Saffron as a Promising Therapy for Inflammatory Bowel Disease
Abstract
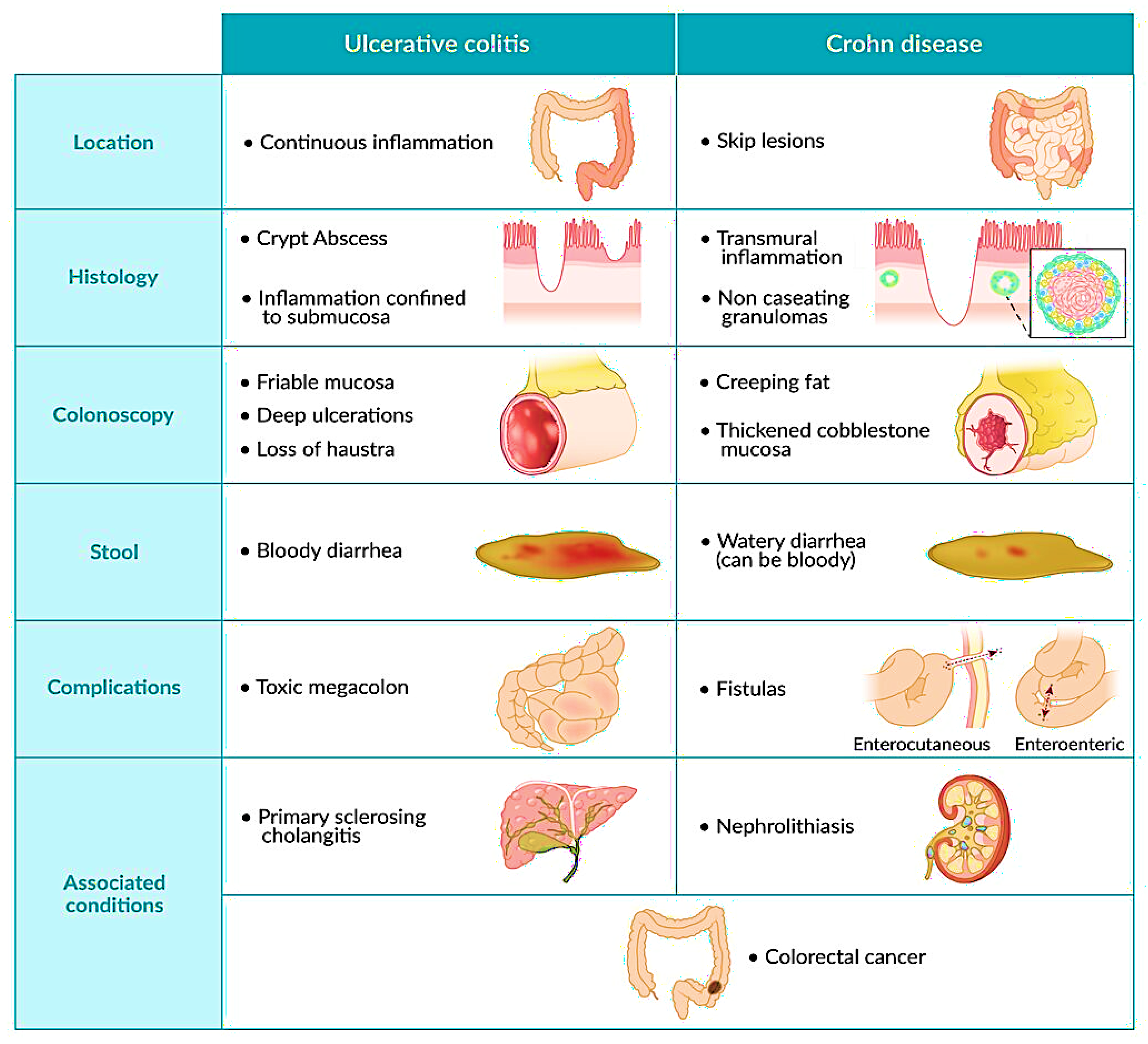
:1. Introduction
2. Current Treatments and Their Limitations
- I.
- Pharmacologic interventions:
- II.
- Non-pharmacologic interventions:
3. The Role of Calprotectin in the Inflammatory Response
4. Preclinical Observations Regarding the Use of Saffron as a Therapeutic Agent
5. Clinical Observations from Using Saffron as a Therapeutic Agent
6. Saffron Is Safe and Cost-Effective and Has Minimal Potential Side Effects
7. Perspective on Saffron Use as an Adjuvant Therapy for Inflammatory Bowel Disease
Supplementary Materials
Funding
Conflicts of Interest
References
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffin, J.M. A tale of two diseases: The history of inflammatory bowel disease. J. Crohns Colitis 2014, 8, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Aufses, A.H., Jr. The History of Crohn’s Disease. Surg. Clin. 2001, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Plichta, D.R.; Graham, D.B.; Subramanian, S.; Xavier, R.J. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell 2019, 178, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Guindi, M.; Riddell, R.H. Indeterminate colitis. J. Clin. Pathol. 2004, 57, 1233–1244. [Google Scholar] [CrossRef]
- Tremaine, W.J. Diagnosis and treatment of indeterminate colitis. Gastroenterol Hepatolm 2011, 7, 826–828. [Google Scholar]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Abegunde, A.T.; Muhammad, B.H.; Bhatti, O.; Ali, T. Environmental risk factors for inflammatory bowel diseases: Evidence based literature review. World J. Gastroenterol. 2016, 22, 6296–6317. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659.e644. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Hanauer, S. Optimizing pharmacologic management of inflammatory bowel disease. Expert. Rev. Clin. Pharmacol. 2017, 10, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Brain, O.; Travis, S.P. Conventional drug therapy for inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.P.; Frias Gomes, C.; Louis, E.; Colombel, J.F.; Satsangi, J. Review article: Withdrawal of 5-aminosalicylates in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Dhaneshwar, S.S. Colon-specific prodrugs of 4-aminosalicylic acid for inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3564–3571. [Google Scholar] [CrossRef]
- Travis, S.P. Editorial: Mesalazine in ulcerative colitis--is it time to revise treatment guidelines in the UK? Aliment. Pharmacol. Ther. 2006, 24 (Suppl. S1), 1. [Google Scholar] [CrossRef]
- Klotz, U. The role of aminosalicylates at the beginning of the new millennium in the treatment of chronic inflammatory bowel disease. Eur. J. Clin. Pharmacol. 2000, 56, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M.; Russel, M.G.; Langholz, E.; Stockbrugger, R.W. Aminosalicylates and colorectal cancer in IBD: A not-so bitter pill to swallow. Am. J. Gastroenterol. 2003, 98, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Irving, P.M. Optimization of conventional therapy in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Hibi, T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr. Pharm. Des. 2003, 9, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.J.; Modi, K.P.; Majumdar, A.S.; Sadarani, B.N. Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytother. Res. 2015, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Le Gall, C.; Lachaux, A.; Boulieu, R. High thiopurine metabolite concentrations associated with lymphopenia in inflammatory bowel disease (IBD) pediatric patients receiving aminosalicylates combined with azathioprine. Int. J. Clin. Pharmacol. Ther. 2010, 48, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.T.; Pan, B.R. Current medical therapy for ulcerative colitis. World J. Gastroenterol. 1999, 5, 64–72. [Google Scholar] [CrossRef]
- Prantera, C.; Marconi, S. Glucocorticosteroids in the treatment of inflammatory bowel disease and approaches to minimizing systemic activity. Ther. Adv. Gastroenterol. 2013, 6, 137–156. [Google Scholar] [CrossRef]
- Hanauer, S.B. New steroids for IBD: Progress report. Gut 2002, 51, 182–183. [Google Scholar] [CrossRef]
- Mulder, C.J.; Tytgat, G.N. Review article: Topical corticosteroids in inflammatory bowel disease. Aliment. Pharmacol. Ther. 1993, 7, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Carpani de Kaski, M.; Hodgson, H.J. Rolling review: Inflammatory bowel disease. Aliment. Pharmacol. Ther. 1993, 7, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Van Ierssel, A.J.; Mieremet-Ooms, M.A.; Van der Zon, J.M.; Van Hogezand, R.A.; Griffioen, G.; Lamers, C.B.; Verspaget, H.W. Suppression of intestinal mucosal natural killer cells by corticosteroids. Aliment. Pharmacol. Ther. 1997, 11, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Wera, O.; Lancellotti, P.; Oury, C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, S.A.; Masterson, J.C.; Fillon, S.; Robinson, Z.D.; Furuta, G.T. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Borghi, A.; Meroni, P.L.; Crosti, C.; Cugno, M. Immune-mediated inflammatory reactions and tumors as skin side effects of inflammatory bowel disease therapy. Autoimmunity 2014, 47, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hathout, Y.; Conklin, L.S.; Seol, H.; Gordish-Dressman, H.; Brown, K.J.; Morgenroth, L.P.; Nagaraju, K.; Heier, C.R.; Damsker, J.M.; van den Anker, J.N.; et al. Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children. Sci. Rep. 2016, 6, 31727. [Google Scholar] [CrossRef]
- Ilan, Y. Oral immune therapy: Targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease. Clin. Transl. Immunol. 2016, 5, e60. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.R.; Kaplan, J.L. Medical management of pediatric inflammatory bowel disease. Semin. Pediatr. Surg. 2017, 26, 360–366. [Google Scholar] [CrossRef]
- Arseneau, K.O.; Cominelli, F. Targeting leukocyte trafficking for the treatment of inflammatory bowel disease. Clin. Pharmacol. Ther. 2015, 97, 22–28. [Google Scholar] [CrossRef]
- Korelitz, B.I.; Reddy, B.; Bratcher, J. Desensitization of patients with allergic reactions to immunosuppressives in the treatment of inflammatory bowel disease. Expert. Opin. Drug Saf. 2010, 9, 379–382. [Google Scholar] [CrossRef] [PubMed]
- van Dieren, J.M.; Kuipers, E.J.; Samsom, J.N.; Nieuwenhuis, E.E.; van der Woude, C.J. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm. Bowel Dis. 2006, 12, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Farrell, R.J. The risk of lymphoma in the treatment of inflammatory bowel disease with immunosuppressive agents. Crit. Rev. Oncol. Hematol. 2005, 56, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Paul, S.; Yakkali, S.; Teresa Selvin, S.; Thomas, S.; Bikeyeva, V.; Abdullah, A.; Radivojevic, A.; Abu Jad, A.A.; Ravanavena, A.; et al. Glucocorticoids-Induced Neuropsychiatric Disorders in Patients with Inflammatory Bowel Disease: A Systematic Review. Cureus 2022, 14, e28981. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Barnes, E.L.; Cameron, A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflamm. Bowel Dis. 2015, 21, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Peyrin-Biroulet, L.; Sokol, H.; Aldeger, X.; Costa, A.; Higgins, P.D.; Joyce, J.C.; Katsanos, K.H.; Lopez, A.; de Xaxars, T.M.; et al. Extra-intestinal malignancies in inflammatory bowel disease: Results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J. Crohns Colitis 2014, 8, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.J.; Dubinsky, M.C.; Ford, A.C.; Ullman, T.A.; Talley, N.J.; Moayyedi, P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: A systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Barta, Z.; Zold, E.; Zeher, M. Pulse cyclophosphamide in steroid-resistant inflammatory bowel disease. Aliment. Pharmacol. Ther. 2007, 25, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.L.; Nguyen, E.T.; Bechtold, M.L. Effect of Immunosuppressive Therapies for the Treatment of Inflammatory Bowel Disease on Response to Routine Vaccinations: A Meta-Analysis. Dig. Dis. Sci. 2015, 60, 2446–2453. [Google Scholar] [CrossRef]
- Melmed, G.Y.; Agarwal, N.; Frenck, R.W.; Ippoliti, A.F.; Ibanez, P.; Papadakis, K.A.; Simpson, P.; Barolet-Garcia, C.; Ward, J.; Targan, S.R.; et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 148–154. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi Porro, G. Biologic therapy for inflammatory bowel disease. Drugs 2005, 65, 2253–2286. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Holleran, G.; Lopetuso, L.; Petito, V.; Graziani, C.; Ianiro, G.; McNamara, D.; Gasbarrini, A.; Scaldaferri, F. The Innate and Adaptive Immune System as Targets for Biologic Therapies in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 2020. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Danese, S.; Peyrin-Biroulet, L. Efficacy of TNF antagonists beyond one year in adult and pediatric inflammatory bowel diseases: A systematic review. Curr. Drug Targets 2010, 11, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Basso, P.J.; Alves, V.B.; Fonseca, M.T.; Bonfa, G.; Nardini, V.; Cardoso, C.R. Classical and recent advances in the treatment of inflammatory bowel diseases. Braz. J. Med. Biol. Res. 2015, 48, 96–107. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, B.R.; Garcia, M.P.; Nogueira, J.B.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.; Tamashiro, W.M.; Simioni, P.U. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediat. Inflamm. 2015, 2015, 493012. [Google Scholar] [CrossRef] [PubMed]
- Khorasanchi, Z.; Shafiee, M.; Kermanshahi, F.; Khazaei, M.; Ryzhikov, M.; Parizadeh, M.R.; Kermanshahi, B.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Crocus sativus a natural food coloring and flavoring has potent anti-tumor properties. Phytomedicine 2018, 43, 21–27. [Google Scholar] [CrossRef]
- Waldron, J.L.; Schworer, S.A.; Kwan, M. Hypersensitivity and Immune-related Adverse Events in Biologic Therapy. Clin. Rev. Allergy Immunol. 2022, 62, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.M.; Del-Alcazar, E. New therapies versus first-generation biologic drugs in psoriasis: A review of adverse events and their management. Expert. Rev. Clin. Immunol. 2018, 14, 259–273. [Google Scholar] [CrossRef]
- France, K.; Yogarajah, S.; Gueiros, L.A.; Valdez, R.; Mays, J.W.; Posey, R.; Payne, A.S.; Setterfield, J.; Sollecito, T.P.; Woo, S.B.; et al. World Workshop on Oral Medicine VII: Oral adverse effects to biologic agents in patients with inflammatory disorders. A scoping review. J. Oral Pathol. Med. 2023, 52, 1–8. [Google Scholar] [CrossRef]
- Soleimani, B.; Murray, K.; Hunt, D. Established and Emerging Immunological Complications of Biological Therapeutics in Multiple Sclerosis. Drug Saf. 2019, 42, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Czekalska, A.; Majewski, D.; Puszczewicz, M. Immunodeficiency and autoimmunity during biological disease-modifying antirheumatic drug therapy. Reumatologia 2019, 57, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Baert, F. Medical therapy of inflammatory bowel disease. Med. Clin. N. Am. 1994, 78, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M.; Varma, M.G. Surgery for inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 2678–2690. [Google Scholar] [CrossRef] [PubMed]
- Meima-van Praag, E.M.; Buskens, C.J.; Hompes, R.; Bemelman, W.A. Surgical management of Crohn’s disease: A state of the art review. Int. J. Color. Dis. 2021, 36, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Kotze, P.G.; Heuthorst, L.; Lightner, A.L.; Damiao, A.; Bemelman, W.A. New insights on the surgical management of ulcerative colitis in the 21st century. Lancet Gastroenterol. Hepatol. 2022, 7, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Grieco, M.J.; Remzi, F.H. Surgical Management of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Schwartz, L.M.; Woloshin, S.; Cole, E.B.; Rubin, D.T.; Vay, T.; Baars, J.; Sands, B.E. When should ulcerative colitis patients undergo colectomy for dysplasia? Mismatch between patient preferences and physician recommendations. Inflamm. Bowel Dis. 2010, 16, 1658–1662. [Google Scholar] [CrossRef]
- DeLeon, M.F.; Stocchi, L. Elective and Emergent Surgery in the Ulcerative Colitis Patient. Clin. Colon Rectal Surg. 2022, 35, 437–444. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Harpaz, N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 2004, 126, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Bohl, J.L.; Sobba, K. Indications and Options for Surgery in Ulcerative Colitis. Surg. Clin. N. Am. 2015, 95, 1211–1232. [Google Scholar] [CrossRef] [PubMed]
- Di Candido, F. Quality of Life in Inflammatory Bowel Diseases (IBDs) Patients after Surgery. Rev. Recent. Clin. Trials 2022, 17, 227–239. [Google Scholar] [CrossRef]
- Godala, M.; Gaszynska, E.; Zatorski, H.; Malecka-Wojciesko, E. Dietary Interventions in Inflammatory Bowel Disease. Nutrients 2022, 14, 4261. [Google Scholar] [CrossRef]
- Nazarenkov, N.; Seeger, K.; Beeken, L.; Ananthakrishnan, A.N.; Khalili, H.; Lewis, J.D.; Konijeti, G.G. Implementing Dietary Modifications and Assessing Nutritional Adequacy of Diets for Inflammatory Bowel Disease. Gastroenterol. Hepatol 2019, 15, 133–144. [Google Scholar]
- Yamamoto, T.; Shimoyama, T. Nutrition and diet in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2023, 39, 110–114. [Google Scholar] [CrossRef]
- De Sousa, J.F.M.; Paghdar, S.; Khan, T.M.; Patel, N.P.; Chandrasekaran, S.; Tsouklidis, N. Stress and Inflammatory Bowel Disease: Clear Mind, Happy Colon. Cureus 2022, 14, e25006. [Google Scholar] [CrossRef]
- Jaghult, S.; Saboonchi, F.; Moller, J.; Johansson, U.B.; Wredling, R.; Kapraali, M. Stress as a Trigger for Relapses in IBD: A Case-Crossover Study. Gastroenterol. Res. 2013, 6, 10–16. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, R.; Wang, B.; Jiang, K.; Cao, H. Stress Triggers Flare of Inflammatory Bowel Disease in Children and Adults. Front. Pediatr. 2019, 7, 432. [Google Scholar] [CrossRef]
- Bhandari, S.; Larson, M.E.; Kumar, N.; Stein, D. Association of Inflammatory Bowel Disease (IBD) with Depressive Symptoms in the United States Population and Independent Predictors of Depressive Symptoms in an IBD Population: A NHANES Study. Gut Liver 2017, 11, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.; Cross, R.K.; Long, M.D. Exercise in patients with inflammatory bowel diseases: Current perspectives. Clin. Exp. Gastroenterol. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Mareschal, J.; Douissard, J.; Genton, L. Physical activity in inflammatory bowel disease: Benefits, challenges and perspectives. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 159–166. [Google Scholar] [CrossRef]
- Bilski, J.; Mazur-Bialy, A.; Brzozowski, B.; Magierowski, M.; Zahradnik-Bilska, J.; Wojcik, D.; Magierowska, K.; Kwiecien, S.; Mach, T.; Brzozowski, T. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol. Rep. 2016, 68, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The role of physical exercise in inflammatory bowel disease. Biomed. Res. Int. 2014, 2014, 429031. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, P.; Vollmer-Conna, U.; Hadzi-Pavlovic, D.; Grimm, M.C. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021, 42, 1603990. [Google Scholar] [CrossRef]
- Cosnes, J. Smoking, physical activity, nutrition and lifestyle: Environmental factors and their impact on IBD. Dig. Dis. 2010, 28, 411–417. [Google Scholar] [CrossRef]
- Jones, D.P.; Richardson, T.G.; Davey Smith, G.; Gunnell, D.; Munafo, M.R.; Wootton, R.E. Exploring the Effects of Cigarette Smoking on Inflammatory Bowel Disease Using Mendelian Randomization. Crohns Colitis 360 2020, 2, otaa018. [Google Scholar] [CrossRef]
- Hijos-Mallada, G.; Sostres, C.; Gomollon, F. NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol. Hepatol. 2022, 45, 215–222. [Google Scholar] [CrossRef]
- McEvoy, L.; Carr, D.F.; Pirmohamed, M. Pharmacogenomics of NSAID-Induced Upper Gastrointestinal Toxicity. Front. Pharmacol. 2021, 12, 684162. [Google Scholar] [CrossRef]
- Kinnucan, J.A.; Rubin, D.T.; Ali, T. Sleep and inflammatory bowel disease: Exploring the relationship between sleep disturbances and inflammation. Gastroenterol. Hepatol. (N.Y.) 2013, 9, 718–727. [Google Scholar]
- Canakis, A.; Qazi, T. Sleep and Fatigue in IBD: An Unrecognized but Important Extra-intestinal Manifestation. Curr. Gastroenterol. Rep. 2020, 22, 8. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mironczuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef]
- Piovezani Ramos, G.; Kane, S. Alcohol Use in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol 2021, 17, 211–225. [Google Scholar]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 167, 120–131. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Nacken, W.; Roth, J.; Sorg, C.; Kerkhoff, C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 2003, 60, 569–580. [Google Scholar] [CrossRef]
- Srikrishna, G. S100A8 and S100A9: New insights into their roles in malignancy. J. Innate Immun. 2012, 4, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [CrossRef]
- Kumar, A.; Steinkasserer, A.; Berchtold, S. Interleukin-10 influences the expression of MRP8 and MRP14 in human dendritic cells. Int. Arch. Allergy Immunol. 2003, 132, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Odink, K.; Cerletti, N.; Bruggen, J.; Clerc, R.G.; Tarcsay, L.; Zwadlo, G.; Gerhards, G.; Schlegel, R.; Sorg, C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 1987, 330, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Dhas, D.B.B.; Bhat, B.V.; Gane, D. Role of Calprotectin in Infection and Inflammation. Curr. Pediatr. Res. 2012, 16, 83–94. [Google Scholar]
- Johne, B.; Fagerhol, M.K.; Lyberg, T.; Prydz, H.; Brandtzaeg, P.; Naess-Andresen, C.F.; Dale, I. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol. Pathol. 1997, 50, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Singh, S.; Sandborn, W.J. Positioning Therapies in the Management of Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zeng, L.; Li, L.J.; Mo, L.H.; Xie, R.D.; Feng, B.S.; Zheng, P.Y.; Liu, Z.G.; Liu, Z.J.; Yang, P.C. Specific immunotherapy ameliorates ulcerative colitis. Allergy Asthma Clin. Immunol. 2016, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Averill, M.M.; Kerkhoff, C.; Bornfeldt, K.E. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bjork, P.; Bjork, A.; Vogl, T.; Stenstrom, M.; Liberg, D.; Olsson, A.; Roth, J.; Ivars, F.; Leanderson, T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009, 7, e97. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Sturfelt, G.; Lood, C.; Ronnblom, L.; van Vollenhoven, R.F.; Axelsson, B.; Sparre, B.; Tuvesson, H.; Ohman, M.W.; Leanderson, T. Pharmacokinetics, tolerability, and preliminary efficacy of paquinimod (ABR-215757), a new quinoline-3-carboxamide derivative: Studies in lupus-prone mice and a multicenter, randomized, double-blind, placebo-controlled, repeat-dose, dose-ranging study in patients with systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 1579–1588. [Google Scholar] [CrossRef]
- Coutant, R.; Landais, P.; Rosilio, M.; Johnsen, C.; Lahlou, N.; Chatelain, P.; Carel, J.C.; Ludvigsson, J.; Boitard, C.; Bougneres, P.F. Low dose linomide in Type I juvenile diabetes of recent onset: A randomised placebo-controlled double blind trial. Diabetologia 1998, 41, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.; Barkhof, F.; Sandberg-Wollheim, M.; Linde, A.; Nordle, O.; Nederman, T.; Laquinimod in Relapsing, M.S.S.G. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 2005, 64, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Mun, S.J.; Kim, H.K.; Ham, Y.S.; Gil, W.J.; Yang, C.S. Colon-targeted S100A8/A9-specific peptide systems ameliorate colitis and colitis-associated colorectal cancer in mouse models. Acta Pharmacol. Sin. 2024, 45, 581–593. [Google Scholar] [CrossRef] [PubMed]
- van Zoelen, M.A.; Vogl, T.; Foell, D.; Van Veen, S.Q.; van Till, J.W.; Florquin, S.; Tanck, M.W.; Wittebole, X.; Laterre, P.F.; Boermeester, M.A.; et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am. J. Respir. Crit. Care Med. 2009, 180, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Monin, L.; Torres, D.; Slight, S.; Mehra, S.; McKenna, K.C.; Fallert Junecko, B.A.; Reinhart, T.A.; Kolls, J.; Baez-Saldana, R.; et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 188, 1137–1146. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Segovia, J.A.; Chang, T.H.; Morris, I.R.; Berton, M.T.; Tessier, P.A.; Tardif, M.R.; Cesaro, A.; Bose, S. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: Role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014, 10, e1003848. [Google Scholar] [CrossRef] [PubMed]
- Trostrup, H.; Lerche, C.J.; Christophersen, L.J.; Thomsen, K.; Jensen, P.O.; Hougen, H.P.; Hoiby, N.; Moser, C. Chronic Pseudomonas aeruginosa biofilm infection impairs murine S100A8/A9 and neutrophil effector cytokines-implications for delayed wound closure? Pathog. Dis. 2017, 75, ftx068. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Woo, J.W.; Kwok, S.K.; Cho, M.L.; Park, S.H. MRP8 promotes Th17 differentiation via upregulation of IL-6 production by fibroblast-like synoviocytes in rheumatoid arthritis. Exp. Mol. Med. 2013, 45, e20. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Zenker, S.; Roth, J. S100-alarmins: Potential therapeutic targets for arthritis. Expert. Opin. Ther. Targets 2017, 21, 739–751. [Google Scholar] [CrossRef]
- Lee, T.H.; Chang, H.S.; Bae, D.J.; Song, H.J.; Kim, M.S.; Park, J.S.; Jun, J.A.; Lee, S.Y.; Uh, S.T.; Kim, S.H.; et al. Role of S100A9 in the development of neutrophilic inflammation in asthmatics and in a murine model. Clin. Immunol. 2017, 183, 158–166. [Google Scholar] [CrossRef]
- Xu, Y.D.; Wang, Y.; Yin, L.M.; Park, G.H.; Ulloa, L.; Yang, Y.Q. S100A8 protein attenuates airway hyperresponsiveness by suppressing the contraction of airway smooth muscle. Biochem. Biophys. Res. Commun. 2017, 484, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Roghani, R.S.; Rohgani, H.; Chirumamilla, L.; Farjana, N.; Rashid, M.; Aduli, F.; Kibreab, A.; Laiyemo, A.; Challa, S.R.; et al. Protective role of saffron to reduce inflammation and improve clinical manifestations in ulcerative colitis patients. Gastroenterology 2024, 166, S12–S13. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Nassiri-Asl, M. Avicenna’s (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): A review. Phytother. Res. 2013, 27, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Albarral, J.A.; de Hoz, R.; Ramirez, A.I.; Lopez-Cuenca, I.; Salobrar-Garcia, E.; Pinazo-Duran, M.D.; Ramirez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent Advances on the Anticancer Properties of Saffron (Crocus sativus L.) and Its Major Constituents. Molecules 2020, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Moratalla-Lopez, N.; Bagur, M.J.; Lorenzo, C.; Salinas, M.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef]
- Pashirzad, M.; Shafiee, M.; Avan, A.; Ryzhikov, M.; Fiuji, H.; Bahreyni, A.; Khazaei, M.; Soleimanpour, S.; Hassanian, S.M. Therapeutic potency of crocin in the treatment of inflammatory diseases: Current status and perspective. J. Cell Physiol. 2019, 234, 14601–14611. [Google Scholar] [CrossRef] [PubMed]
- Hashemzaei, M.; Mamoulakis, C.; Tsarouhas, K.; Georgiadis, G.; Lazopoulos, G.; Tsatsakis, A.; Shojaei Asrami, E.; Rezaee, R. Crocin: A fighter against inflammation and pain. Food Chem. Toxicol. 2020, 143, 111521. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Arab, F.L.; Rastin, M.; Tabasi, N.S.; Nikkhah, K.; Mahmoudi, M. Comparative assessment of immunomodulatory, proliferative, and antioxidant activities of crocin and crocetin on mesenchymal stem cells. J. Cell. Biochem. 2021, 122, 29–42. [Google Scholar] [CrossRef]
- Li, Y.; Kakkar, R.; Wang, J. In vivo and in vitro Approach to Anti-arthritic and Anti-inflammatory Effect of Crocetin by Alteration of Nuclear Factor-E2-Related Factor 2/hem Oxygenase (HO)-1 and NF-kappaB Expression. Front. Pharmacol. 2018, 9, 1341. [Google Scholar] [CrossRef]
- Wen, Y.L.; He, Z.; Hou, D.X.; Qin, S. Crocetin Exerts Its Anti-inflammatory Property in LPS-Induced RAW264.7 Cells Potentially via Modulation on the Crosstalk between MEK1/JNK/NF-kappaB/iNOS Pathway and Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6631929. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qian, Z.; Yang, Y.; Sheng, L.; Ji, H.; Zhou, C.; Kazi, H.A. Involvement of Ca2+ in the inhibition by crocetin of platelet activity and thrombosis formation. J. Agric. Food Chem. 2008, 56, 9429–9433. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhao, J.; Kang, Y.; Liu, L.; He, W.; Xie, Y.; Wang, R.; Shan, L.; Li, X.; Ma, K. Effect and mechanism of safranal on ISO-induced myocardial injury based on network pharmacology. J. Ethnopharmacol. 2023, 305, 116103. [Google Scholar] [CrossRef]
- Gupta, M.; Wani, A.; Ahsan, A.U.; Ali, M.; Chibber, P.; Singh, S.; Digra, S.K.; Datt, M.; Bharate, S.B.; Vishwakarma, R.A.; et al. Safranal inhibits NLRP3 inflammasome activation by preventing ASC oligomerization. Toxicol. Appl. Pharmacol. 2021, 423, 115582. [Google Scholar] [CrossRef]
- Lertnimitphun, P.; Zhang, W.; Fu, W.; Yang, B.; Zheng, C.; Yuan, M.; Zhou, H.; Zhang, X.; Pei, W.; Lu, Y.; et al. Safranal Alleviated OVA-Induced Asthma Model and Inhibits Mast Cell Activation. Front. Immunol. 2021, 12, 585595. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, X.; Liu, Z.; Li, Y. Crocetin Potentiates Neurite Growth in Hippocampal Neurons and Facilitates Functional Recovery in Rats with Spinal Cord Injury. Neurosci. Bull. 2017, 33, 695–702. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Qiao, Y.; Feng, C.; Zhao, X. Crocetin attenuates spared nerve injury-induced neuropathic pain in mice. J. Pharmacol. Sci. 2017, 135, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.K.; Qiao, L.Y.; Wen, X.N. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap1/Nrf2 signaling pathway. Cell. Mol. Biol. (Noisy-le-grand) 2016, 62, 11–17. [Google Scholar] [CrossRef]
- Nanda, S.; Madan, K. The role of Safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: A systematic review. Heliyon 2021, 7, e06117. [Google Scholar] [CrossRef]
- Esmaealzadeh, D.; Moodi Ghalibaf, A.; Shariati Rad, M.; Rezaee, R.; Razavi, B.M.; Hosseinzadeh, H. Pharmacological effects of Safranal: An updated review. Iran. J. Basic Med. Sci. 2023, 26, 1131–1143. [Google Scholar] [CrossRef]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Ishisaka, M.; Tsujii, S.; Tsuruma, K.; Shimazawa, M.; Kubo, K.; Umigai, N.; Iwawaki, T.; Hara, H. Crocetin protects ultraviolet A-induced oxidative stress and cell death in skin in vitro and in vivo. Eur. J. Pharmacol. 2016, 789, 244–253. [Google Scholar] [CrossRef]
- Li Puma, S.; Landini, L.; Macedo, S.J., Jr.; Seravalli, V.; Marone, I.M.; Coppi, E.; Patacchini, R.; Geppetti, P.; Materazzi, S.; Nassini, R.; et al. TRPA1 mediates the antinociceptive properties of the constituent of Crocus sativus L., safranal. J. Cell. Mol. Med. 2019, 23, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Hazman, O.; Ovali, S. Investigation of the anti-inflammatory effects of safranal on high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Inflammation 2015, 38, 1012–1019. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, 64–72. [Google Scholar] [CrossRef]
- Tamaddonfard, E.; Erfanparast, A.; Farshid, A.A.; Imani, M.; Mirzakhani, N.; Salighedar, R.; Tamaddonfard, S. Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sci. 2019, 224, 88–94. [Google Scholar] [CrossRef]
- Lertnimitphun, P.; Jiang, Y.; Kim, N.; Fu, W.; Zheng, C.; Tan, H.; Zhou, H.; Zhang, X.; Pei, W.; Lu, Y.; et al. Safranal Alleviates Dextran Sulfate Sodium-Induced Colitis and Suppresses Macrophage-Mediated Inflammation. Front. Pharmacol. 2019, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, Q.; Liu, L.; Wang, S.; Wu, Z.; Tao, Y.; Huang, P.; Wang, P. Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability. Int. J. Mol. Sci. 2022, 23, 3832. [Google Scholar] [CrossRef]
- Liu, M.; Amini, A.; Ahmad, Z. Safranal and its analogs inhibit Escherichia coli ATP synthase and cell growth. Int. J. Biol. Macromol. 2017, 95, 145–152. [Google Scholar] [CrossRef]
- Wang, M.Z.; Gao, J.; Chu, Y.; Niu, J.; Chen, M.; Shang, Q.; Peng, L.H.; Jiang, Z.H. Synthesis of crocetin derivatives and their potent inhibition in multiple tumor cells proliferation and inflammatory property of macrophage. BMC Complement. Med. Ther. 2020, 20, 29. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, J.; Niu, J.; Huang, Y.F.; Chen, M.; Wang, M.Z.; Shang, Q.; Lu, W.Q.; Peng, L.H.; Jiang, Z.H. Synthesis, characterization and inhibitory effects of crocetin derivative compounds in cancer and inflammation. Biomed. Pharmacother. 2018, 98, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kam, H.T.; Chen, Y.; Gong, G.; Hoi, M.P.; Skalicka-Wozniak, K.; Dias, A.C.P.; Lee, S.M. Crocetin and Its Glycoside Crocin, Two Bioactive Constituents from Crocus sativus L. (Saffron), Differentially Inhibit Angiogenesis by Inhibiting Endothelial Cytoskeleton Organization and Cell Migration through VEGFR2/SRC/FAK and VEGFR2/MEK/ERK Signaling Pathways. Front. Pharmacol. 2021, 12, 675359. [Google Scholar] [CrossRef]
- Vafaei, S.; Wu, X.; Tu, J.; Nematollahi-Mahani, S.N. The Effects of Crocin on Bone and Cartilage Diseases. Front. Pharmacol. 2021, 12, 830331. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Alonso, G.-L.; Coca-Prados, M.; Fernandez, J.-A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996, 100, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, Y.; Abdalla, A.; Hamza, A.A.; Amin, A. Safranal Prevents Liver Cancer through Inhibiting Oxidative Stress and Alleviating Inflammation. Front. Pharmacol. 2021, 12, 777500. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Golechha, M.; Kumari, S.; Siddiqui, K.M.; Arya, D.S. Akt/GSK-3beta/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur. J. Nutr. 2012, 51, 719–727. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Saadat, S.; Rahmanian Devin, P.; Jebalbarezy, A.; Moqaddam, M.; Boskabady, M.H.; Askari, V.R. Crocetin regulates Th1/Th2 and Th17/Treg balances, nitric oxide production, and nuclear localization of NF-kappaB in Th2-provoked and normal situations in human-isolated lymphocytes. Biofactors 2023, 49, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Kang, C.; Sun, Y.; Huang, W.; Liu, W.; Qian, Z. Crocetin Inhibits Lipopolysaccharide-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. Cell Physiol. Biochem. 2016, 40, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Feyzi, R.; Boskabady, M.H.; Seyedhosseini Tamijani, S.M.; Rafatpanah, H.; Rezaei, S.A. The Effect of Safranal on Th1/Th2 Cytokine Balance. Iran. J. Immunol. 2016, 13, 263–273. [Google Scholar]
- Singh, G.; Haileselassie, Y.; Ji, A.R.; Maecker, H.T.; Sinha, S.R.; Brim, H.; Habtezion, A.; Ashktorab, H. Protective Effect of Saffron in Mouse Colitis Models through Immune Modulation. Dig. Dis. Sci. 2022, 67, 2922–2935. [Google Scholar] [CrossRef]
- Ashktorab, H.; Soleimani, A.; Singh, G.; Amin, A.; Tabtabaei, S.; Latella, G.; Stein, U.; Akhondzadeh, S.; Solanki, N.; Gondre-Lewis, M.C.; et al. Saffron: The Golden Spice with Therapeutic Properties on Digestive Diseases. Nutrients 2019, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Brim, H.; Kwon, Y.H.; Singh, G.; Sinha, S.R.; Wang, H.; Khan, W.I.; Ashktorab, H. Saffron Pre-Treatment Promotes Reduction in Tissue Inflammatory Profiles and Alters Microbiome Composition in Experimental Colitis Mice. Molecules 2021, 26, 3351. [Google Scholar] [CrossRef] [PubMed]
- Tahvilian, N.; Masoodi, M.; Faghihi Kashani, A.; Vafa, M.; Aryaeian, N.; Heydarian, A.; Hosseini, A.; Moradi, N.; Farsi, F. Effects of saffron supplementation on oxidative/antioxidant status and severity of disease in ulcerative colitis patients: A randomized, double-blind, placebo-controlled study. Phytother. Res. 2021, 35, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Pachikian, B.; Copine, S.; Suchareau, M.; Deldicque, L. Effects of Saffron Extract on Sleep Quality: A Randomized Double-Blind Controlled Clinical Trial. Nutrients 2021, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Metse, A.P.; Drummond, P.D. Effects of saffron on sleep quality in healthy adults with self-reported poor sleep: A randomized, double-blind, placebo-controlled trial. J. Clin. Sleep. Med. 2020, 16, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Hood, S.D.; Drummond, P.D. Efficacy of a standardised saffron extract (affron(R)) as an add-on to antidepressant medication for the treatment of persistent depressive symptoms in adults: A randomised, double-blind, placebo-controlled study. J. Psychopharmacol. 2019, 33, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D.; Inarejos-Garcia, A.M.; Prodanov, M. affron((R)), a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2018, 232, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Neishabouri, S.M.; Velayati, N.; Jahangard, L.; Matinnia, N.; Haghighi, M.; Ghaleiha, A.; Afarideh, M.; Salimi, S.; Meysamie, A.; et al. Crocus sativus L. versus Citalopram in the Treatment of Major Depressive Disorder with Anxious Distress: A Double-Blind, Controlled Clinical Trial. Pharmacopsychiatry 2017, 50, 152–160. [Google Scholar] [CrossRef]
- Kashani, L.; Eslatmanesh, S.; Saedi, N.; Niroomand, N.; Ebrahimi, M.; Hosseinian, M.; Foroughifar, T.; Salimi, S.; Akhondzadeh, S. Comparison of Saffron versus Fluoxetine in Treatment of Mild to Moderate Postpartum Depression: A Double-Blind, Randomized Clinical Trial. Pharmacopsychiatry 2017, 50, 64–68. [Google Scholar] [CrossRef]
- Talaei, A.; Hassanpour Moghadam, M.; Sajadi Tabassi, S.A.; Mohajeri, S.A. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: A randomized, double-blind, placebo-controlled, pilot clinical trial. J. Affect. Disord. 2015, 174, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Tahmacebi-Pour, N.; Noorbala, A.-A.; Amini, H.; Fallah-Pour, H.; Jamshidi, A.-H.; Khani, M. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother. Res. 2005, 19, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Noorbala, A.A.; Akhondzadeh, S.; Tahmacebi-Pour, N.; Jamshidi, A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005, 97, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J. An examination into the mental and physical effects of a saffron extract (affron(R)) in recreationally-active adults: A randomized, double-blind, placebo-controlled study. J. Int. Soc. Sports Nutr. 2022, 19, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Moazen-Zadeh, E.; Abbasi, S.H.; Safi-Aghdam, H.; Shahmansouri, N.; Arjmandi-Beglar, A.; Hajhosseinn Talasaz, A.; Salehiomran, A.; Forghani, S.; Akhondzadeh, S. Effects of Saffron on Cognition, Anxiety, and Depression in Patients Undergoing Coronary Artery Bypass Grafting: A Randomized Double-Blind Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 361–368. [Google Scholar] [CrossRef]
- Baziar, S.; Aqamolaei, A.; Khadem, E.; Mortazavi, S.H.; Naderi, S.; Sahebolzamani, E.; Mortezaei, A.; Jalilevand, S.; Mohammadi, M.-R.; Shahmirzadi, M.; et al. Crocus sativus L. versus Methylphenidate in Treatment of Children with Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind Pilot Study. J. Child Adolesc. Psychopharmacol. 2019, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Shafiee Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Shahmansouri, N.; Farokhnia, M.; Abbasi, S.H.; Kassaian, S.E.; Noorbala Tafti, A.A.; Gougol, A.; Yekehtaz, H.; Forghani, S.; Mahmoodian, M.; Saroukhani, S.; et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014, 155, 216–222. [Google Scholar] [CrossRef]
- Ahmadikhatir, S.; Ostadrahimi, A.; Safaiyan, A.; Ahmadikhatir, S.; Farrin, N. Saffron (Crocus sativus L.) supplements improve quality of life and appetite in atherosclerosis patients: A randomized clinical trial. J. Res. Med. Sci. 2022, 27, 30. [Google Scholar] [CrossRef]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Haleem Al-Qaim, Z.; Hadi, A.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Ostadrahimi, A. The effect of saffron (Crocus sativus L.) on glycemia, lipid profile, and antioxidant status in patients with type-2 diabetes mellitus: A randomized placebo-controlled trial. Phytother. Res. 2023, 37, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Fadai, F.; Mousavi, B.; Ashtari, Z.; Ali beigi, N.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Bathaie, S.Z. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacopsychiatry 2014, 47, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, Z.; Aryaeian, N.; Abolghasemi, J.; Shirani, F.; Hadidi, M.; Fallah, S.; Moradi, N. The effect of saffron supplement on clinical outcomes and metabolic profiles in patients with active rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2020, 34, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Kashani, L.; Aslzadeh, S.; Shokraee, K.; Shamabadi, A.; Tadayon Najafabadi, B.; Jafarinia, M.; Esalatmanesh, S.; Akhondzadeh, S. Crocus sativus (saffron) in the treatment of female sexual dysfunction: A three-center, double-blind, randomized, and placebo-controlled clinical trial. Avicenna J. Phytomedicine 2022, 12, 257–268. [Google Scholar] [CrossRef]
- Mohammadzadeh-Moghadam, H.; Nazari, S.M.; Shamsa, A.; Kamalinejad, M.; Esmaeeli, H.; Asadpour, A.A.; Khajavi, A. Effects of a Topical Saffron (Crocus sativus L.) Gel on Erectile Dysfunction in Diabetics: A Randomized, Parallel-Group, Double-Blind, Placebo-Controlled Trial. J. Evid. Based Complementary Altern. Med. 2015, 20, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, M.; Kashani, L.; Aleyaseen, A.; Ghoreishi, A.; Rahmanpour, H.; Zarrinara, A.; Akhondzadeh, S. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG: An. Int. J. Obstet. Gynaecol. 2008, 115, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Salmanroghani, R.; Salmanroghani, H.; Oskrochi, R.; Rashid, M.; Laiyemo, A.; Challa, S.R.; Oppong-Twene, P.; Farjana, N.; Kibreab, A.; et al. Abstract 7462: Interventional dietary saffron drives antitumor immunity inInterventional dietary saffron drives antitumor immunity in high risk colorectal cancer IBD Patients, A Multisite Clinical Trial Study. Cancer Research 2024, 84, 7462. [Google Scholar] [CrossRef]
- Kianbakht, S.; Ghazavi, A. Immunomodulatory effects of saffron: A randomized double-blind placebo-controlled clinical trial. Phytother. Res. 2011, 25, 1801–1805. [Google Scholar] [CrossRef]
- Piccardi, M.; Fadda, A.; Martelli, F.; Marangoni, D.; Magli, A.; Minnella, A.M.; Bertelli, M.; Di Marco, S.; Bisti, S.; Falsini, B. Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy. Nutrients 2019, 11, 2461. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; McCluskey, P.; Hong, T.; Schlub, T.E.; Chang, A.A. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: A randomised clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 31–40. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Modaghegh, M.H.; Shahabian, M.; Esmaeili, H.A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, R.; Thirunavukkarasu, C.; Gunasekaran, P.; Ramamurty, N.; Sakthisekaran, D. In vitro studies on the selective cytotoxic effect of crocetin and quercetin. Fitoterapia 2000, 71, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.B.; Mehri, S.; Hosseinzadeh, H. Toxicology effects of saffron and its constituents: A review. Iran. J. Basic. Med. Sci. 2017, 20, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Muosa, F.; Al-Rekabi, K.; Askar, S.; Yousif, E. Evaluation of the toxic effect of ethanolic extract of saffron in male mice after subchronic exposure. Donnish J. Pharm. Pharmacol. 2015, 1, 1–7. [Google Scholar]
- Wuthrich, B.; Schmid-Grendelmeyer, P.; Lundberg, M. Anaphylaxis to saffron. Allergy 1997, 52, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Rafieian, M.; Baradaran, A.; Rafieian, S.; Rafieian-Kopaei, M. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J. Nephropathol. 2014, 3, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Shamabadi, A.; Hasanzadeh, A.; Akhondzadeh, S. The neuropsychotropic effects of Crocus sativus L. (saffron): An overview of systematic reviews and meta-analyses investigating its clinical efficacy in psychiatric and neurological disorders. Avicenna J. Phytomed 2022, 12, 475–488. [Google Scholar] [CrossRef]
- Milajerdi, A.; Djafarian, K.; Hosseini, B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J. Nutr. Intermed. Metab. 2016, 3, 23–32. [Google Scholar] [CrossRef]
- Zeynali, F.; Dashti, M.H.; Anvari, M.; Hosseini, S.M.; Mohsen, M.S. Studing teratogenic and abortificant effects of different doses of saffron (crocus sativus) decoction in whole gestational period and the 3rd trimester of gestational period in mice. Int. J. Reprod. Biomed. 2009, 7. Available online: https://sid.ir/paper/295421/en (accessed on 18 June 2024).
- Al-Qudsi, F.M.; Ayedh, A.S.; Arabia, S. Effect of saffron on mouse embryo development. J. Am. Sci. 2012, 8, 1554–1568. [Google Scholar]
- Mehri, S.; Razavi, B.-M.; Hosseinzadeh, H. Chapter 34—Safety and toxicity of saffron. In Saffron; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 517–530. [Google Scholar]
- Shakeri, M.; Hashemi Tayer, A.; Shakeri, H.; Sotoodeh Jahromi, A.; Moradzadeh, M.; Hojjat-Farsangi, M. Toxicity of Saffron Extracts on Cancer and Normal Cells: A Review Article. Asian Pac. J. Cancer Prev. 2020, 21, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Güllü, N.; Kobelt, D.; Brim, H.; Rahman, S.; Timm, L.; Smith, J.; Soleimani, A.; Di Marco, S.; Bisti, S.; Ashktorab, H. Saffron crudes and compounds restrict MACC1-dependent cell proliferation and migration of colorectal cancer cells. Cells 2020, 9, 1829. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.D.; Tarantilis, P.A.; Tajmir-Riahi, H.A.; Polissiou, M.G. DNA interaction with saffron’s secondary metabolites safranal, crocetin, and dimethylcrocetin. DNA Cell Biol. 2007, 26, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Brim, H.; Ashktorab, H. Saffron, Its Active Components, and Their Association with DNA and Histone Modification: A Narrative Review of Current Knowledge. Nutrients 2022, 14, 3317. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Ma, Q.; Kim, S.; Wang, D.H.; Shoyama, Y.; Yuan, C.S. Effects of saffron and its active constituent crocin on cancer management: A narrative review. Longhua Chin. Med. 2022, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.C.; Pannikar, B.; Panikkar, K.R. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991, 57, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Comitato, R.; Manzi, P. Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells. Molecules 2022, 27, 1995. [Google Scholar] [CrossRef]
- Khan, M.; Hearn, K.; Parry, C.; Rasid, M.; Brim, H.; Ashktorab, H.; Kwabi-Addo, B. Mechanism of Antitumor Effects of Saffron in Human Prostate Cancer Cells. Nutrients 2023, 16, 114. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Nikola, O.; Marka, S.; Koniari, E.; Kakouri, E.; Zografaki, M.E.; Mavrikou, S.S.; Kanakis, C.; Flemetakis, E.; Chrousos, G.P.; et al. An In Vitro Study of Saffron Carotenoids: The Effect of Crocin Extracts and Dimethylcrocetin on Cancer Cell Lines. Antioxidants 2022, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Bettiga, A.; DI Marco, F.; Vago, R.; Romani, A.; Vignolini, P.; Vita, C.; Fiorio, F.; Liguori, F.; Ieri, F.; Campo, M.; et al. FC021: Saffron-Derived Bioactive Molecules and Their in-vitro Activity on Kidney and Bladder Tumoral Cells. Nephrol. Dial. Transplant. 2022, 37, gfac098.004. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. Saffron; An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Farkhondeh, T. Antiinflammatory, Antioxidant, and Immunomodulatory Effects of Crocus sativus L. and its Main Constituents. Phytother. Res. 2016, 30, 1072–1094. [Google Scholar] [CrossRef] [PubMed]
- Poursamimi, J.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohajeri, S.A.; Mohammadi, M. Crocus Sativus (Saffron): An Immunoregulatory Factor in the Autoimmune and Non-autoimmune Diseases. Iran. J. Allergy Asthma Immunol. 2020, 19, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Li, S.; Yang, J.; Lin, D.; Feng, Y.; Lu, J.; Shao, Q. Phytochemistry, pharmacology, and potential clinical applications of saffron: A review. J. Ethnopharmacol. 2021, 281, 114555. [Google Scholar] [CrossRef] [PubMed]
- Ann Hausenblas, H.; Heekin, K.; Mutchie, H.L.; Anton, S. A systematic review of randomized controlled trials examining the effectiveness of saffron (Crocus sativus L.) on psychological and behavioral outcomes. J. Integr. Med. 2015, 13, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zilaee, M.; Hosseini, S.A.; Jafarirad, S.; Abolnezhadian, F.; Cheraghian, B.; Namjoyan, F.; Ghadiri, A. An evaluation of the effects of saffron supplementation on the asthma clinical symptoms and asthma severity in patients with mild and moderate persistent allergic asthma: A double-blind, randomized placebo-controlled trial. Respir. Res. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, M.; Khazdair, M.R. Possible therapeutic effects of Crocus sativus stigma and its petal flavonoid, kaempferol, on respiratory disorders. Pharm. Biol. 2020, 58, 1140–1149. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Sadeghi, O.; Rigi, S.; Tan, S.C.; Shokri, A.; Mousavi, S.M. Effects of saffron (Crocus sativus L.) supplementation on inflammatory biomarkers: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 20–32. [Google Scholar] [CrossRef]
- Singh, G.; Brim, H.; Haileselassie, Y.; Varma, S.; Habtezion, A.; Rashid, M.; Sinha, S.R.; Ashktorab, H. Microbiomic and Metabolomic Analyses Unveil the Protective Effect of Saffron in a Mouse Colitis Model. Curr. Issues Mol. Biol. 2023, 45, 5558–5574. [Google Scholar] [CrossRef] [PubMed]
- Khoshandam, A.; Razavi, B.M.; Hosseinzadeh, H. Interaction of saffron and its constituents with Nrf2 signaling pathway: A review. Iran. J. Basic Med. Sci. 2022, 25, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and anti-inflammatory properties of Crocus sativus (Saffron) and its main active constituents: A review. Iran. J. Basic Med. Sci. 2019, 22, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Farrukh, A.; Murali, C.; Soleimani, A.; Praz, F.; Graziani, G.; Brim, H.; Ashktorab, H. Saffron and its major ingredients’ effect on colon cancer cells with mismatch repair deficiency and microsatellite instability. Molecules 2021, 26, 3855. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Shafiee Sabet, M.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Kamalipour, M.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Human Psychopharmacol. Clin. Exp. 2014, 29, 351–359. [Google Scholar] [CrossRef]
- Mirzaei, H.; Gharehgozlou, R.; Heydarirad, G.; Fahimi, S.; Ghafari, S.; Mosavat, S.H.; Moghani, M.M.; Hajian, P. Efficacy and Safety of Jollab (a Saffron-Based Beverage) on Cancer-Related Fatigue in Breast Cancer Patients: A Double-Blind Randomized Clinical Trial. Complement. Med. Res. 2022, 29, 437–445. [Google Scholar] [CrossRef]
| Bioactive Compound | Pharmacological Effect | Health Benefits | References |
|---|---|---|---|
| Crocetin |
|
| [127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152] |
| Crocin |
|
| [150,151,152,153,154,155] |
| Safranal |
|
| [132,133,134,135,144,145,146,147,148,149,156,157,158,159] |
| Role of Saffron | Country | Year | Concentration | Participants | Clinical Trial # | Ref. |
|---|---|---|---|---|---|---|
| SLEEP QUALITY | ||||||
| Reports have shown that saffron intake was associated with improvements in sleep quality in adults with self-reported sleep complaints. | Belgium | 2021 | 15.5 mg per day for 6 weeks | 34 | NCT04750681 | [164] |
| Australia | 2020 | 14 mg twice daily for 28 days | 33 | ACTRN12619000863134 | [165] | |
| NEUROPSYCHIATRIC CONDITIONS | ||||||
| Several lines of evidence have shown that supplementation with saffron showed potential SSRI-like activity and neuroprotective properties, implying that saffron could serve as a safe adjunctive medication to alleviate symptoms, particularly in MDD and postpartum depression, with a notable impact on anxiety disorders and a minimal occurrence of side effects. | Australia | 2019 | 14 mg b.i.d. for 8 weeks | 72 | NA | [166] |
| Australia | 2018 | 14 mg b.i.d. for 8 weeks | 40 | ACTRN12617000155392 | [167] | |
| Australia | 2017 | 28 mg/day and 22 mg/day for 4 weeks | 121 | NA | [168] | |
| Iran | 2017 | 30 mg/day for 6 weeks | 30 | NA | [169] | |
| Iran | 2017 | 15 mg twice daily for 6 weeks | 34 | NA | [170] | |
| Iran | 2016 | 50 mg twice daily for 12 weeks | 54 | NA | [167] | |
| Iran | 2015 | 30 mg/day and 15 mg b.i.d. for 4 weeks | 23 | IRCT20130418013058N11 | [171] | |
| Iran | 2005 | 30 mg/day (b.i.d.) for 6 weeks | 20 | NA | [172] | |
| Iran | 2005 | 30 mg/day capsule for 6 weeks | 20 | NA | [173] | |
| Australia | 2020 | 28 mg daily for 6 weeks | 31 | ACTRN12621000501842 | [174,175] | |
| Studies have shown that saffron exhibited efficacy equivalent to methylphenidate in treating ADHD in children, suggesting its potential as a candidate for ADHD therapy due to its ability to impact both monoaminergic and glutamatergic systems, yielding satisfactory outcomes. | Iran | 2019 | 20–30 mg/day for 6 weeks | 27 | IRCT201701131556N94 | [176] |
| Other reports have shown saffron to be both safe and effective in the short-term for individuals with mild-to-moderate AD, attributed to its ability to inhibit the aggregation and deposition of amyloid β in the human brain, thereby potentially treating neurodegenerative damage caused by oxidative stress. | Iran | 2009 | 30 mg/day (15 mg twice per day) | 27 | IRCT138711051556N1 | [177] |
| Iran | 2010 | 30 mg/day for 16 weeks | 23 | NA | [178] | |
| CARDIOVASCULAR EFFECTS | ||||||
| Saffron, possessing antioxidant, anti-inflammatory, antihyperlipidemic, hypotensive, and weight-lowering properties, can aid in supporting cardiovascular health and ameliorating symptoms in atherosclerosis patients, including physical disability, sexual dysfunction, and psychological disorders, enhancing quality of life. | Iran | 2014 | 30 mg/day capsule for 6 weeks | 22 | NA | [179] |
| Iran | 2022 | 100 mg/day for 6 weeks | 33 |
NA IRCT201511192017N25 | [180] | |
| METABOLIC DISORDERS | ||||||
| Saffron’s potent antidiabetic, antiobesity, hypotensive, and hypolipidemic effects suggest its potential importance in managing MetS, with studies demonstrating improvements in FBG, hemoglobin A1C, glycemic status, lipid profile, oxidative status, and liver function tests in diabetic profiles. | Iran | 2022 | 100 mg/day for 8 weeks | 35 | NA | [181] |
| Iran | 2014 | 30 mg daily for 12 weeks | 44 | NA | [182] | |
| RHEUMATOID ARTHRITIS | ||||||
| A study showed the potential benefits of saffron supplementation in enhancing disease activity and clinical outcomes in RA by decreasing inflammatory ILs, highlighting its anti-inflammatory properties and ability to alleviate acute and chronic pain. | Iran | 2020 | 100 mg/day for 12 weeks | 31 | NA | [183] |
| REPRODUCTIVE HEALTH | ||||||
| Studies have explored saffron’s aphrodisiac effects in men, indicating improvements in erectile function and overall sexual health, especially in diabetes. Similarly, in women, saffron has proven effective in alleviating sexual dysfunction and relieving symptoms of premenstrual syndrome, dysmenorrhea, and irregular menstruation, potentially modulating the secretion of steroid hormones. | Iran | 2022 | 15 mg twice daily for 2 weeks | 34 | IRCT20090117001556N110 | [184] |
| Iran | 2015 | 1% topical saffron gel | 25 | IRCT ID: 201404071769N1 | [185] | |
| Iran | 2008 | 30 mg/day for 2 menstrual cycles | 25 | NA | [186] | |
| Inflammatory Bowel Disease | ||||||
| Reports have shown that saffron supplementation among patients with UC may be effective in improving antioxidant status and reducing disease severity. Our multiple-center IBD clinical trial has suggested that saffron treatment led to a decrease in pro-inflammatory (TNFα, INF-γ, IL-6, IL-2, and IL-17a) and an increase in anti-inflammatory (IL-10 and TGF-β) cytokines, along with reduced fecal calprotectin (CP) and serum CRP levels in patients with mild-to-moderate UC | Iran USA | 2020 Presently active | 100 mg/daily 50mg/b.i.d | 40 62 | NA NCT04749576 | [163] [122,187] |
| IMMUNOREGULATORY | ||||||
| Saffron may have effects on the immune system and hematological parameters. | Iran | 2011 | 100 mg daily for 6 weeks | 45 | NA | [188] |
| Saffron may have mental and physical effects in healthy recreationally active adults | Australia | 2020 | 28 mg daily for 6 weeks | 31 | ACTRN12621000501842 | [174] |
| OCULAR DISEASES | ||||||
| Saffron supplementation modestly improved visual function in participants with AMD, including those using AREDS supplements. Additionally, saffron supplementation shows promise in slowing down the progression of central retinal dysfunction in ABCA4-related STG/FF. | Italy | 2019 | 20 mg over 180 days | 14 | NCT01278277 | [189] |
| Australia and New Zealand | 2019 | 20 mg/day for 3 months | 50 | ACTRN 12612000729820 | [190] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.; Rashid, R.; Saroya, S.; Deverapalli, M.; Brim, H.; Ashktorab, H. Saffron as a Promising Therapy for Inflammatory Bowel Disease. Nutrients 2024, 16, 2353. https://doi.org/10.3390/nu16142353
Rashid M, Rashid R, Saroya S, Deverapalli M, Brim H, Ashktorab H. Saffron as a Promising Therapy for Inflammatory Bowel Disease. Nutrients. 2024; 16(14):2353. https://doi.org/10.3390/nu16142353
Chicago/Turabian StyleRashid, Mudasir, Rumaisa Rashid, Sabtain Saroya, Mrinalini Deverapalli, Hassan Brim, and Hassan Ashktorab. 2024. "Saffron as a Promising Therapy for Inflammatory Bowel Disease" Nutrients 16, no. 14: 2353. https://doi.org/10.3390/nu16142353





