γδ+ T-Cells Is a Useful Biomarker for the Differential Diagnosis between Celiac Disease and Non-Celiac Gluten Sensitivity in Patients under Gluten Free Diet
Abstract
1. Introduction
2. Materials and Methods
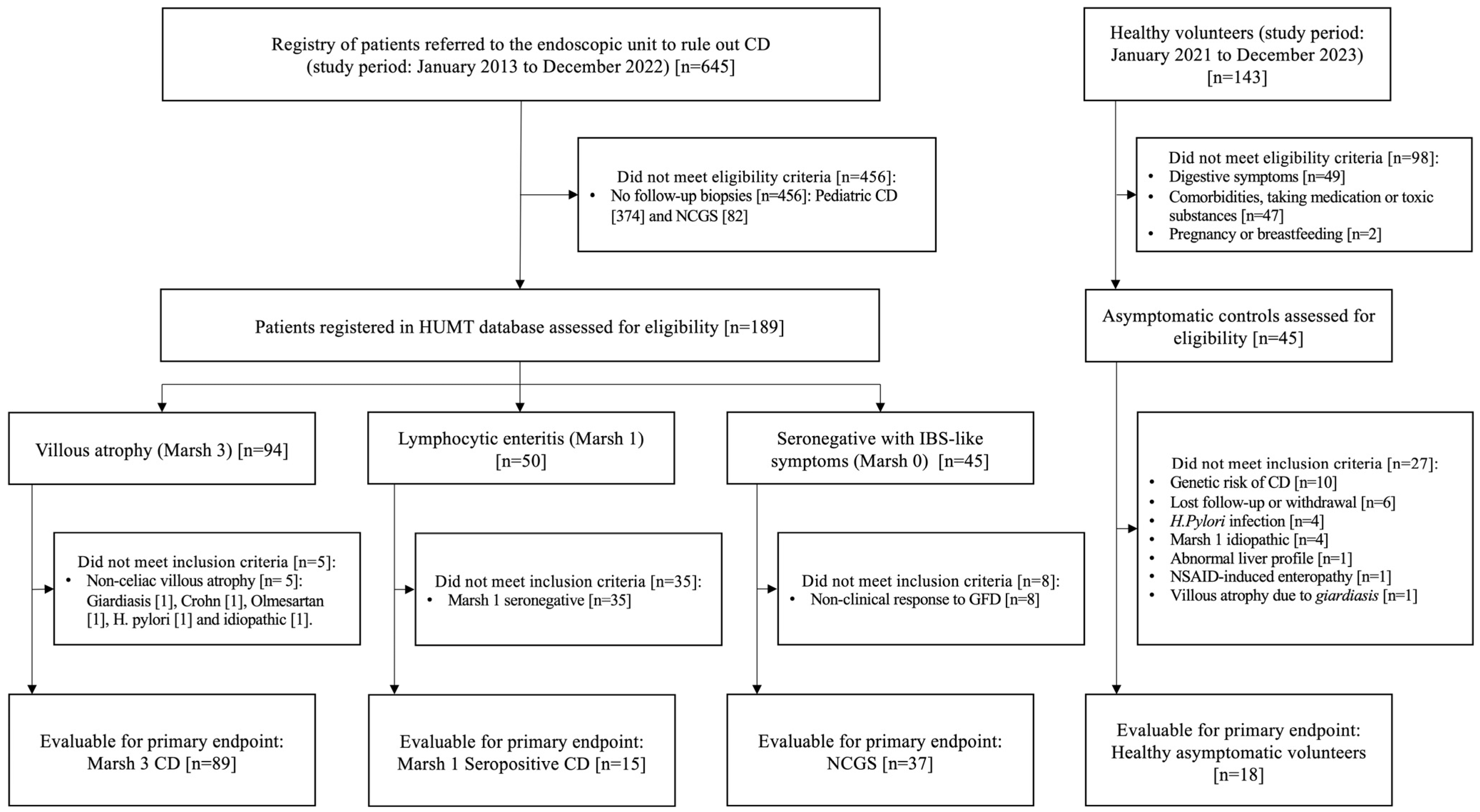
2.1. Study Design, Definitions, Patients and Controls
2.2. Duodenal Sample Collection
2.3. Histopathological Assessment
2.4. Celiac Serology
2.5. HLA-DQ Genotyping
2.6. Intestinal Lymphocyte Isolation and Quantification by Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Clinical, Histological, and Serological Evolution of Patients at Baseline and after a GFD
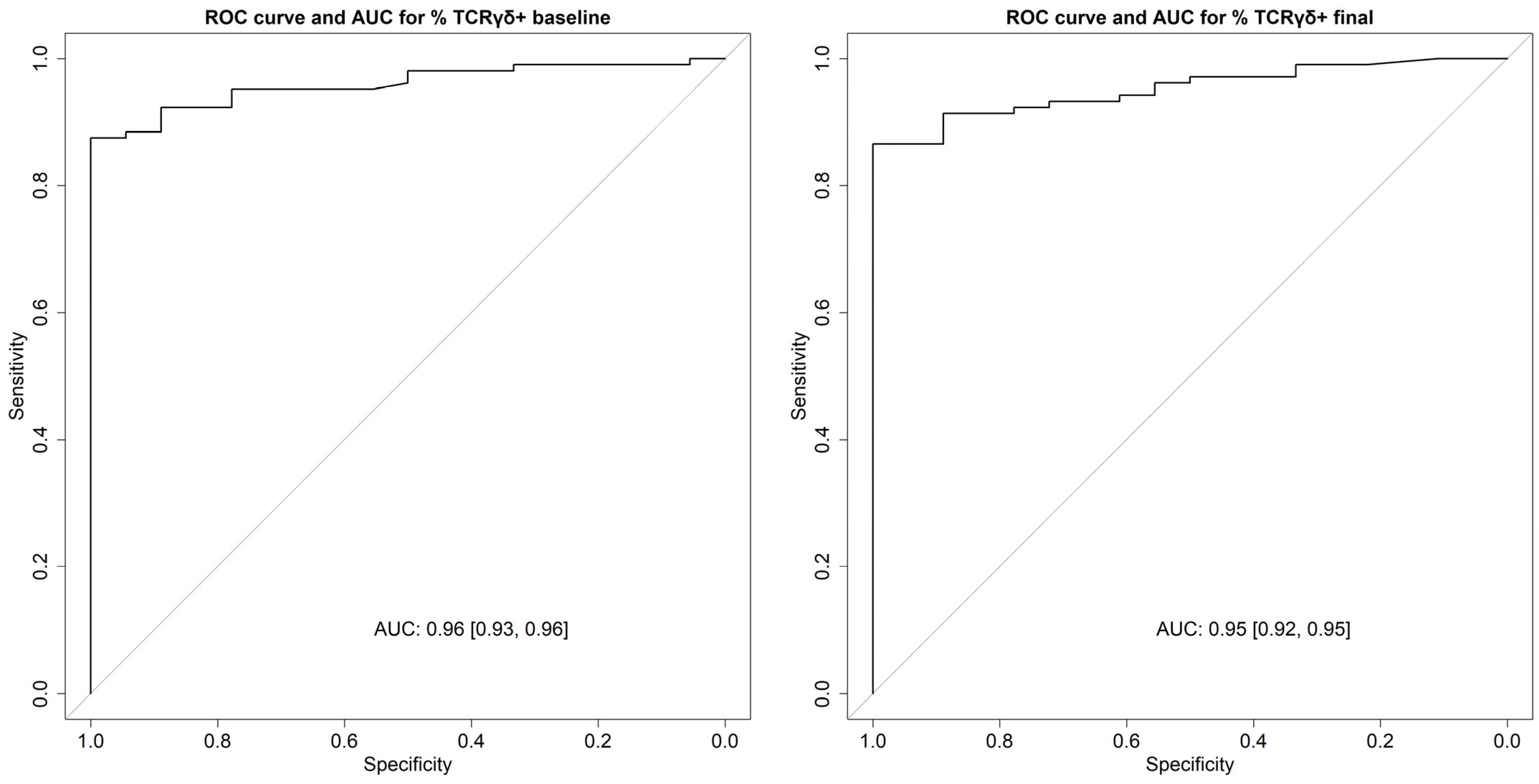
3.3. Diagnostic Accuracy of γδ T Cells and Celiac Lymphogram for Identifying Patients with CD with or without Gluten Intake and the Differential Diagnosis with NCGS
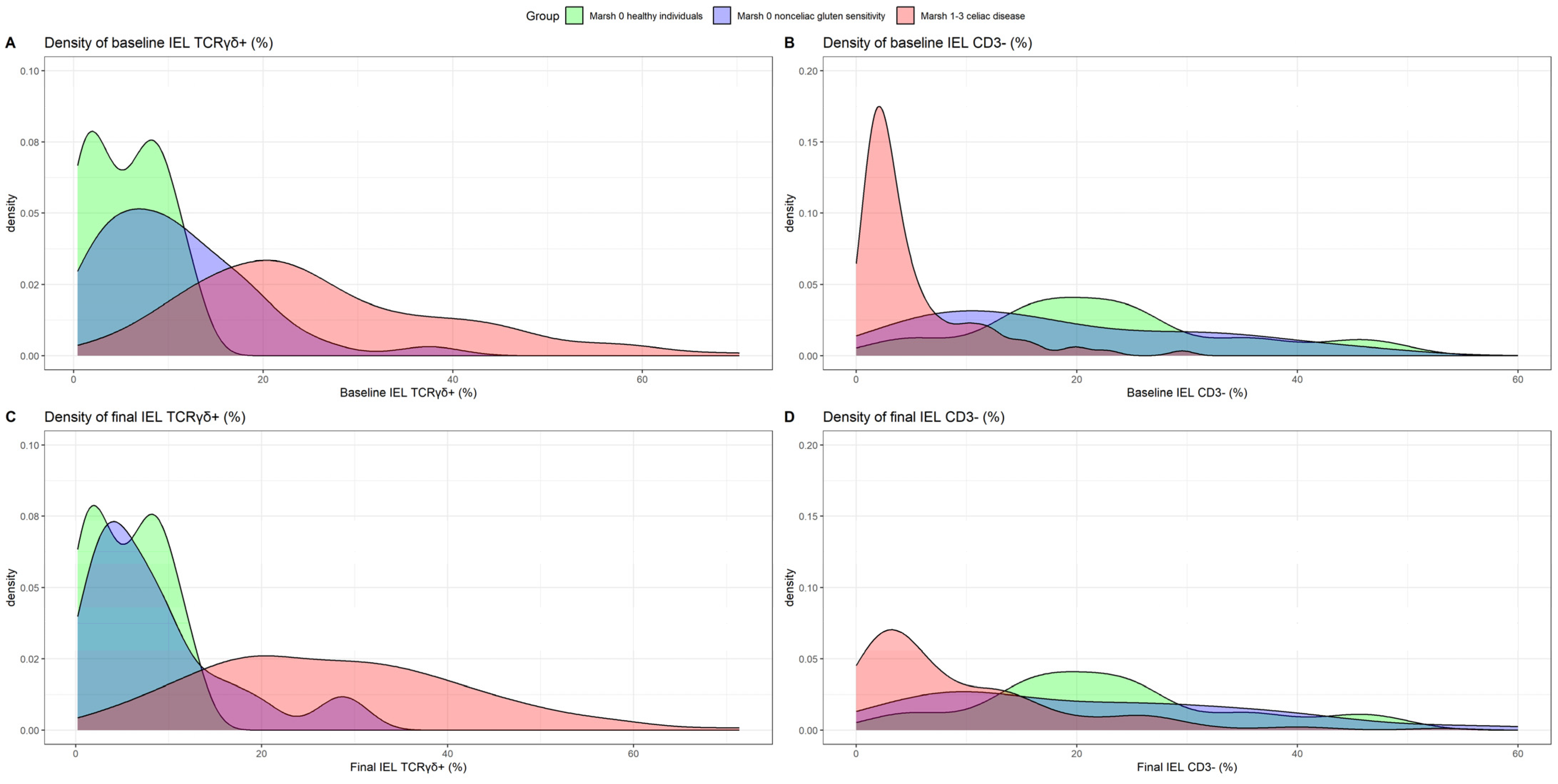
3.4. TCRγδ+ Kinetics at Baseline and in the Long Term under a GFD in Patients with CD and NCGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Ferch, C.C.; Chey, W.D. Irritable Bowel Syndrome and Gluten Sensitivity without Celiac Disease: Separating the Wheat from the Chaff. Gastroenterology 2012, 142, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients after Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Hadjivassiliou, M.; Sanders, D.S. The Spectrum of Noncoeliac Gluten Sensitivity. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Newell, E.W.; Glanville, J.; Fernandez-Becker, N.; Khosla, C.; Chien, Y.H.; Davis, M.M. Dietary Gluten Triggers Concomitant Activation of CD4+ and CD8+ Aβ T Cells and Γλ T Cells in Celiac Disease. Proc. Natl. Acad. Sci. USA 2013, 110, 13073–13078. [Google Scholar] [CrossRef] [PubMed]
- Risnes, L.F.; Eggesbø, L.M.; Zühlke, S.; Dahal-Koirala, S.; Neumann, R.S.; Lundin, K.E.A.; Christophersen, A.; Sollid, L.M. Circulating CD103+ Γδ and CD8+ T Cells Are Clonally Shared with Tissue-Resident Intraepithelial Lymphocytes in Celiac Disease. Mucosal. Immunol. 2021, 14, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bañares, F.; Carrasco, A.; Martín, A.; Esteve, M. Systematic Review and Meta-Analysis: Accuracy of Both Gamma Delta+ Intraepithelial Lymphocytes and Coeliac Lymphogram Evaluated by Flow Cytometry for Coeliac Disease Diagnosis. Nutrients 2019, 11, 1992. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Fernández-Bañares, F.; Corzo, M.; Gómez-Aguililla, S.; García-Hoz, C.; Núñez, C. Intestinal and Blood Lymphograms as New Diagnostic Tests for Celiac Disease. Front. Immunol. 2023, 13, 1081955. [Google Scholar] [CrossRef]
- Mayassi, T.; Ladell, K.; Gudjonson, H.; McLaren, J.E.; Shaw, D.G.; Tran, M.T.; Rokicka, J.J.; Lawrence, I.; Grenier, J.C.; van Unen, V.; et al. Chronic Inflammation Permanently Reshapes Tissue-Resident Immunity in Celiac Disease. Cell 2019, 176, 967–981.e19. [Google Scholar] [CrossRef]
- Mandile, R.; Maglio, M.; Mosca, C.; Marano, A.; Discepolo, V.; Troncone, R.; Auricchio, R. Mucosal Healing in Celiac Disease: Villous Architecture and Immunohistochemical Features in Children on a Long-Term Gluten Free Diet. Nutrients 2022, 14, 3696. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.R.; Elliott, L.; Hussey, S.; Mahmud, N.; Kelly, J.; Doherty, D.G.; Feighery, C.F. Persistent Changes in Circulating and Intestinal Γδ T Cell Subsets, Invariant Natural Killer T Cells and Mucosal-Associated Invariant T Cells in Children and Adults with Coeliac Disease. PLoS ONE 2013, 8, e76008. [Google Scholar] [CrossRef] [PubMed]
- García-Hoz, C.; Crespo, L.; Pariente, R.; De Andrés, A.; Rodríguez-Ramos, R.; Roy, G. Intraepithelial Lymphogram in the Diagnosis of Celiac Disease in Adult Patients: A Validation Cohort. Nutrients 2024, 16, 1117. [Google Scholar] [CrossRef] [PubMed]
- Camarero, C.; Eiras, P.; Asensio, A.; Leon, F.; Olivares, F.; Escobar, H.; Roy, G. Intraepithelial Lymphocytes and Coeliac Disease: Permanent Changes in CD3−/CD7+ and T Cell Receptor Γβ Subsets Studied by Flow Cytometry. Acta Paediatr. 2000, 89, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Saborido, R.; Martinón, N.; Regueiro, A.; Crujeiras, V.; Eiras, P.; Leis, R. Intraepithelial Lymphocyte Immunophenotype: A Useful Tool in the Diagnosis of Celiac Disease. J. Physiol. Biochem. 2018, 74, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Basu, K.; Creasey, H.; Bruggemann, N.; Stevens, J.; Bloxham, D.; Woodward, J.M. Diagnosis of Coeliac Disease by Flow Cytometry of Intraepithelial Lymphocytes: A New ‘Gold’ Standard? Frontline Gastroenterol. 2022, 13, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Nijeboer, P.; van Gils, T.; Reijm, M.; Ooijevaar, R.; Lissenberg-Witte, B.I.; Bontkes, H.J.; Mulder, C.J.J.; Bouma, G. Gamma-Delta T Lymphocytes in the Diagnostic Approach of Coeliac Disease. J. Clin. Gastroenterol. 2019, 53, e208–e213. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bañares, F.; Beltrán, B.; Salas, A.; Comino, I.; Ballester-Clau, R.; Ferrer, C.; Molina-Infante, J.; Rosinach, M.; Modolell, I.; Rodríguez-Moranta, F.; et al. Persistent Villous Atrophy in De Novo Adult Patients with Celiac Disease and Strict Control of Gluten-Free Diet Adherence: A Multicenter Prospective Study (CADER Study). Am. J. Gastroenterol. 2021, 116, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Sanders, D.S.; Baiardi, P.; Caio, G.; Ciacci, C.; Kaukinen, K.; Lebwohl, B.; Leffler, D.; Malamut, G.; Murray, J.A.; et al. Nomenclature and Diagnosis of Seronegative Coeliac Disease and Chronic Non-Coeliac Enteropathies in Adults: The Paris Consensus. Gut 2022, 71, 2218–2225. [Google Scholar] [CrossRef]
- Walker, M.M.; Murray, J.A. An Update in the Diagnosis of Coeliac Disease. Histopathology 2010, 59, 166–179. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Carrasco, A.; García-Puig, R.; Rosinach, M.; González, C.; Alsina, M.; Loras, C.; Salas, A.; Viver, J.M.; Esteve, M. Intestinal Intraepithelial Lymphocyte Cytometric Pattern Is More Accurate than Subepithelial Deposits of Anti-Tissue Transglutaminase IgA for the Diagnosis of Celiac Disease in Lymphocytic Enteritis. PLoS ONE 2014, 9, e101249. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The Histopathology of Coeliac Disease: Time for a Standardized Report Scheme for Pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, S.; Villanacci, V.; Sansotta, N.; Scarfì, R.; Bassotti, G.; Vieni, G.; Princiotta, A.; Sferlazzas, C.; Magazzù, G.; Tuccari, G. Redefining the Intraepithelial Lymphocytes Threshold to Diagnose Gluten Sensitivity in Patients with Architecturally Normal Duodenal Histology. Aliment. Pharmacol. Ther. 2011, 33, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.; Cairns, A.; Dixon, M.F.; O’Mahony, S. Quantitation of Intraepithelial Lymphocytes in Human Duodenum: What Is Normal? J. Clin. Pathol. 2002, 55, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Ciacci, C.; Green, P.H.R.; Kaukinen, K.; Korponay-Szabo, I.R.; Kurppa, K.; Murray, J.A.; Lundin, K.E.A.; Maki, M.J.; Popp, A.; et al. Outcome Measures in Coeliac Disease Trials: The Tampere Recommendations. Gut 2018, 67, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.D.L.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of Gluten Immunogenic Peptides in the Urine of Patients with Coeliac Disease Reveals Transgressions in the Gluten-Free Diet and Incomplete Mucosal Healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.K.; Guandalini, S.; Semrad, C.; Kupfer, S.S. A Clinician’s Guide to Celiac Disease HLA Genetics. Am. J. Gastroenterol. 2019, 114, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Leon, F. Intestinal Intraepithelial Lymphocytes and Anti-Transglutaminase in a Screening Algorithm for Coeliac Disease. Gut 2002, 50, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Calleja, S.; Vivas, S.; Santiuste, M.; Arias, L.; Hernando, M.; Nistal, E.; Casqueiro, J.; Ruiz de Morales, J.G. Dynamics of Non-Conventional Intraepithelial Lymphocytes—NK, NKT, and Γδ T—In Celiac Disease: Relationship with Age, Diet, and Histopathology. Dig. Dis. Sci. 2011, 56, 2042–2049. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo Definitions for Coeliac Disease and Related Terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Bianchi, P.I.; Vattiato, C.; Marchese, A.; Trotta, L.; Badulli, C.; De Silvestri, A.; Martinetti, M.; Corazza, G.R. Influence of HLA-DQ2 and DQ8 on Severity in Celiac Disease. J. Clin. Gastroenterol. 2012, 46, 46–50. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; López-Palacios, N.; Corzo, M.; Arau, B.; Rubio, M.; Fernández-Prieto, M.; Tristán, E.; Pujals, M.; Farrais, S.; Horta, S.; et al. Activated Gut-Homing CD8+ T Cells for Coeliac Disease Diagnosis on a Gluten-Free Diet. BMC Med. 2021, 19, 237. [Google Scholar] [CrossRef]
- Leonard, M.M.; Silvester, J.A.; Leffler, D.; Fasano, A.; Kelly, C.P.; Lewis, S.K.; Goldsmith, J.D.; Greenblatt, E.; Kwok, W.W.; McAuliffe, W.J.; et al. Evaluating Responses to Gluten Challenge: A Randomized, Double-Blind, 2-Dose Gluten Challenge Trial. Gastroenterology 2021, 160, 720–733.e8. [Google Scholar] [CrossRef] [PubMed]
- Tye-Din, J.A.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Acaster, S.; Goldstein, K.E.; Dzuris, J.L.; Neff, K.M.; Truitt, K.E.; et al. Elevated Serum Interleukin-2 after Gluten Correlates with Symptoms and Is a Potential Diagnostic Biomarker for Coeliac Disease. Aliment. Pharmacol. Ther. 2019, 50, 901–910. [Google Scholar] [CrossRef]
- Sarna, V.K.; Lundin, K.E.A.; Mørkrid, L.; Qiao, S.-W.; Sollid, L.M.; Christophersen, A. HLA-DQ–Gluten Tetramer Blood Test Accurately Identifies Patients with and without Celiac Disease in Absence of Gluten Consumption. Gastroenterology 2018, 154, 886–896.e6. [Google Scholar] [CrossRef]
- Ontiveros, N.; Tye-Din, J.A.; Hardy, M.Y.; Anderson, R.P. Ex-Vivo Whole Blood Secretion of Interferon (IFN)-γ and IFN-γ-Inducible Protein-10 Measured by Enzyme-Linked Immunosorbent Assay Are as Sensitive as IFN-γ Enzyme-Linked Immunospot for the Detection of Gluten-Reactive T Cells in Human Leucocyte Antigen (HLA)-DQ2·5(+) -associated coeliac disease. Clin. Exp. Immunol. 2014, 175, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Dunne, M.R.; Byrne, G.; Chirdo, F.G.; Feighery, C. Coeliac Disease Pathogenesis: The Uncertainties of a Well-Known Immune Mediated Disorder. Front. Immunol. 2020, 11, 1374. [Google Scholar] [CrossRef]
- Camarero, C.; De Andrés, A.; García-Hoz, C.; Roldán, B.; Muriel, A.; León, F.; Roy, G. Assessment of Duodenal Intraepithelial Lymphocyte Composition (Lymphogram) for Accurate and Prompt Diagnosis of Celiac Disease in Pediatric Patients. Clin. Transl. Gastroenterol. 2021, 12, e00426. [Google Scholar] [CrossRef]


| Variables | Marsh 3 Celiac Disease (n = 89) | Seropositive Marsh 1 Celiac Disease (n = 15) | Marsh 0 Non-Celiac Gluten Sensitivity (n = 37) | Marsh 0 Healthy Individuals (n = 18) |
|---|---|---|---|---|
| Age (years) a | 34.00 [19.00; 44.00] | 37.00 [21.00; 53.00] | 41.00 [35.00; 46.00] | 25.00 [22.25; 26.75] |
| Female (n, %) | 69 (77.53%) | 10 (66.67%) | 30 (81.08%) | 12 (66.67%) |
| Duration of GFD to 2nd biopsy (years) a | 2.00 [2.00; 3.00] | 2.00 [1.50; 2.50] | 2.00 [1.00; 4.00] | NA [NA; NA] |
| Celiac serology (n, %) | ||||
| Positive anti-tTG2 b | 85 (95.51%) | 15 (100%) | 0 (0.00%) | 0 (0.00%) |
| Negative anti-tTG2 | 4 (4.49%) | 0 (0.00%) | 37 (100.00%) | 18 (100.00%) |
| %TCRγδ+ cells a | 22.65 [16.90; 32.64] | 25.42 [13.70; 35.75] | 9.43 [4.10; 14.66] | 5.75 [1.80; 8.59] |
| %CD3− cells a | 2.78 [1.66; 5.00] | 6.41 [2.44; 10.96] | 16.56 [8.65; 28.16] | 21.97 [16.78; 26.40] |
| %IEL/100 epithelial cells a | 50.00 [40.00; 63.00] | 35.00 [29.37; 47.00] | 19.00 [13.00; 22.00] | 14.50 [13.00; 17.75] |
| HLA-DQ genotype (n, %) | ||||
| HLA-DQ2.5 | 71 (86.59%) | 15 (100.00%) | 15 (40.54%) | 0 (0.00%) |
| HLA-DQ8 | 5 (6.10%) | 0 (0.00%) | 16 (43.24%) | 0 (0.00%) |
| HLA-DQ2.2 | 6 (7.32%) | 0 (0.00%) | 4 (10.81%) | 0 (0.00%) |
| HLA-DQ7.5 | 0 (0.00%) | 0 (0.00%) | 1 (2.70%) | 0 (0.00%) |
| All negative | 0 (0.00%) | 0 (0.00%) | 1 (2.70%) | 18 (100%) |
| Marsh 3 Celiac Disease | Seropositive Marsh 1 Celiac Disease | Marsh 0 Non-Celiac Gluten Sensitivity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Baseline (n = 89) | Final (n = 89) | p Value | Baseline (n = 15) | Final (n = 15) | p Value | Baseline (n = 37) | Final (n = 37) | p Value |
| Histology (n, %) | |||||||||
| Marsh 0 | 0 (0.00%) | 31 (34.83%) | 0 (0.00%) | 14 (93.33%) | 37 (100.00%) | 37 (100.00%) | |||
| Marsh 1 | 0 (0.00%) | 42 (47.19%) | 15 (100.00%) | 1 (6.67%) | 0 (0.00%) | 0 (0.00%) | |||
| Marsh 3 | 89 (100.00%) | 16 (17.98%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |||
| %IEL/100 epithelial cells a | NA [NA; NA] | NA [NA; NA] | 35.00 [29.37; 47.00] | 22.00 [20.50; 24.70] | <0.001 | 19.00 [13.00; 22.00] | 18.00 [13.40; 21.00] | 0.475 | |
| Celiac serology (n, %) | |||||||||
| Positive Anti-tTG2 b | 85 (95.51%) | 19 (22.10%) | 15 (100%) | 2 (14.28%) | 0 (0.00%) | 0 (0.00%) | |||
| IEL flow cytometry a | |||||||||
| %TCRγδ+ cells a | 22.65 [16.90; 32.64] | 26.99 [17.40; 36.69] | 0.020 c | 25.42 [13.70; 35.75] | 25.17 [18.91; 34.66] | >0.999 c | 9.43 [4.10; 14.66] | 6.40 [3.20; 11.00] | 0.022 c |
| %CD3− cells a | 2.78 [1.66; 5.00] | 4.80 [2.50; 13.28] | <0.001 c | 6.41 [2.44; 10.96] | 11.20 [3.98; 14.33] | 0.003 c | 16.56 [8.65; 28.16] | 19.37 [8.80; 30.53] | 0.200 c |
| Isolated Increase TCRγδ+ | Celiac Lymphogram a | |||
|---|---|---|---|---|
| Best Cutoff Value | Youden Index | Logistic Model | Youden Index | Logistic Model |
| Diagnostic accuracy with GCD (baseline) | %TCRγδ+ > 12.91 | %TCRγδ+ > 8.64 | %TCRγδ+ > 12.91 and %CD3− ≤ 13.33 | %TCRγδ+ > 8.64 and %CD3− ≤ 16.3 |
| Sensitivity | 0.87 [0.78, 0.92] | 0.95 [0.89, 0.98] | 0.82 [0.73, 0.88] | 0.91 [0.84, 0.96] |
| Specificity | 1 [0.78, 1] | 0.78 [0.52, 0.93] | 1 [0.78, 1] | 0.94 [0.71, 1] |
| PPV | 1 [0.95, 1] | 0.96 [0.90, 0.99] | 1 [0.95, 1] | 0.99 [0.94, 1.00] |
| NPV | 0.56 [0.38, 0.73] | 0.74 [0.49, 0.90] | 0.49 [0.32, 0.65] | 0.65 [0.44, 0.82] |
| Accuracy | 0.89 [0.81, 0.93] | 0.93 [0.86, 0.96] | 0.84 [0.77, 0.90] | 0.92 [0.85, 0.96] |
| Diagnostic accuracy with GFD (final) | %TCRγδ+ > 13.31 | %TCRγδ+ > 8.67 | %TCRγδ+ > 13.31 and %CD3− ≤ 13.33 | %TCRγδ+ > 8.67 and %CD3− ≤ 16.6 |
| Sensitivity | 0.86 [0.77, 0.91] | 0.92 [0.86, 0.96] | 0.65 [0.55, 0.74] | 0.81 [0.72, 0.88] |
| Specificity | 1 [0.78, 1] | 0.78 [0.52, 0.93] | 1 [0.78, 1] | 0.94 [0.71, 1.00] |
| PPV | 1 [0.95, 1] | 0.96 [0.89, 0.99] | 1 [0.93, 1] | 0.99 [0.93, 1.00] |
| NPV | 0.55 [0.37, 0.72] | 0.64 [0.41, 0.82] | 0.33 [0.22, 0.48] | 0.46 [0.30, 0.63] |
| Accuracy | 0.88 [0.80, 0.93] | 0.90 [0.83, 0.95] | 0.70 [0.61, 0.78] | 0.83 [0.75, 0.89] |
| Age | Gender | Main Clinical Feature | First-Degree Relatives with CD | HLA-DQ Genotype | Baseline %TCRγδ+ Cells | Final %TCRγδ+ Cells |
|---|---|---|---|---|---|---|
| 67 | Female | Bloating | No | DQ8 | 13.51 | 7.50 |
| 30 | Female | Abdominal pain | Yes | DQ2.5 | 14.29 | 11.50 |
| 39 | Female | Diarrhea | Yes | DQ8 | 14.66 | 11.00 |
| 64 | Female | Dyspepsia | No | DQ2.5 | 15.22 | 7.73 |
| 63 | Female | Diarrhea | No | DQ8 | 16.36 | 1.88 |
| 39 | Female | Bloating | No | DQ8 | 18.16 | 19.52 |
| 41 | Female | Bloating | Yes | DQ8 | 18.34 | 14.86 |
| 36 | Female | Dyspepsia | Unknown | DQ2.2 | 18.73 | 28.83 |
| 60 | Female | Bloating | No | DQ2.5 and DQ8 | 19.00 | 27.40 |
| 51 | Female | Bloating | Yes | DQ8 | 21.90 | 15.80 |
| 35 | Female | Dyspepsia | No | DQ2.5 | 25.94 | 20.00 |
| 63 | Male | Diarrhea | Yes | DQ8 | 37.51 | 29.79 |
| 46 | Female | Diarrhea | No | DQ8 | 11.04 | 15.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Cardona, A.; Carrasco, A.; Arau, B.; Vidal, J.; Tristán, E.; Ferrer, C.; Gonzalez-Puglia, G.; Pallarès, N.; Tebé, C.; Farrais, S.; et al. γδ+ T-Cells Is a Useful Biomarker for the Differential Diagnosis between Celiac Disease and Non-Celiac Gluten Sensitivity in Patients under Gluten Free Diet. Nutrients 2024, 16, 2294. https://doi.org/10.3390/nu16142294
Martín-Cardona A, Carrasco A, Arau B, Vidal J, Tristán E, Ferrer C, Gonzalez-Puglia G, Pallarès N, Tebé C, Farrais S, et al. γδ+ T-Cells Is a Useful Biomarker for the Differential Diagnosis between Celiac Disease and Non-Celiac Gluten Sensitivity in Patients under Gluten Free Diet. Nutrients. 2024; 16(14):2294. https://doi.org/10.3390/nu16142294
Chicago/Turabian StyleMartín-Cardona, Albert, Anna Carrasco, Beatriz Arau, Judith Vidal, Eva Tristán, Carme Ferrer, Gerardo Gonzalez-Puglia, Natàlia Pallarès, Cristian Tebé, Sergio Farrais, and et al. 2024. "γδ+ T-Cells Is a Useful Biomarker for the Differential Diagnosis between Celiac Disease and Non-Celiac Gluten Sensitivity in Patients under Gluten Free Diet" Nutrients 16, no. 14: 2294. https://doi.org/10.3390/nu16142294
APA StyleMartín-Cardona, A., Carrasco, A., Arau, B., Vidal, J., Tristán, E., Ferrer, C., Gonzalez-Puglia, G., Pallarès, N., Tebé, C., Farrais, S., Núñez, C., Fernández-Bañares, F., & Esteve, M. (2024). γδ+ T-Cells Is a Useful Biomarker for the Differential Diagnosis between Celiac Disease and Non-Celiac Gluten Sensitivity in Patients under Gluten Free Diet. Nutrients, 16(14), 2294. https://doi.org/10.3390/nu16142294







