The Nigerian Dietary Screening Tool: A Step toward Improved Patient-Clinician Communication in Nigerian Hospitals: A Pilot Implementation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sample Size
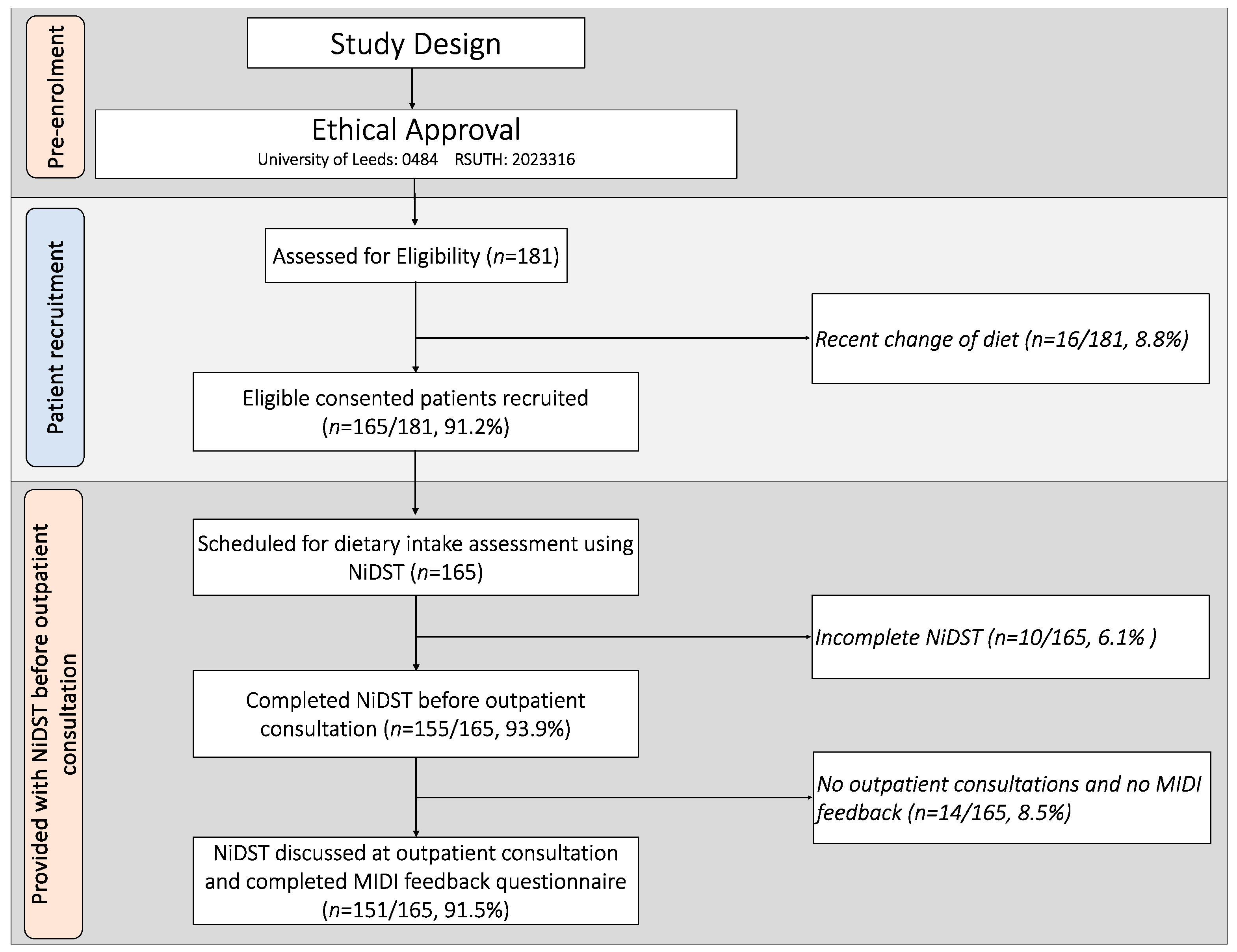
2.3. Participant Recruitment
2.4. Innovation—The Nigerian Dietary Screening Tool (NiDST)
2.5. Data Collection
2.5.1. Pre-Implementation Stage
2.5.2. Implementation Stage
2.5.3. Post-Implementations Stage
2.6. Outcome Measures
2.6.1. Fidelity of Implementation
2.6.2. Barriers to and Facilitators of Implementation
2.7. Data Analyses
2.7.1. Quantitative Data
2.7.2. Qualitative Data
3. Results
3.1. Participant Characteristics
3.2. Food Intake Assessment
3.3. Completion and Consultation Time
3.4. Fidelity of the Use of the NiDST
3.5. Barriers to and Facilitators of Implementation of the NiDST
3.5.1. Barriers Perceived by Clinicians
“We only offer little dietary support to patients in the clinic. The number of patients we see on each clinic day is so much as such there is no time to fully assess patient food intake and provide one-on-one dietary counselling. Personally, I refer patients to see the dietician for the purpose of nutritional assessment and counselling”. (Female, 28 years).
“In my opinion, the tool was very useful, but the challenge is the additional time needed to counsel and make recommendations to the patients because you can imagine spending 20–45 min with one patient in a busy clinic”. (Male, 38 years)
“Well, it takes me some time to go through the tool and understand the patient’s food intake before I can start discussing it with them and advise them, but I think if the tool can be made simple and training be given on how to use it, then it will be easy to apply it without taken much time”. (Male, 36 years)
3.5.2. Facilitators Perceived by Clinicians
“For me, the tool integrated well into our routine workflow and training clinicians on how to use the tool, especially how to interpret the tool provided some sort of dietary guidelines for each food group. Yeah, this can help clinicians more focused on the dietary discussion with the patient, and this can shorten the consultation time” (Female, 48 years)
“From my experience, I’ve found that the NiDST really helps me dive into understanding my patients’ dietary habits better. It’s like having a comprehensive roadmap right in front of me during consultations. And you know what’s great? It doesn’t just stop there. Using the NiDST has actually improved my communication with patients. It’s like it bridges that gap between us, making our conversations more meaningful and productive. It’s been a game-changer for me, honestly”. (Female, 42 years)
“Yeah, I saw most of the patient were satisfied because the dietary discussion was focused on their usual food intake. They were able to ask questions on the right food they eat to reduce their risk of hypertension. Like, there was this one patient who mentioned how surprised to know that excess use of Maggi can worsen hypertension. They were like, ‘I use plenty Maggi when cooking my stews and soups”. (Female, 36 years)
3.5.3. Facilitators Perceived by Patients
“[It] was very straightforward and easy to fill out. The instructions at the top clearly explained what was needed, and the arrangement with the columns was simple to understand”. (Female, 45 years, >5 years hypertensive).
“I was able to complete the questionnaire in just a few minutes while waiting to see my doctor. It didn’t feel long or burdensome at all. The length and time needed fit perfectly into the normal clinic visit”. (Male, 61 years, non-hypertensive)
“Filling the form and discussing with my doctor made me more aware of how often I eat food prepared by mama put and red meats each week. It was eye-opening, and this will motivate me to improve my diet. For me, I will complete the form next time I come to the hospital” (Male, 47 years, 1 year hypertensive)
“I think the use of the complete form really helps my doctor to understand the food I usually eat. I was really happy to hear the doctor telling me to reduce some food that I eat because I can contribute to my BP not being controlled”. (Female, 56 years > 5 years hypertensive)
4. Discussion
4.1. Patient–Clinician Communication
4.2. Barriers to and Facilitators of Implementation
4.3. Practical Implication and Clinical Relevance
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Bayaraa, N.; Azahar, N.M.; Kitaoka, K.; Kobayashi, Y.; Yano, Y. African Control of Hypertension through Innovative Epidemiology and a Vibrant Ecosystem (ACHIEVE): A holistic approach for hypertension control in Africa. J. Hum. Hypertens. 2023, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Peer, N.; Uthman, O.A.; Kengne, A.P. Rising prevalence, and improved but suboptimal management, of hypertension in South Africa: A comparison of two national surveys. Glob. Epidemiol. 2021, 3, 100063. [Google Scholar] [CrossRef] [PubMed]
- Bosu, W.K.; Reilly, S.T.; Aheto, J.M.K.; Zucchelli, E. Hypertension in older adults in Africa: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0214934. [Google Scholar] [CrossRef] [PubMed]
- Akinkugbe, O.O. Non-Communicable Diseases in Nigeria: National Survey (Final Report) on Hypertension, Coronary Heart Disease, Diabetes Mellitus, Haemoglobinopathies, G6PD Deficiency and Anaemia. National Expert Committee on Non-Communicable Diseases; Federal Ministry of Health and Social Services: Lagos, Nigeria, 1997. [Google Scholar]
- Odili, A.N.; Chori, B.S.; Danladi, B.; Nwakile, P.C.; Okoye, I.C.; Abdullahi, U.; Nwegbu, M.N.; Zawaya, K.; Essien, I.; Sada, K.; et al. Prevalence, Awareness, Treatment and Control of Hypertension in Nigeria: Data from a Nationwide Survey 2017. Glob. Heart 2020, 15, 47. [Google Scholar] [CrossRef]
- Adeloye, D.; Owolabi, E.O.; Ojji, D.B.; Auta, A.; Dewan, M.T.; Olanrewaju, T.O.; Ogah, O.S.; Omoyele, C.; Ezeigwe, N.; Mpazanje, R.G.; et al. Prevalence, awareness, treatment, and control of hypertension in Nigeria in 1995 and 2020: A systematic analysis of current evidence. J. Clin. Hypertens. 2021, 23, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, E.; Dogbe, W. The cause and effect of the nutrition transition in Nigeria: Analysis of the value of indigenous knowledge and traditional foods in Enugu State, Igboland. J. Ethn. Foods 2023, 10, 30. [Google Scholar] [CrossRef]
- Oguoma, V.M.; Nwose, E.U.; Skinner, T.C.; Digban, K.A.; Onyia, I.C.; Richards, R.S. Prevalence of cardiovascular disease risk factors among a Nigerian adult population: Relationship with income level and accessibility to CVD risks screening. BMC Public Health 2015, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Ogah, O.S.; Okpechi, I.; Chukwuonye, I.I.; Akinyemi, J.O.; Onwubere, B.J.; Falase, A.O.; Stewart, S.; Sliwa, K. Blood pressure, prevalence of hypertension and hypertension related complications in Nigerian Africans: A review. World J. Cardiol. 2012, 4, 327–340. [Google Scholar] [CrossRef]
- Petrikova, I.; Bhattacharjee, R.; Fraser, P.D. The ‘Nigerian Diet’ and Its Evolution: Review of the Existing Literature and Household Survey Data. Foods 2023, 12, 443. [Google Scholar] [CrossRef]
- Batubo, N.P.; Moore, J.B.; Zulyniak, M.A. Dietary factors and hypertension risk in West Africa: A systematic review and meta-analysis of observational studies. J. Hypertens. 2023, 41, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S. The Role of Nutrition in Chronic Disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef]
- Adesina, M.A.; Oladele, R.I.; Olufadewa, I.I.; Onothoja, O.F.; Oladipo, D.R.; Iyiola, O.P.; Ekott, M.B.; Nwachukwu, P.C.; Baru, A.; Akinloye, S.J. Addressing the high burden of noncommunicable diseases in Nigeria: A commentary. J. Health Res. 2021, 35, 457–462. [Google Scholar] [CrossRef]
- Scrafford, C.G.; Bi, X.; Multani, J.K.; Murphy, M.M.; Schmier, J.K.; Barraj, L.M. Health Economic Evaluation Modeling Shows Potential Health Care Cost Savings with Increased Conformance with Healthy Dietary Patterns among Adults in the United States. J. Acad. Nutr. Diet. 2019, 119, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Krebs-Smith, S.M.; Subar, A.F.; George, S.M.; Harmon, B.E.; Neuhouser, M.L.; Boushey, C.J.; Schap, T.E.; Reedy, J. The Dietary Patterns Methods Project: Synthesis of findings across cohorts and relevance to dietary guidance. J. Nutr. 2015, 145, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; De Lucia, F.; Olivieri, M.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonanni, A.; Moli-sani Project, I. Nutrition knowledge is associated with higher adherence to Mediterranean diet and lower prevalence of obesity. Results from the Moli-sani study. Appetite 2013, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.E.; Hebert, J.R.; Hurley, T.G.; Resnicow, K.; Thompson, F.E.; Greene, G.W.; Shaikh, A.R.; Yaroch, A.L.; Williams, G.C.; Salkeld, J.; et al. Accuracy and precision of two short screeners to assess change in fruit and vegetable consumption among diverse populations participating in health promotion intervention trials. J. Nutr. 2008, 138, 218S–225S. [Google Scholar] [CrossRef] [PubMed]
- Vadiveloo, M.; Lichtenstein, A.H.; Anderson, C.; Aspry, K.; Foraker, R.; Griggs, S.; Hayman, L.L.; Johnston, E.; Stone, N.J.; Thorndike, A.N.; et al. Rapid Diet Assessment Screening Tools for Cardiovascular Disease Risk Reduction Across Healthcare Settings: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e000094. [Google Scholar] [CrossRef] [PubMed]
- Lara-Breitinger, K.M.; Medina Inojosa, J.R.; Li, Z.; Kunzova, S.; Lerman, A.; Kopecky, S.L.; Lopez-Jimenez, F. Validation of a Brief Dietary Questionnaire for Use in Clinical Practice: Mini-EAT (Eating Assessment Tool). J. Am. Heart Assoc. 2023, 12, e025064. [Google Scholar] [CrossRef] [PubMed]
- Batubo, N.P.; Carolyn, I.A.; Moore, J.B.; Zulyniak, M.A. Validity and reproducibility of a culturally tailored dietary screening tool for hypertension risk in Nigerian healthcare. medRxiv 2024. [Google Scholar] [CrossRef]
- Abaraogu, U.O.; Ogaga, M.O.; Odidika, E.; Frantz, J. Promotion of healthy nutrition in clinical practice: A cross-sectional survey of practices and barriers among physiotherapists in southeast Nigeria. Hong Kong Physiother. J. 2016, 35, 21–29. [Google Scholar] [CrossRef]
- Wang, Z.; Norris, S.L.; Bero, L. The advantages and limitations of guideline adaptation frameworks. Implement. Sci. 2018, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Ebell, M.H.; Shaughnessy, A.F.; Slawson, D.C. Why Are We So Slow to Adopt Some Evidence-Based Practices? Am. Fam. Physician 2018, 98, 709–710. [Google Scholar]
- Fischer, F.; Lange, K.; Klose, K.; Greiner, W.; Kraemer, A. Barriers and Strategies in Guideline Implementation-A Scoping Review. Healthcare 2016, 4, 36. [Google Scholar] [CrossRef]
- Kolasa, K.M.; Rickett, K. Barriers to providing nutrition counseling cited by physicians: A survey of primary care practitioners. Nutr. Clin. Pract. 2010, 25, 502–509. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Delgado, M.; Saxena, A. Trends and disparities in the prevalence of physicians’ counseling on diet and nutrition among the U.S. adult population, 2000–2011. Prev. Med. 2016, 89, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Aspry, K.E.; Van Horn, L.; Carson, J.A.S.; Wylie-Rosett, J.; Kushner, R.F.; Lichtenstein, A.H.; Devries, S.; Freeman, A.M.; Crawford, A.; Kris-Etherton, P.; et al. Medical Nutrition Education, Training, and Competencies to Advance Guideline-Based Diet Counseling by Physicians: A Science Advisory From the American Heart Association. Circulation 2018, 137, e821–e841. [Google Scholar] [CrossRef]
- Kushner, R.F. Barriers to providing nutrition counseling by physicians: A survey of primary care practitioners. Prev. Med. 1995, 24, 546–552. [Google Scholar] [CrossRef]
- Kelley, M.M.; Foraker, R.E.; Lin, E.-J.D.; Kulkarni, M.; Lustberg, M.B.; Weaver, K.E. Oncologists’ Perceptions of a Digital Tool to Improve Cancer Survivors’ Cardiovascular Health. ACI Open 2019, 3, e78–e87. [Google Scholar] [CrossRef]
- Batubo, N.P.; Nwanze, N.M.; Alikor, C.A.; Auma, C.I.; Moore, J.B.; Zulyniak, M.A. Empowering healthcare professionals in West Africa—A feasibility study and qualitative assessment of a dietary screening tool to identify adults at high risk of hypertension. PLoS ONE 2024, 19, e0294370. [Google Scholar] [CrossRef]
- Vadiveloo, M.K.; Thorndike, A.N.; Lichtenstein, A.H. Integrating Diet Screening Into Routine Clinical Care: The Time Is Now. J. Am. Heart Assoc. 2023, 12, e028583. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Zolnierek, K.B.; Dimatteo, M.R. Physician communication and patient adherence to treatment: A meta-analysis. Med. Care 2009, 47, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, A.M.; Quitério, C.F.; Félix, I.B.; Guerreiro, M.P. Communication and Person-Centred Behaviour Change. In A Practical Guide on Behaviour Change Support for Self-Managing Chronic Disease; Guerreiro, M.P., Brito Félix, I., Moreira Marques, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 81–112. [Google Scholar]
- Batubo, N.P.; Moore, J.B.; Zulyniak, M.A. Dietary assessment and prevention of hypertension in Nigeria: Protocol for a retrospective cross-sectional study for the development and validation of a food frequency questionnaire for clinical use. PLoS ONE 2024, 19, e0292561. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Gupta, K.K.; Attri, J.P.; Singh, A.; Kaur, H.; Kaur, G. Basic concepts for sample size calculation: Critical step for any clinical trials! Saudi J. Anaesth. 2016, 10, 328–331. [Google Scholar] [CrossRef]
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef]
- Hennink, M.; Kaiser, B.N. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc. Sci. Med. 2022, 292, 114523. [Google Scholar] [CrossRef]
- Guest, G.; Bunce, A.; Johnson, L. How Many Interviews Are Enough?: An Experiment with Data Saturation and Variability. Field Methods 2006, 18, 59–82. [Google Scholar] [CrossRef]
- Hennink, M.M.; Kaiser, B.N.; Marconi, V.C. Code Saturation Versus Meaning Saturation: How Many Interviews Are Enough? Qual. Health Res. 2017, 27, 591–608. [Google Scholar] [CrossRef]
- Vasileiou, K.; Barnett, J.; Thorpe, S.; Young, T. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, M.A.; Paulussen, T.G.; Van Dommelen, P.; Van Buuren, S. Towards a measurement instrument for determinants of innovations. Int. J. Qual. Health Care 2014, 26, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, M.; Wiefferink, K.; Paulussen, T. Determinants of innovation within health care organizations: Literature review and Delphi study. Int. J. Qual. Health Care 2004, 16, 107–123. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Patton, M.Q. Qualitative Research & Evaluation Methods: Integrating Theory and Practice, 4th ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Lumivero. QSR International. NVivo (Version 14); Lumivero: Denver, CO, USA, 2020. [Google Scholar]
- Smith, T.G.; Castro, K.M.; Troeschel, A.N.; Arora, N.K.; Lipscomb, J.; Jones, S.M.; Treiman, K.A.; Hobbs, C.; McCabe, R.M.; Clauser, S.B. The rationale for patient-reported outcomes surveillance in cancer and a reproducible method for achieving it. Cancer 2016, 122, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.M.; Lucas, R.; Cowie, M.R. Assessing health-related quality of life in heart failure patients attending an outpatient clinic: A pragmatic approach. ESC Heart Fail. 2019, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Haverman, L.; van Oers, H.A.; Limperg, P.F.; Hijmans, C.T.; Schepers, S.A.; Sint Nicolaas, S.M.; Verhaak, C.M.; Bouts, A.H.M.; Fijnvandraat, K.; Peters, M.; et al. Implementation of electronic Patient Reported Outcomes in pediatric daily clinical practice: The KLIK experience. Tijdschr. Voor Kindergeneeskd. 2013, 81, 48–49. [Google Scholar] [CrossRef]
- Schepers, S.A.; Sint Nicolaas, S.M.; Haverman, L.; Wensing, M.; Schouten van Meeteren, A.Y.N.; Veening, M.A.; Caron, H.N.; Hoogerbrugge, P.M.; Kaspers, G.J.L.; Verhaak, C.M.; et al. Real-world implementation of electronic patient-reported outcomes in outpatient pediatric cancer care. Psychooncology 2017, 26, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J. Acad. Nutr. Diet. 2014, 114, 613–621. [Google Scholar] [CrossRef]
- Ardoin, T.W.; Hamer, D.; Mason, N.; Reine, A.; Barleycorn, L.; Francis, D.; Johnson, A. Effectiveness of a Patient-Centered Dietary Educational Intervention. Ochsner J. 2022, 22, 113–128. [Google Scholar] [CrossRef]
- Epstein, R.M.; Street, R.L., Jr. Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering; NIH Publication No. 07-6225; National Cancer Institute: Bethesda, MD, USA, 2007. [Google Scholar]
- Weldring, T.; Smith, S.M. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, 61–68. [Google Scholar] [CrossRef]
- Fabbri, M.; Finney Rutten, L.J.; Manemann, S.M.; Boyd, C.; Wolff, J.; Chamberlain, A.M.; Weston, S.A.; Yost, K.J.; Griffin, J.M.; Killian, J.M.; et al. Patient-centered communication and outcomes in heart failure. Am. J. Manag. Care 2020, 26, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Mejdahl, C.T.; Schougaard, L.M.V.; Hjollund, N.H.; Riiskjaer, E.; Lomborg, K. Patient-reported outcome measures in the interaction between patient and clinician—A multi-perspective qualitative study. J. Patient Rep. Outcomes 2020, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ou, L.; Hollis, S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv. Res. 2013, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, G.; Kearney, N.; Maguire, R.; Harrow, A.; Di Domenico, D.; Croy, S.; MacGillivray, S. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J. Clin. Oncol. 2014, 32, 1480–1501. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.A.; Roque, M.; Mateos-Campos, R.; Figueiras, A.; Herdeiro, M.T.; Roque, F. Barriers and facilitators of health professionals in adopting digital health-related tools for medication appropriateness: A systematic review. Digit. Health 2024, 10, 20552076231225133. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tan, J.B.; Liu, X.L.; Zhao, I. Barriers and enablers to implementing clinical practice guidelines in primary care: An overview of systematic reviews. BMJ Open 2023, 13, e062158. [Google Scholar] [CrossRef] [PubMed]
- Palacholla, R.S.; Fischer, N.; Coleman, A.; Agboola, S.; Kirley, K.; Felsted, J.; Katz, C.; Lloyd, S.; Jethwani, K. Provider- and Patient-Related Barriers to and Facilitators of Digital Health Technology Adoption for Hypertension Management: Scoping Review. JMIR Cardio 2019, 3, e11951. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Grossman, D.C.; et al. Behavioral Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 1163–1171. [Google Scholar] [CrossRef]
- NHS. The Eatwell Guide. National Health Service. Available online: https://www.nhs.uk/live-well/eat-well/food-guidelines-and-food-labels/the-eatwell-guide/ (accessed on 29 June 2024).
- Fayet-Moore, F.; Pearson, S. Interpreting the Australian dietary guideline to “limit” into practical and personalised advice. Nutrients 2015, 7, 2026–2043. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age between 18 and 70 years | Individuals <18 years or >70 years of age |
| Men and women | Pregnant or breastfeeding women, or those intending to become pregnant |
| Hypertensive or non-hypertensive individuals | Diagnosis of other chronic diseases such as cancer, diabetes, renal failure, endocrine diseases, and previous and recent incidence of cardiovascular disease (CVD) and stroke |
| Individuals who have been residents in Nigeria for the past 2 years | Individuals who have been residents in Nigeria for less than 2 years |
| Ability to read, write, and communicate over the phone in English | Individuals with dietary restrictions or with recent changes to their diet or food |
| Individuals who gave their consent to participate | Individuals who did not give their consent to participate or were currently enrolled in other studies |
| Characteristics | Overall (n = 151) | Hypertensive (n = 88, 58.3%) | Non-Hypertensive (n = 63, 41.7%) | p-Value |
|---|---|---|---|---|
| Patients (n = 151) | ||||
| Sex, n (%) | 0.010 | |||
| Male | 75 (49.7) | 52 (59.1%) | 23 (36.5%) | |
| Female | 76 (50.3) | 36 (40.9%) | 40 (63.5%) | |
| Age (years) | 44.4 ± 11.1 | 46.0 ± 10.2 | 42.1 ± 12.2 | 0.098 |
| Education, n (%) | 0.472 | |||
| No formal | 1 (0.6) | 1 (1.1) | 0 (0) | |
| Primary | 26 (17.2) | 14 (15.9) | 12 (19.1) | |
| Secondary | 62 (41.1) | 40 (45.5) | 22 (34.9) | |
| Tertiary | 62(41.1) | 33 (37.5) | 29 (46.0) | |
| Marital Status, n (%) | 0.072 | |||
| Divorced | 3 (2.0) | 2 (2.3) | 1 (1.6) | |
| Married | 119 (78.8) | 75 (85.2) | 44 (69.8) | |
| Single | 18 (11.9) | 6 (6.8) | 12 (19.0) | |
| Widowed | 11 (7.3) | 5 (5.7) | 6 (9.5) | |
| Employment, n (%) | 0.057 | |||
| Employed | 25 (16.6) | 14 (15.9) | 11 (17.5) | |
| Homemaker | 1 (0.6) | 0 (0) | 1 (1.6) | |
| Retired | 9 (6.0) | 7 (8.0) | 2 (3.2) | |
| Self-employed | 103 (68.2) | 63 (71.6) | 40 (63.5) | |
| Student | 8 (5.3) | 1(0.6) | 7 (11.1) | |
| Unemployed | 5 (3.3) | 3 (3.4) | 2 (3.2) | |
| Family history of HTN, n (%) | 34 (22.5) | 26 (29.5) | 8 (12.7) | |
| Physical activity level, n (%) | 0.542 | |||
| Active | 23 (15.2) | 12 (13.6) | 11 (17.5) | |
| Moderately active | 15 (9.9) | 7 (8.0) | 8 (12.7) | |
| Moderately inactive | 41 (27.2) | 27 (30.7) | 14 (22.2) | |
| Inactive | 72 (47.7) | 42 (47.7) | 30 (47.6) | |
| BMI (kg/m2) | 29.0 ± 6.2 | 29.1 ± 6.3 | 29.1 ± 5.7 | 0.934 |
| Waist circumference (cm) | 95.4 ± 14.5 | 95.49 ± 14.6 | 96.0 ± 14.2 | 0.740 |
| Blood pressure (mmHg) | ||||
| Systolic blood pressure | 142.5 ± 24.2 | 159.4 ± 15.7 | 119.0 ± 10.0 | <0.001 |
| Diastolic blood pressure | 98.0 ± 71.5 | 113.0 ± 9.8 | 77.1 ± 7.2 | <0.001 |
| Mean arterial pressur | 127.7 ± 32.3 | 143.9 ± 33.3 | 105.0 ± 8.0 | <0.001 |
| NiDST completion time (minutes) | 5.5 ± 1.7 | 5.5 ± 1.6 | 5.5 ± 1.7 | 0.905 |
| NiDST consultation time (minutes) | 9.6 ± 1.5 | 9.6 ± 1.5 | 9.6 ± 1.5 | 0.920 |
| Clinicians (n = 20) | ||||
| Overall | Men (n = 6) | Women (n = 14) | p-value | |
| Age (years) | 42.6 ± 6.0 | 43.2 ± 6.0 | 42.3 ± 6.2 | 0.771 |
| Years of experience | 13.0 ± 4.7 | 11.2 ± 2.0 | 13.7 ± 5.4 | 0.279 |
| Specialty | <0.001 | |||
| Internal Medicine n (%) | 9 (45) | 5 (25) | 4 (20) | |
| Family Medicine n (%) | 11 (55) | 1 (5)) | 10 (50) | |
| Food Items/Groups (Servings/Day) | Hypertensive (n = 88, 58%) | Non-Hypertensive (n = 63, 42%) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | IQR | Mean | Median | IQR | ||
| Healthy (non-atherogenic) food groups | |||||||
| Fruits | 0.36 | 0.21 | 0.36 | 0.39 | 0.21 | 0.36 | 0.687 |
| Vegetables | 0.49 | 0.57 | 0.36 | 0.60 | 0.57 | 0.79 | 0.052 |
| Rice and pasta | 0.50 | 0.57 | 0.36 | 0.57 | 0.57 | 0.58 | 0.199 |
| Wheat products | 0.53 | 0.57 | 0.36 | 0.55 | 0.57 | 0.79 | 0.913 |
| Fibre-rich cereals | 0.02 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.005 |
| Beans and lentils | 0.38 | 0.21 | 0.36 | 0.44 | 0.57 | 0.36 | 0.199 |
| Nuts and seeds | 0.43 | 0.57 | 0.36 | 0.50 | 0.57 | 0.36 | 0.215 |
| White (lean) meat | 0.36 | 0.21 | 0.36 | 0.31 | 0.21 | 0.36 | 0.426 |
| Starchy tubers | 0.67 | 0.57 | 0.43 | 0.68 | 0.57 | 0.43 | 0.474 |
| Fish and seafoods | 0.60 | 0.57 | 0.79 | 0.79 | 0.57 | 0.43 | 0.155 |
| Tea and coffee | 0.43 | 0.57 | 0.68 | 0.35 | 0.21 | 0.57 | 0.167 |
| Dairy (Milk) | 0.54 | 0.57 | 0.79 | 0.66 | 0.57 | 0.79 | 0.253 |
| Unhealthy (atherogenic) food groups | |||||||
| Red meat | 0.66 | 0.57 | 0.43 | 0.43 | 0.57 | 0.36 | 0.001 |
| Processed meat | 0.27 | 0.21 | 0.57 | 0.14 | 0.00 | 0.21 | 0.003 |
| Eggs and egg products | 0.40 | 0.21 | 0.36 | 0.23 | 0.21 | 0.00 | 0.001 |
| Fried foods | 0.49 | 0.57 | 0.36 | 0.29 | 0.21 | 0.36 | <0.001 |
| Fast foods | 0.40 | 0.21 | 0.57 | 0.26 | 0.00 | 0.57 | 0.005 |
| Soups and stews | 0.83 | 1.00 | 0.43 | 0.68 | 0.57 | 0.43 | 0.027 |
| Fats and oils | 0.94 | 1.00 | 0.43 | 0.77 | 1.00 | 0.43 | 0.035 |
| Desserts and sweets | 0.32 | 0.21 | 0.57 | 0.14 | 0.21 | 0.21 | 0.001 |
| Soft drinks | 0.54 | 0.57 | 0.79 | 0.28 | 0.21 | 0.36 | <0.001 |
| Alcoholic drinks | 0.51 | 0.21 | 1.00 | 0.12 | 0.00 | 0.21 | <0.001 |
| Salt and seasonings | 0.92 | 1.00 | 0.43 | 0.71 | 0.57 | 0.43 | <0.001 |
| MIDI Scale and Determinants | Disagree/ Totally Disagree (%) | Neutral (%) | Agree/ Totally Agree (%) |
|---|---|---|---|
| Nigerian Dietary Screening Tool | |||
| Procedural clarity: Activities for the use of the NiDST were clearly described | 0 | 0 | 100 |
| Completeness: The food list in the NiDST is comprehensive | 0 | 0 | 100 |
| Complexity: The NiDST is too complex for me to use | 100 | 0 | 0 |
| Compatibility: Compatible with workflows | 15 | 0 | 85 |
| Relevance for patient: Intervention is relevant for my patients | 0 | 0 | 100 |
| Clinicians | |||
| Personal benefits: I think using the NiDST will help me to assess patient dietary intake | 0 | 10 | 90 |
| Personal communication: The NiDST will help me to engage and improve engagement and communication with patients | 0 | 0 | 100 |
| Outcomes expectations: I think using the NiDST will support me to offer dietary advice to patients | 0 | 5 | 95 |
| Professional obligation: I feel it is my responsibility to use the NiDST to assess dietary intake and offer dietary advice to patients routinely | 0 | 5 | 95 |
| Patient satisfaction: Patients will be satisfied when using the tool with their dietary intake | 0 | 0 | 100 |
| Patient cooperation: Patient will generally cooperate when using the tool having their dietary intake | 0 | 0 | 100 |
| Nutritional knowledge: I have enough knowledge to use and interpret the dietary assessment tool as intended | 25 | 0 | 75 |
| Time available: I have enough time to assess patient dietary intake and offer dietary counselling | 25 | 0 | 75 |
| MIDI Scale and Items | Disagree/ Totally Disagree (%) | Neutral (%) | Agree/ Totally Agree (%) |
|---|---|---|---|
| Personal benefits: The tool was quick to complete within one clinical visit. | 2.0 | 2.0 | 96.0 |
| Personal communication: Helped me in communicating with doctor. | 0.0 | 0.7 | 99.3 |
| Patient engagement: NiDST helped me discuss with doctors. | 0.0 | 1.3 | 98.7 |
| Outcome expectations: Helped me understand and reflect on my food intake. | 0.7 | 8.6 | 90.7 |
| Awareness of content: The frequency response options allowed me to accurately report my usual food intake. | 0.0 | 4.0 | 96.0 |
| Time available: The time taken to complete the NiDST was acceptable to me. | 0.0 | 0.7 | 99.3 |
| Patient satisfaction: I am satisfied with using the tool and consultation. | 1.3 | 2.7 | 96.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batubo, N.P.; Auma, C.I.; Moore, J.B.; Zulyniak, M.A. The Nigerian Dietary Screening Tool: A Step toward Improved Patient-Clinician Communication in Nigerian Hospitals: A Pilot Implementation Study. Nutrients 2024, 16, 2286. https://doi.org/10.3390/nu16142286
Batubo NP, Auma CI, Moore JB, Zulyniak MA. The Nigerian Dietary Screening Tool: A Step toward Improved Patient-Clinician Communication in Nigerian Hospitals: A Pilot Implementation Study. Nutrients. 2024; 16(14):2286. https://doi.org/10.3390/nu16142286
Chicago/Turabian StyleBatubo, Nimisoere P., Carolyn I. Auma, J. Bernadette Moore, and Michael A. Zulyniak. 2024. "The Nigerian Dietary Screening Tool: A Step toward Improved Patient-Clinician Communication in Nigerian Hospitals: A Pilot Implementation Study" Nutrients 16, no. 14: 2286. https://doi.org/10.3390/nu16142286
APA StyleBatubo, N. P., Auma, C. I., Moore, J. B., & Zulyniak, M. A. (2024). The Nigerian Dietary Screening Tool: A Step toward Improved Patient-Clinician Communication in Nigerian Hospitals: A Pilot Implementation Study. Nutrients, 16(14), 2286. https://doi.org/10.3390/nu16142286







