroorkar, P.N.; Afzal, O.; Kazmi, I.; Al-Abbas, F.A.; Altamimi, A.S.A.; Gubbiyappa, K.S.; Sreeharsha, N. Cardioprotective effect of tangeretin by inhibiting PTEN/AKT/mTOR axis in experimental sepsis-induced myocardial dysfunction. Molecules 2020, 25, 5622. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Song, H.; Fan, M.; You, F.; Zhang, L.; Luo, J.; Li, J.; Wang, L.; Li, C.; Yuan, M. Luteolin attenuates sepsis-induced myocardial injury by enhancing autophagy in mice. Int. J. Mol. Med. 2020, 45, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; He, Y.; Wang, L.; Luo, C.; Liu, Y.; Li, R. Puerarin alleviates LPS-induced H9C2 cell injury by inducing mitochondrial autophagy. J. Cardiovasc. Pharmacol. 2022, 80, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chuankayan, P.; Rimlumduan, T.; Svasti, J.; Ketudat Cairns, J.R. Hydrolysis of soybean isoflavonoid glycosides by dalbergia β-glucosidases. J. Agric. Food Chem. 2007, 55, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.F.; Zhang, Q.; Raheem, K.S.; O’Hagan, D.; O’Connell, M.A.; Kay, C.D. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J. Nutr. 2016, 146, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, B.; Siqin, B.; He, Q.; Zhang, R.; Meng, X.; Zhang, N.; Zhang, N.; Li, M. Gut microbiota: A novel regulator of cardiovascular disease and key factor in the therapeutic effects of flavonoids. Front. Pharmacol. 2021, 12, 651926. [Google Scholar] [CrossRef]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of several flavonoids on human gut microbiota and its metabolism by in vitro simulated fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Qing, Y.; Li, H.; Sun, W.; Yu, X.; Hui, H.; Guo, Q.; Xu, J. FV-429 induces autophagy blockage and lysosome-dependent cell death of T-cell malignancies via lysosomal dysregulation. Cell Death Dis. 2021, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.F.; Zhang, D.; Wang, Z.Y.; Wang, L. Quercetin alleviates Cadmium-induced autophagy inhibition via TFEB-dependent lysosomal restoration in primary proximal tubular cells. Ecotoxicol. Environ. Saf. 2021, 208, 111743. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, W.; Guo, L.; Zhang, H.; Guan, L.; Yan, X.; Zhai, Y.; Qiao, Y.; Wang, Z.; Zhao, J.; et al. Quercetin protects against palmitate-induced pancreatic β-cell apoptosis by restoring lysosomal function and autophagic flux. J. Nutr. Biochem. 2022, 107, 09060. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Guo, L.; Zhang, H.; Liu, H.; Zhou, W.; Zhai, Y.; Yan, X.; Men, X.; Peng, L. Naringin protects against non-alcoholic fatty liver disease by promoting autophagic flux and lipophagy. Mol. Nutr. Food Res. 2024, 68, e2200812. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, Y.; Zhang, X.; Chen, X.; Liu, Z.; Tian, X. Isoquercetin ameliorates myocardial infarction through anti-inflammation and anti-apoptosis factor and regulating TLR4-NF-κB signal pathway. Mol. Med. Rep. 2018, 17, 6675–6680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Chen, R.R.; Li, J.H.; Chen, J.Y.; Li, W.; Niu, X.L.; Wang, F.F.; Wang, J.; Yang, J.X. Puerarin protects against myocardial ischemia/reperfusion injury by inhibiting inflammation and the NLRP3 inflammasome: The role of the SIRT1/NF-kappaB pathway. Int. Immunopharmacol. 2020, 89, 107086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, Z.; Zhu, C.; Fu, Z.; Yu, D. Luteolin alleviates myocardial ischemia reperfusion injury in rats via Siti1/NLRP3/NF-κB pathway. Int. Immunopharmacol. 2020, 85, 106680. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, L.; Li, X.; Zhao, L.; Hu, X.; Ma, Z. Short-term pretreatment of naringin isolated from Citrus wilsonii Tanaka attenuates rat myocardial ischemia/reperfusion injury. Naunyn Schmiedeb. Arch. Pharmacol. 2022, 395, 1047–1059. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’Abbate, S.; Kusmic, C. The Protective Effect of Flavonoids in the Diet on Autophagy-Related Cardiac Impairment. Nutrients 2024, 16, 2207. https://doi.org/10.3390/nu16142207
L’Abbate S, Kusmic C. The Protective Effect of Flavonoids in the Diet on Autophagy-Related Cardiac Impairment. Nutrients. 2024; 16(14):2207. https://doi.org/10.3390/nu16142207
Chicago/Turabian StyleL’Abbate, Serena, and Claudia Kusmic. 2024. "The Protective Effect of Flavonoids in the Diet on Autophagy-Related Cardiac Impairment" Nutrients 16, no. 14: 2207. https://doi.org/10.3390/nu16142207
APA StyleL’Abbate, S., & Kusmic, C. (2024). The Protective Effect of Flavonoids in the Diet on Autophagy-Related Cardiac Impairment. Nutrients, 16(14), 2207. https://doi.org/10.3390/nu16142207







