A Novel Nutraceutical Supplement Lowers Postprandial Glucose and Insulin Levels upon a Carbohydrate-Rich Meal or Sucrose Drink Intake in Healthy Individuals—A Randomized, Placebo-Controlled, Crossover Feeding Study
Highlights
- GLUBLOCTM reduced post-prandial blood glucose levels by 49.78% (iAUC0–60min) and 43.36% (iAUC0–120min) in the Sucrose drink arm.
- GLUBLOCTM reduced post-prandial blood glucose levels by 41.13% (iAUC0–60min) and 20.26% (iAUC0–120min) in the carbohydrate-rich meal intake arm.
- The use of GLUBLOCTM in lowering the impact of dietary carbohydrates on post-prandial blood glucose and insulin levels.
- The study highlights the potential application of GLUBLOCTM as a novel dietary supplement in effectively managing post-meal blood glucose spikes in prediabetes and diabetes and possibly helping manage insulin-resistant weight gain.
Abstract
:1. Introduction
2. Methods
Sample Size
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulati, S.; Misra, A. Sugar Intake, Obesity, and Diabetes in India. Nutrients 2014, 6, 5955–5974. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Atre, S. The burden of diabetes in India. Lancet Glob. Health 2019, 7, e418. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Akhtar, S.; Upadhyay, A.K.; Mohanty, S.K. Socioeconomic inequality in awareness, treatment and control of diabetes among adults in India: Evidence from National Family Health Survey of India (NFHS), 2019–2021. Sci. Rep. 2023, 13, 2971. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, P.; Bhansali, A.; Ravikiran, M.; Bhansali, S.; Walia, R.; Shanmugasundar, G.; Thakur, J.; Bhadada, S.K.; Dutta, P. Prevalence and risk factors of diabetes in a community-based study in North India: The Chandigarh Urban Diabetes Study (CUDS). Diabetes Metab. 2011, 37, 216–221. [Google Scholar] [CrossRef]
- Ruijgrok, C.; Blaak, E.E.; Egli, L.; Dussort, P.; Vinoy, S.; Rauh, S.P.; Beulens, J.W.; Robertson, M.D.; Alssema, M. Reducing postprandial glucose in dietaryintervention studies and the magnitude of the effect on diabetes-related risk factors: Asystematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. BioFactors 2013, 39, 448–456. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef]
- Tattersall, R. Alpha-glucosidase Inhibition as an Adjunct to the Treatment of Type 1 Diabetes. Diabet. Med. 1993, 10, 688–693. [Google Scholar] [CrossRef]
- Wright, E.M.; Hirayama, B.A.; Loo, D.F. Active sugar transport in health and disease. J. Intern. Med. 2007, 261, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef]
- Bashkin, A.; Ghanim, M.; Abu-Farich, B.; Rayan, M.; Miari, R.; Srouji, S.; Rayan, A.; Falah, M. Forty-One Plant Extracts Screened for Dual Antidiabetic and Antioxidant Functions: Evaluating the Types of Correlation between α-Amylase Inhibition and Free Radical Scavenging. Molecules 2021, 26, 317. [Google Scholar] [CrossRef]
- Takasu, S.; Parida, I.S.; Onose, S.; Ito, J.; Ikeda, R.; Yamagishi, K.; Higuchi, O.; Tanaka, F.; Kimura, T.; Miyazawa, T.; et al. Evaluation of the antihyperglycemic effect and safety of microorganism 1-deoxynojirimycin. PLoS ONE 2018, 13, e0199057. [Google Scholar] [CrossRef]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism: Mulberry DNJ and postprandial glycemia. J. Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Kojima, Y.; Kimura, T.; Nakagawa, K.; Asai, A.; Hasumi, K.; Oikawa, S.; Miyazawa, T. Effects of Mulberry Leaf Extract Rich in 1-Deoxynojirimycin on Blood Lipid Profiles in Humans. J. Clin. Biochem. Nutr. 2010, 47, 155–161. [Google Scholar] [CrossRef]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of mulberry leaves’ hypoglycemic properties and hypoglycemic mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Yu, T.; Seong, S.H.; Kuk, E.B.; Jung, H.A.; Choi, J.S. Protein Tyrosine Phosphatase 1B Inhibition and Glucose Uptake Potentials of Mulberrofuran G, Albanol B, and Kuwanon G from Root Bark of Morus alba L. in Insulin-Resistant HepG2 Cells: An In Vitro and In Silico Study. IJMS 2018, 19, 1542. [Google Scholar] [CrossRef]
- Niu, S.-L.; Tong, Z.-F.; Zhang, Y.; Liu, T.-L.; Tian, C.-L.; Zhang, D.-X.; Liu, M.-C.; Li, B.; Tian, J.-L. Novel Protein Tyrosine Phosphatase 1B Inhibitor-Geranylated Flavonoid from Mulberry Leaves Ameliorates Insulin Resistance. J. Agric. Food Chem. 2020, 68, 8223–8231. [Google Scholar] [CrossRef]
- Velasquez-Mieyer, P.A.; Cowan, P.A.; Arheart, K.L.; Buffington, C.K.; Spencer, K.A.; Connelly, B.E.; Cowan, G.W.; Lustig, R.H. Suppression of insulin secretion is associated with weight loss and altered macronutrient intake and preference in a subset of obese adults. Int. J. Obes. 2003, 27, 219–226. [Google Scholar] [CrossRef]
- Ci, Z.; Kikuchi, K.; Hatsuzawa, A.; Nakai, A.; Jiang, C.; Itadani, A.; Kojima, M. Antioxidant Activity, and α-Glucosidase, α-Amylase and Lipase Inhibitory Activity of Polyphenols in Flesh, Peel, Core and Seed from Mini Apple. AJFST 2018, 6, 258–262. [Google Scholar] [CrossRef]
- Okada, J.; Yamada, E.; Okada, K.; Okada, S.; Yamada, M. Comparing the efficacy of apple peels and a sodium-glucose cotransporter 2 inhibitor (ipragliflozin) on interstitial glucose levels: A pilot case study. Curr. Ther. Res. 2020, 93, 100597. [Google Scholar] [CrossRef]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary intake of phloridzin from natural occurrence in foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, W. Natural Products as Lead Compounds for Sodium Glucose Cotransporter (SGLT) Inhibitors. Planta Med. 2017, 83, 985–993. [Google Scholar] [CrossRef]
- Yu, C.H.; Migicovsky, Z.; Song, J.; Rupasinghe, H.V. (Poly)phenols of apples contribute to in vitro antidiabetic properties: Assessment of Canada’s Apple Biodiversity Collection. Plants People Planet 2023, 5, 225–240. [Google Scholar] [CrossRef]
- Venugopal, G.; Khan, Z.H.; Dash, R.; Tulsian, V.; Agrawal, S.; Rout, S.; Mahajan, P.; Ramadass, B. Predictive association of gut microbiome and NLR in anemic low middle-income population of Odisha—A cross-sectional study. Front. Nutr. 2023, 10, 1200688. [Google Scholar] [CrossRef] [PubMed]
- Lacey, S. Incremental Area Under the Curve; Zenodo: Geneve, Switzerland, 2022; Available online: https://zenodo.org/record/6951357 (accessed on 24 November 2023).
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M.S. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Hansawasdi, C.; Kawabata, J. α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar] [CrossRef]
- Yatsunami, K.; Ichida, M.; Onodera, S. The relationship between 1-deoxynojirimycin content and α-glucosidase inhibitory activity in leaves of 276 mulberry cultivars (Morus spp.) in Kyoto, Japan. J. Nat. Med. 2007, 62, 63–66. [Google Scholar] [CrossRef]
- Khanam, S.; Mishra, A.; Shahid, A.; Pujari, N.M. Therapeutic indication of Phloridzin: A new Gleam for metabolic disorders. Phytomed. Plus 2022, 2, 100200. [Google Scholar] [CrossRef]
- Thondre, P.S.; Lightowler, H.; Ahlstrom, L.; Gallagher, A. Mulberry leaf extract improves glycaemic response and insulaemic response to sucrose in healthy subjects: Results of a randomized, double blind, placebo-controlled study. Nutr. Metab. 2021, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ok, H.M.; Kim, J.; Park, S.W.; Kwon, S.W.; Kwon, O. Mulberry Leaf Extract Improves Postprandial Glucose Response in Prediabetic Subjects: A Randomized, Double-Blind Placebo-Controlled Trial. J. Med. Food 2015, 18, 306–313. [Google Scholar] [CrossRef]
- Lown, M.; Fuller, R.; Lightowler, H.; Fraser, A.; Gallagher, A.; Stuart, B.; Byrne, C.; Lewith, G. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo-controlled study. PLoS ONE 2017, 12, e0172239. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Ye, H.; Chen, Y.; Yu, J.; Yang, J.; Zhang, X. Randomized, Double-Blinded, Double-Dummy, Active-Controlled, and Multiple-Dose Clinical Study Comparing the Efficacy and Safety of Mulberry Twig (Ramulus Mori, Sangzhi) Alkaloid Tablet and Acarbose in Individuals with Type 2 Diabetes Mellitus. Evid.-Based Complement. Altern. Med. 2016, 2016, 7121356. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women: Results From 3 Prospective Cohort Studies. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef]
- Mudra, M.; Ercan-Fang, N.; Zhong, L.; Furne, J.; Levitt, M. Influence of Mulberry Leaf Extract on the Blood Glucose and Breath Hydrogen Response to Ingestion of 75 g Sucrose by Type 2 Diabetic and Control Subjects. Diabetes Care 2007, 30, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-N.; Kwon, Y.-I.; Jang, H.-D. Mulberry Leaf Extract Reduces Postprandial Hyperglycemia with Few Side Effects by Inhibiting α-Glucosidase in Normal Rats. J. Med. Food 2011, 14, 712–717. [Google Scholar] [CrossRef]
- Bonsembiante, L.; Targher, G.; Maffeis, C. Type 2 Diabetes and Dietary Carbohydrate Intake of Adolescents and Young Adults: What Is the Impact of Different Choices? Nutrients 2021, 13, 3344. [Google Scholar] [CrossRef]
- Hosseini, F.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Dietary carbohydrate and the risk of type 2 diabetes: An updated systematic review and dose–response meta-analysis of prospective cohort studies. Sci. Rep. 2022, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Rahman, S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, A.; Hannan, A.; Uddin, J.; Pang, M.-G. Role of Insulin in Health and Disease: An Update. IJMS 2021, 22, 6403. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Shakappa, D.; Naik, R.; Sobhana, P.P. Glycemic carbohydrates, glycemic index, and glycemic load of commonly consumed South Indian breakfast foods. J. Food Sci. Technol. 2022, 59, 3619–3626. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, B.; Rani, B.S.; Pugazhendhi, S.; John, K.R.; Ramakrishna, B.S. Faecal microbiota of healthy adults in south India: Comparison of a tribal & a rural population. Indian. J. Med. Res. 2017, 145, 237–246. [Google Scholar] [PubMed]
- Antony, M.A.; Chowdhury, A.; Edem, D.; Raj, R.; Nain, P.; Joglekar, M.; Verma, V.; Kant, R. Gut microbiome supplementation as therapy for metabolic syndrome. World J. Diabetes 2023, 14, 1502–1513. [Google Scholar] [CrossRef]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
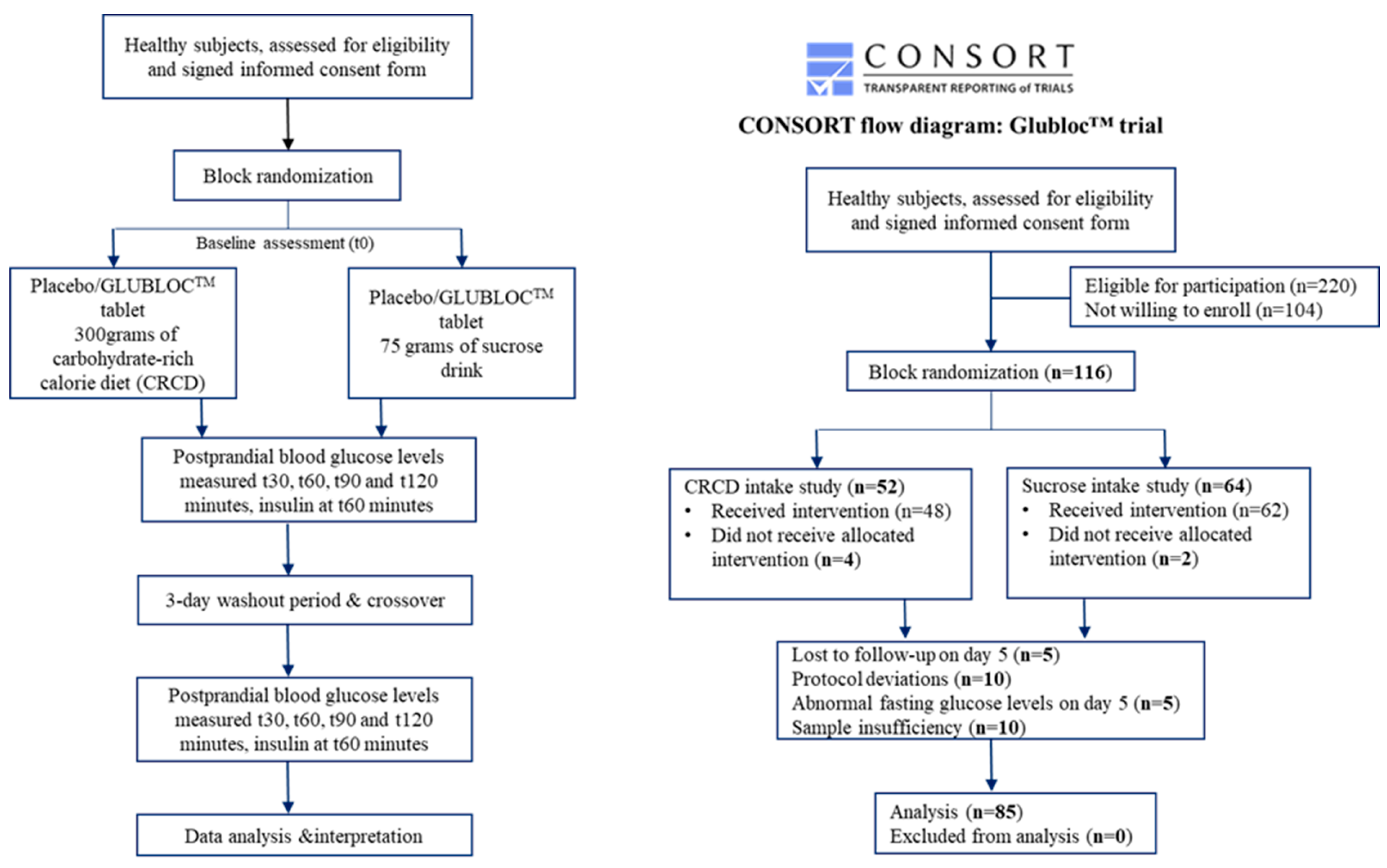

| Variable | Sucrose Drink Intake Group (n = 45) | CRCD Intake Group (n = 40) | ||||
|---|---|---|---|---|---|---|
| Day 1 (n = 45) | Day 5 (n = 45) | p-Value | Day 1 (n = 40) | Day 5 (n = 40) | p-Value | |
| Age (Yrs) | 28.6 ± 0.96 | __ | NA | 27.7 ± 1.14 | __ | NA |
| Gender M, F (%) | 39, 6 | __ | NA | 33, 7 | __ | NA |
| BMI (Kg/m2) | 21.9 ± 0.21 | __ | NA | 21.7 ± 0.26 | __ | NA |
| Fasting glucose (mg/dL) | 89.6 ± 1.43 | 85.9 ± 1.3 | 0.22 | 85.9 ± 1.06 | 88.2 ± 1 | 0.22 |
| Fasting insulin (µU/mL) | 7.38 ± 1.09 | 6.31 ± 0.69 | 0.41 | 7.45 ± 0.73 | 9.79 ± 1.57 | 0.24 |
| Parameter | Sucrose Drink Intake Group (n = 45) | ||||
|---|---|---|---|---|---|
| GLUBLOC™ | Placebo | Percentage Change (%) | p-Value | ||
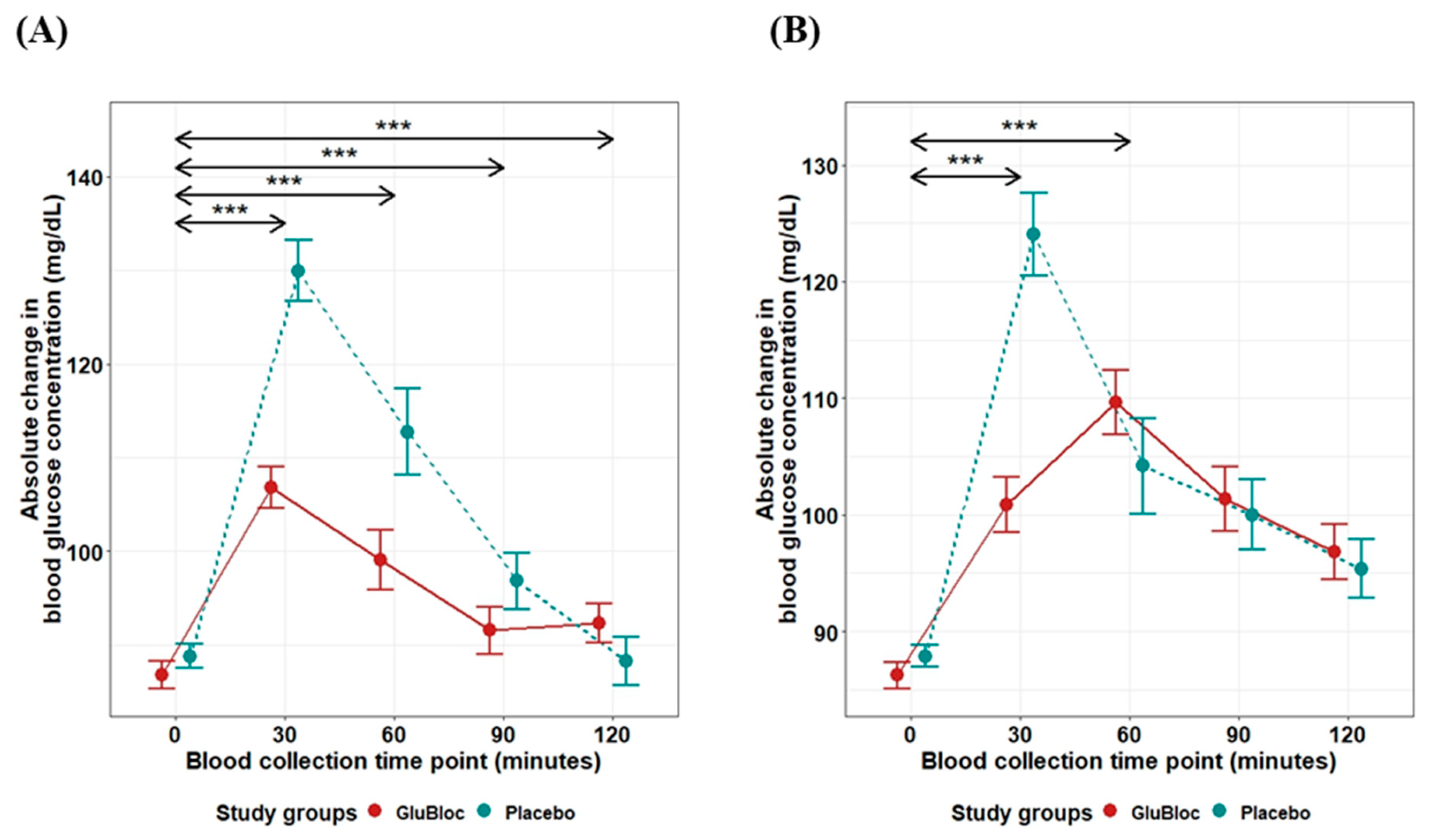
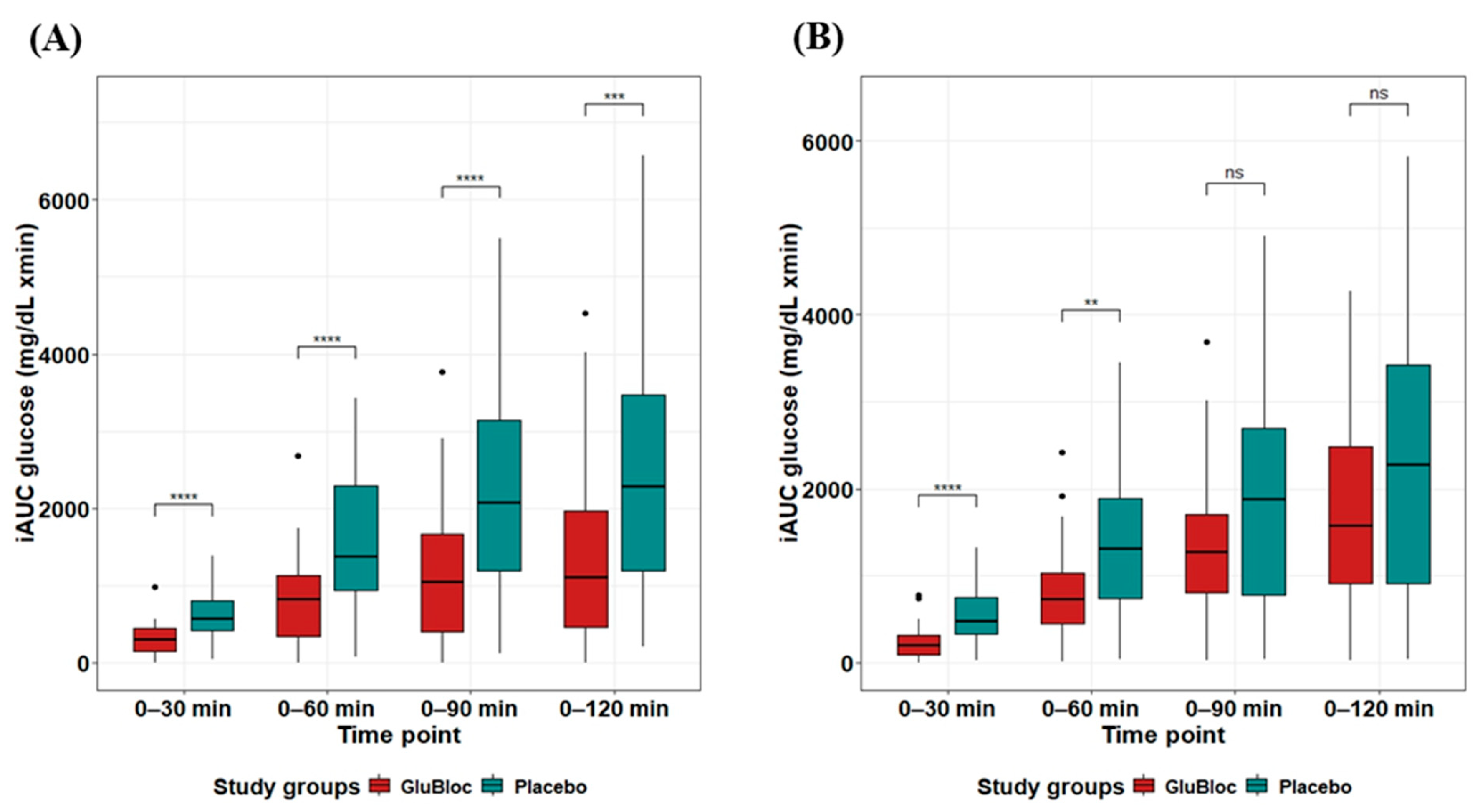
| Glucose mg/dL × min | 0–30 min | 304 ± 30.68 | 618 ± 44.99 | 50.81 | <0.0001 |
| 0–60 min | 816.47 ± 86.01 | 1625.81 ± 134.82 | 49.78 | <0.0001 | |
| 0–90 min | 1161.10 ± 137.09 | 2219.18 ± 204.52 | 47.68 | <0.0001 | |
| 0–120 min | 1406.19 ± 178.25 | 2483.04 ± 238.98 | 43.36 | <0.001 | |
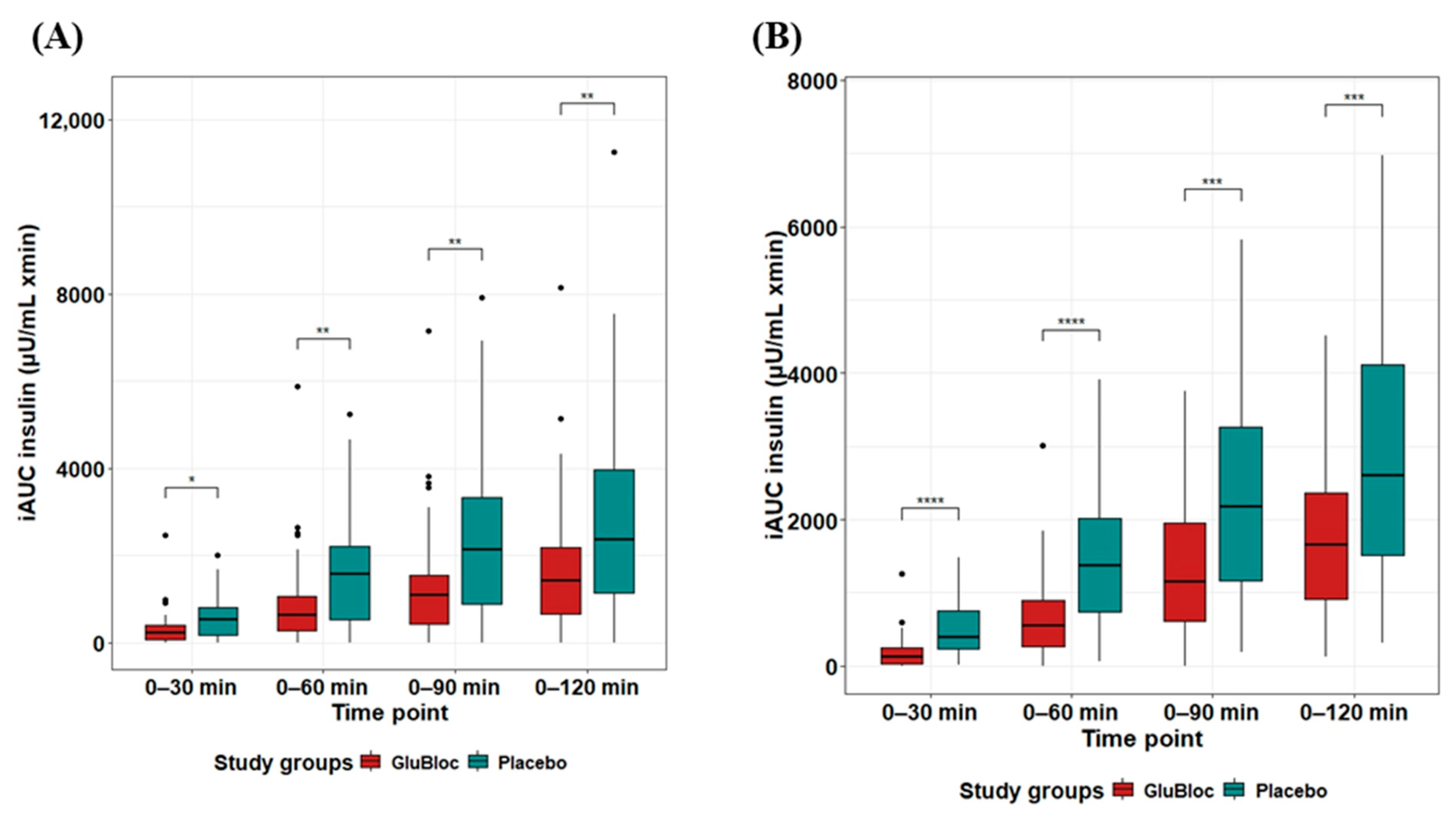
| Insulin µU/mL × min | 0–30 min | 323.85 ± 61.76 | 547.95 ± 66.17 | 40.9 | <0.05 |
| 0–60 min | 900.49 ± 152.7 | 1547.86 ± 177.41 | 41.82 | <0.01 | |
| 0–90 min | 1331.7 ± 197.48 | 2333.05 ± 27.62 | 42.92 | <0.001 | |
| 0–120 min | 1406.19 ± 178.25 | 2483.04 ± 238.98 | 40.96 | <0.01 | |
| Parameter | CRCD Intake Group (n = 40) | ||||
| GLUBLOC™ | Placebo | Percentage Change (%) | p-Value | ||
| Glucose mg/dL × min | 0–30 min | 227.63 ± 30.12 | 542.25 ± 51.7 | 58.02 | <0.0001 |
| 0–60 min | 802.76 ± 84.5 | 1363.62 ± 141.7 | 41.13 | <0.001 | |
| 0–90 min | 1395.47 ± 134.31 | 1887.52 ± 208.08 | 26.07 | 0.05 | |
| 0–120 min | 1812.80 ± 173 | 2273.40 ± 252.84 | 20.26 | 0.13 | |
| Insulin µU/mL × min | 0–30 min | 201.73 ± 37.96 | 502.04 ± 56.38 | 59.82 | <0.0001 |
| 0–60 min | 700.76 ± 98.24 | 1465.56 ± 151.35 | 52.19 | <0.0001 | |
| 0–90 min | 1286.82 ± 142.15 | 2323.62 ± 234.58 | 44.62 | <0.001 | |
| 0–120 min | 1693.02 ± 232.60 | 2867.92 ± 345.45 | 41.3 | <0.001 | |
| Sucrose Drink Intake Group (n = 45) | CRCD Intake Group (n = 40) | |||||
|---|---|---|---|---|---|---|
| Cmax (mg/dL) | Cmax (mg/dL) | |||||
| Parameter | GLUBLOCTM (n = 45) | Placebo (n = 45) | p-Value (FDR-Corrected) | GLUBLOCTM (n = 40) | Placebo (n = 40) | p-Value (FDR-Corrected) |
| Glucose (mg/dL) | 111.8 ± 2.3 | 133.87 ± 3.54 | 0.000001 | 114.38 ± 2.53 | 127.13 ± 3.46 | 0.00401 |
| Insulin (µU/mL) | 34.83 ± 4.56 | 50.09 ± 5.06 | 0.0276 | 40.51 ± 3.79 | 51.47 ± 4.22 | 0.0571 |
| Tmax (min) | Tmax (min) | |||||
| Glucose (mg/dL) | 41.33 ± 3.06 | 42 ± 2.76 | 0.872 | 65.25 ± 4.41 | 42 ± 3.69 | 0.000127 |
| Insulin (µU/mL) | 54.67 ± 4.60 | 49.33 ± 4.17 | 0.393 | 69.75 ± 4.35 | 57.75 ± 4.21 | 0.051 |
| Diet | Parameter | Variable | Mean Difference | Lower 95 CI | Upper 95 CI | p-Value |
|---|---|---|---|---|---|---|
| Sucrose drink intake (n = 45) | Glucose | Cmax (mg/dL) | 22.07 | 15.72 | 28.41 | 1.11 × 10−8 |
| Tmax (min) | 0.67 | −6.12 | 7.46 | 0.84 | ||
| Insulin | Cmax (µU/mL) | 15.26 | 1.21 | 29.3 | 0.03 | |
| Tmax (min) | −5.33 | −18.55 | 7.88 | 0.42 | ||
| CRCD intake (n = 40) | Glucose | Cmax (mg/dL) | 12.75 | 6.46 | 19.04 | 0.0002 |
| Tmax (min) | 23.25 | 11.06 | 35.44 | 0.0004 | ||
| Insulin | Cmax (µU/mL) | 10.95 | 1.88 | 20.02 | 0.01 | |
| Tmax (min) | −12 | −24.06 | 0.06 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopal, G.; Dash, R.; Agrawal, S.; Ray, S.; Kumar Sahoo, P.; Ramadass, B. A Novel Nutraceutical Supplement Lowers Postprandial Glucose and Insulin Levels upon a Carbohydrate-Rich Meal or Sucrose Drink Intake in Healthy Individuals—A Randomized, Placebo-Controlled, Crossover Feeding Study. Nutrients 2024, 16, 2237. https://doi.org/10.3390/nu16142237
Venugopal G, Dash R, Agrawal S, Ray S, Kumar Sahoo P, Ramadass B. A Novel Nutraceutical Supplement Lowers Postprandial Glucose and Insulin Levels upon a Carbohydrate-Rich Meal or Sucrose Drink Intake in Healthy Individuals—A Randomized, Placebo-Controlled, Crossover Feeding Study. Nutrients. 2024; 16(14):2237. https://doi.org/10.3390/nu16142237
Chicago/Turabian StyleVenugopal, Giriprasad, Rishikesh Dash, Siwani Agrawal, Sayantan Ray, Prasanta Kumar Sahoo, and Balamurugan Ramadass. 2024. "A Novel Nutraceutical Supplement Lowers Postprandial Glucose and Insulin Levels upon a Carbohydrate-Rich Meal or Sucrose Drink Intake in Healthy Individuals—A Randomized, Placebo-Controlled, Crossover Feeding Study" Nutrients 16, no. 14: 2237. https://doi.org/10.3390/nu16142237
APA StyleVenugopal, G., Dash, R., Agrawal, S., Ray, S., Kumar Sahoo, P., & Ramadass, B. (2024). A Novel Nutraceutical Supplement Lowers Postprandial Glucose and Insulin Levels upon a Carbohydrate-Rich Meal or Sucrose Drink Intake in Healthy Individuals—A Randomized, Placebo-Controlled, Crossover Feeding Study. Nutrients, 16(14), 2237. https://doi.org/10.3390/nu16142237









