Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction Process
3. Results and Discussion
3.1. Aging and Vitamins D and K
3.2. Cardiovascular Health
3.2.1. Vitamins D and K in Cardiovascular Health
3.2.2. Randomized Controlled Trials of Vitamins D and K in Cardiovascular Health
3.3. Bone Health
3.3.1. Osteoporosis and Vitamins D and K
3.3.2. Randomized Controlled Trials of Vitamins D and K in Bone Health
3.4. Findings and Recommendations
| Parameter | Number of Studies | References |
|---|---|---|
| OC; cOC; ucOC; cOC/ucOC ratio | 20 | [73,76,92,94,95,96,97,98,99,100,102,103,104,105,110,111,113,114,115,118] |
| BMD | 15 | [93,94,96,99,101,102,103,105,106,112,113,114,116,117,118] |
| PINP, CTX; CTX/P1NP | 4 | [76,116,117,119] |
| IGF-1 | 3 | [98,100,102] |
| MGP; dp-ucMGP | 3 | [72,74,134] |
| BAP | 2 | [113,118] |
| Adiponectin | 1 | [76] |
| CC; DC; IMT | 1 | [71] |
| DPD | 1 | [102] |
| NO; hROS | 1 | [75] |
| NTX | 1 | [97] |
3.5. Mechanisms of Action
3.6. Strength and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrucci, L.; Cooper, R.; Shardell, M.; Simonsick, E.M.; Schrack, J.A.; Kuh, D. Age-Related Change in Mobility: Perspectives from Life Course Epidemiology and Geroscience. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Lynch, N.A.; Ryan, A.S.; Berman, D.M.; Sorkin, J.D.; Nicklas, B.J. Comparison of VO2 Max and Disease Risk Factors between Perimenopausal and Postmenopausal Women. Menopause 2002, 9, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Nicklas, B.J.; Elahi, D. A Cross-Sectional Study on Body Composition and Energy Expenditure in Women Athletes during Aging. Am. J. Physiol. 1996, 271 Pt 1, E916–E921. [Google Scholar] [CrossRef]
- Ryan, A.S.; Nicklas, B.J. Age-Related Changes in Fat Deposition in Mid-Thigh Muscle in Women: Relationships with Metabolic Cardiovascular Disease Risk Factors. Int. J. Obes. 1999, 23, 126–132. [Google Scholar] [CrossRef]
- Ryan, A.S.; Muller, D.C.; Elahi, D. Sequential Hyperglycemic-Euglycemic Clamp to Assess Beta-Cell and Peripheral Tissue: Studies in Female Athletes. J. Appl. Physiol. 2001, 91, 872–881. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Ryan, A.S.; Katzel, L.I. Lipoprotein Subfractions in Women Athletes: Effects of Age, Visceral Obesity and Aerobic Fitness. Int. J. Obes. 1999, 23, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Crandall, C. The Women’s Health Initiative: A Landmark Resource for Skeletal Research Since 1992. J. Bone Miner. Res. 2020, 35, 845–860. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Vlase, L.; Popa, D.S. Antioxidants in Age-Related Diseases and Anti-Aging Strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet Women and Cardiovascular Disease Commission: Reducing the Global Burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Nappi, R.; Chedraui, P.; Lambrinoudaki, I.; Simoncini, T. Menopause: A Cardiometabolic Transition. Lancet Diabetes Endocrinol. 2022, 10, 442–456. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, E506–E532. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management—The Plaque Hypothesis: A Narrative Review. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.; Cubero Salazar, I.; Kazzi, B.; Hays, A.G.; Choi, A.D.; Arbab-Zadeh, A.; Michos, E.D. Sex-Specific Plaque Signature: Uniqueness of Atherosclerosis in Women. Curr. Cardiol. Rep. 2021, 23, 84. [Google Scholar] [CrossRef] [PubMed]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Koshy, F.S.; George, K.; Poudel, P.; Chalasani, R.; Goonathilake, M.R.; Waqar, S.; George, S.; Jean-Baptiste, W.; Yusuf Ali, A.; Inyang, B.; et al. Exercise Prescription and the Minimum Dose for Bone Remodeling Needed to Prevent Osteoporosis in Postmenopausal Women: A Systematic Review. Cureus 2022, 4, e25993. [Google Scholar] [CrossRef] [PubMed]
- Muniyasamy, R.; Manjubala, I. Insight into the Mechanism of Osteoporosis and the Available Treatment Options. Curr. Pharm. Biotechnol. 2023, 25, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Shane, E. Postmenopausal Osteoporosis. N. Engl. J. Med. 2023, 389, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Anam, A.K.; Insogna, K. Update on Osteoporosis Screening and Management. Med. Clin. N. Am. 2021, 105, 1117–1134. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Wen, Z.; Wang, G. Romosozumab in Osteoporosis: Yesterday, Today and Tomorrow. J. Transl. Med. 2023, 21, 668. [Google Scholar] [CrossRef]
- Mateș, L.; Rusu, M.E.; Popa, D.-S. Phytochemicals and Biological Activities of Walnut Septum: A Systematic Review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.-S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Bigman, G.; Shea, K.M.; Rusu, M.E.; Ryan, A.S. Intake of Dark Green Vegetables May Benefit Specific Cognitive Domains in US Men and Women Aged 60 Years or Older. Nutr. Healthy Aging 2023, 8, 67–77. [Google Scholar] [CrossRef]
- El Menyiy, N.; Aboulaghras, S.; Bakrim, S.; Moubachir, R.; Taha, D.; Khalid, A.; Abdalla, A.N.; Algarni, A.S.; Hermansyah, A.; Ming, L.C.; et al. Genkwanin: An Emerging Natural Compound with Multifaceted Pharmacological Effects. Biomed. Pharmacother. 2023, 165, 115159. [Google Scholar] [CrossRef] [PubMed]
- Feduniw, S.; Korczyńska, L.; Górski, K.; Zgliczyńska, M.; Bączkowska, M.; Byrczak, M.; Kociuba, J.; Ali, M.; Ciebiera, M. The Effect of Vitamin E Supplementation in Postmenopausal Women—A Systematic Review. Nutrients 2023, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.Z.; Wu, S.K.; Zailani, H.; Chiu, W.C.; Liu, W.C.; Su, K.P.; Lee, S. Da Effects of Omega-3 Polyunsaturated Fatty Acids Intake on Vasomotor Symptoms, Sleep Quality and Depression in Postmenopausal Women: A Systematic Review. Nutrients 2023, 15, 4231. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The Health Effects of Vitamin D Supplementation: Evidence from Human Studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Chedraui, P.; Pilz, S. Vitamin D Supplementation after the Menopause. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820931291. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Nardin, S.; Cao, D.; Chiarito, M.; Kedhi, E.; Galasso, G.; Condorelli, G.; De Luca, G. Vitamin D and Cardiovascular Diseases: From Physiology to Pathophysiology and Outcomes. Biomedicines 2024, 12, 768. [Google Scholar] [CrossRef]
- Holick, M.F. The One-Hundred-Year Anniversary of the Discovery of the Sunshine Vitamin D3: Historical, Personal Experience and Evidence-Based Perspectives. Nutrients 2023, 15, 593. [Google Scholar] [CrossRef]
- Alonso, N.; Meinitzer, A.; Fritz-Petrin, E.; Enko, D.; Herrmann, M. Role of Vitamin K in Bone and Muscle Metabolism. Calcif. Tissue Int. 2023, 112, 178–196. [Google Scholar] [CrossRef]
- Tsugawa, N.; Shiraki, M. Vitamin K Nutrition and Bone Health. Nutrients 2020, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Y. The Potential of Vitamin K as a Regulatory Factor of Bone Metabolism—A Review. Nutrients 2023, 15, 4935. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Zhang, B.; Gu, M.; Chen, C.; Zhang, Q.; Zhang, G.; Cao, X. Vitamin K Intake and the Risk of Fractures. Medicine 2017, 96, e6725. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Ma, Z.; He, Y.; Sun, H.; Yang, B.; Ruan, B.; Zhan, W.; Li, S.; Dong, H.; Wang, Y.; et al. Efficacy of Vitamin K2 in the Prevention and Treatment of Postmenopausal Osteoporosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Public Health 2022, 10, 979649. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.C.W.; Li, R.W.S.; Seto, S.W.; Kong, S.K.; Ho, H.P.; Hoi, M.P.M.; Lee, S.M.Y.; Ngai, S.M.; Chan, S.W.; Leung, G.P.H.; et al. In Vitro Vitamin K2 and 1α,25-Dihydroxyvitamin D3 Combination Enhances Osteoblasts Anabolism of Diabetic Mice. Eur. J. Pharmacol. 2015, 767, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Torbergsen, A.C.; Watne, L.O.; Wyller, T.B.; Frihagen, F.; Strømsøe, K.; Bøhmer, T.; Mowe, M. Vitamin K1 and 25(OH)D Are Independently and Synergistically Associated with a Risk for Hip Fracture in an Elderly Population: A Case Control Study. Clin. Nutr. 2015, 34, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Hmamouchi, I.; Allali, F.; Khazzani, H.; Bennani, L.; El Mansouri, L.; Ichchou, L.; Cherkaoui, M.; Abouqal, R.; Hajjaj-Hassouni, N. Low Bone Mineral Density Is Related to Atherosclerosis in Postmenopausal Moroccan Women. BMC Public Health 2009, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Saverino, A.; Del Sette, M.; Conti, M.; Ermirio, D.; Ricca, M.; Rovetta, G.; Gandolfo, C. Hyperechoic Plaque: An Ultrasound Marker for Osteoporosis in Acute Stroke Patients with Carotid Disease. Eur. Neurol. 2006, 55, 31–36. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Rusu, M.; Simedrea, R.; Gheldiu, A.-M.; Mocan, A.; Vlase, L.; Popa, D.-S.; Ferreira, I.C.F.R. Benefits of Tree Nut Consumption on Aging and Age-Related Diseases: Mechanisms of Actions. Trends Food Sci. Technol. 2019, 88, 104–120. [Google Scholar] [CrossRef]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Mandatori, D.; Pelusi, L.; Schiavone, V.; Pipino, C.; Di Pietro, N.; Pandolfi, A. The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification. Nutrients 2021, 13, 1222. [Google Scholar] [CrossRef]
- Martini, N.; Streckwall, L.; McCarthy, A.D. Osteoporosis and Vascular Calcifications. Endocr. Connect. 2023, 12, e230305. [Google Scholar] [CrossRef]
- Rodríguez, A.J.; Leow, K.; Szulc, P.; Scott, D.; Ebeling, P.; Sim, M.; Wong, G.; Lim, W.H.; Schousboe, J.T.; Kiel, D.P.; et al. Abdominal Aortic Calcification, Bone Mineral Density and Fractures: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2019, 9, e026232. [Google Scholar] [CrossRef] [PubMed]
- Campos-Obando, N.; Kavousi, M.; Roeters van Lennep, J.E.; Rivadeneira, F.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Zillikens, M.C. Bone Health and Coronary Artery Calcification: The Rotterdam Study. Atherosclerosis 2014, 241, 278–283. [Google Scholar] [CrossRef]
- Asadi, M.; Razi, F.; Fahimfar, N.; Shirani, S.; Behzad, G.; Salari, P. The Association of Coronary Artery Calcium Score and Osteoporosis in Postmenopausal Women: A Cross-Sectional Study. J. Bone Metab. 2022, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.L.; Koo, M.; Wang, Y.F. Association of Bone Mineral Density and Coronary Artery Calcification in Patients with Osteopenia and Osteoporosis. Diagnostics 2020, 10, 699. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Liu, Y.; Bai, L.; Liu, L.; He, L.; Deng, H.; Li, T.; Xu, S.; Chen, L.; et al. Associations between Bone Mineral Density in Different Measurement Locations and Coronary Artery Disease: A Cross-Sectional Study. Arch. Osteoporos. 2021, 16, 100. [Google Scholar] [CrossRef]
- Vassalle, C.; Mazzone, A. Bone Loss and Vascular Calcification: A Bi-Directional Interplay? Vasc. Pharmacol. 2016, 86, 77–86. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-Soluble Vitamins: Updated Review of Their Role and Orchestration in Human Nutrition throughout Life Cycle with Sex Differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef]
- Arora, J.; Wang, J.; Weaver, V.; Zhang, Y.; Cantorna, M.T. Novel Insight into the Role of the Vitamin D Receptor in the Development and Function of the Immune System. J. Steroid Biochem. Mol. Biol. 2022, 219, 106084. [Google Scholar] [CrossRef] [PubMed]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An Overview of Gene Regulation, Ranging from Metabolism to Genomic Effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin d on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Kimball, S.M.; Holick, M.F. Official Recommendations for Vitamin D through the Life Stages in Developed Countries. Eur. J. Clin. Nutr. 2020, 74, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Mafra, D.; Shiels, P.G.; Hackeng, T.M.; Stenvinkel, P.; Schurgers, L.J. Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome. Nutrients 2023, 15, 2727. [Google Scholar] [CrossRef]
- Elshaikh, A.O.; Shah, L.; Joy Mathew, C.; Lee, R.; Jose, M.T.; Cancarevic, I. Influence of Vitamin K on Bone Mineral Density and Osteoporosis. Cureus 2020, 12, e10816. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef]
- Margier, M.; Antoine, T.; Siriaco, A.; Nowicki, M.; Halimi, C.; Maillot, M.; Georgé, S.; Reboul, E. The Presence of Pulses within a Meal Can Alter Fat-Soluble Vitamin Bioavailability. Mol. Nutr. Food Res. 2019, 63, e1801323. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Biltoft-Jensen, A.P.; Jakobsen, J. In Vitro Bioaccessibility of Vitamin K (Phylloquinone and Menaquinones) in Food and Supplements Assessed by INFOGEST 2.0—Vit K. Curr. Res. Food Sci. 2022, 5, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Flore, R.; Ponziani, F.R.; Di Rienzo, T.A.; Zocco, M.A.; Flex, A.; Gerardino, L.; Lupascu, A.; Santoro, L.; Santoliquido, A.; Di Stasio, E.; et al. Something More to Say about Calcium Homeostasis: The Role of Vitamin K2 in Vascular Calcification and Osteoporosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2433–2440. [Google Scholar] [PubMed]
- Chen, Y.; Zhao, X.; Wu, H. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arter. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and Women’s Cardiovascular Health: Is It Really an Obvious Relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.R.; Malhotra, R.; Hilaire, C.S.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.U.; Shanahan, C.M.; et al. Molecular Mechanisms of Vascular Health: Insights from Vascular Aging and Calcification. Arter. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Kinuta, M. Coronary Artery Calcium in Assessment of Atherosclerotic Cardiovascular Disease Risk and Its Role in Primary Prevention. J. Atheroscler. Thromb. 2023, 30, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Kumric, M.; Borovac, J.A.; Kurir, T.T.; Martinovic, D.; Separovic, I.F.; Baric, L.; Bozic, J. Role of Matrix Gla Protein in the Complex Network of Coronary Artery Disease: A Comprehensive Review. Life 2021, 11, 737. [Google Scholar] [CrossRef]
- Shioi, A.; Morioka, T.; Shoji, T.; Emoto, M. The Inhibitory Roles of Vitamin k in Progression of Vascular Calcification. Nutrients 2020, 12, 583. [Google Scholar] [CrossRef]
- Braam, L.A.J.L.M.; Hoeks, A.P.G.; Brouns, F.; Halmuyák, K.; Gerichhausen, M.J.W.; Vermeer, C. Beneficial Effects of Vitamins D and K on the Elastic Properties of the Vessel Wall in Postmenopausal Women: A Follow-up Study. Thromb. Haemost. 2004, 91, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K Supplementation and Progression of Coronary Artery Calcium in Older Men and Women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.J.; Braam, L.A.J.L.M.; Teunissen, K.J.; Zwijsen, R.M.L.; Theuwissen, E.; Vermeer, C. Yogurt Drink Fortified with Menaquinone-7 Improves Vitamin K Status in a Healthy Population. J. Nutr. Sci. 2015, 4, e35. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.J.; Braam, L.A.J.L.M.; Teunissen, K.J.; Van’t Hoofd, C.M.; Zwijsen, R.M.L.; Van Den Heuvel, E.G.H.M.; Vermeer, C. Steady-State Vitamin K2 (Menaquinone-7) Plasma Concentrations after Intake of Dairy Products and Soft Gel Capsules. Eur. J. Clin. Nutr. 2016, 70, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Vignini, A.; Nanetti, L.; Raffaelli, F.; Sabbatinelli, J.; Salvolini, E.; Quagliarini, V.; Cester, N.; Mazzanti, L. Effect of 1-y Oral Supplementation with Vitaminized Olive Oil on Platelets from Healthy Postmenopausal Women. Nutrition 2017, 42, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rønn, S.H.; Harsløf, T.; Pedersen, S.B.; Langdahl, B.L. Vitamin K2 (Menaquinone-7) Increases Plasma Adiponectin but Does Not Affect Insulin Sensitivity in Postmenopausal Women: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2021, 75, 1661–1667. [Google Scholar] [CrossRef]
- Manson, J.E.; Allison, M.A.; Carr, J.J.; Langer, R.D.; Cochrane, B.B.; Hendrix, S.L.; Hsia, J.; Hunt, J.R.; Lewis, C.E.; Margolis, K.L.; et al. Calcium/Vitamin D Supplementation and Coronary Artery Calcification in the Women’s Health Initiative. Menopause 2010, 17, 683–691. [Google Scholar] [CrossRef]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Plasma Desphospho-Uncarboxylated Matrix Gla Protein as a Marker of Kidney Damage and Cardiovascular Risk in Advanced Stage of Chronic Kidney Disease. Kidney Blood Press. Res. 2016, 41, 231–239. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; van Putten, S.R.; Visser, M.; Beulens, J.W.; Hoogendijk, E.O. Vitamin K Status and Physical Decline in Older Adults—The Longitudinal Aging Study Amsterdam. Maturitas 2018, 113, 73–79. [Google Scholar] [CrossRef]
- Machado-Fragua, M.D.; Hoogendijk, E.O.; Struijk, E.A.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Beulens, J.W.; van Ballegooijen, A.J. High Dephospho-Uncarboxylated Matrix Gla Protein Concentrations, a Plasma Biomarker of Vitamin K, in Relation to Frailty: The Longitudinal Aging Study Amsterdam. Eur. J. Nutr. 2020, 59, 1243–1251. [Google Scholar] [CrossRef]
- Shea, M.K.; Loeser, R.F.; Hsu, F.C.; Booth, S.L.; Nevitt, M.; Simonsick, E.M.; Strotmeyer, E.S.; Vermeer, C.; Kritchevsky, S.B. Vitamin K Status and Lower Extremity Function in Older Adults: The Health Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Kritchevsky, S.B.; Loeser, R.F.; Booth, S.L. Vitamin K Status and Mobility Limitation and Disability in Older Adults: The Health, Aging, and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Jamilian, M.; Karamali, M.; Bahmani, F.; Aghadavod, E.; Asemi, Z. The Effects of Vitamin D-K-Calcium Co-Supplementation on Endocrine, Inflammation, and Oxidative Stress Biomarkers in Vitamin D-Deficient Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2016, 48, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, E.; Beulens, J.W.J.; Elders, P.; Rutters, F.; Stehouwer, C.D.A.; Van Der Heijden, A.A.; Van Ballegooijen, A.J. The Association of Vitamin D and Vitamin K Status with Subclinical Measures of Cardiovascular Health and All-Cause Mortality in Older Adults: The Hoorn Study. J. Nutr. 2020, 150, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- van Ballegooijen, A.J.; Beulens, J.W.J.; Kieneker, L.M.; de Borst, M.H.; Gansevoort, R.T.; Kema, I.P.; Schurgers, L.J.; Vervloet, M.G.; Bakker, S.J.L. Combined Low Vitamin D and K Status Amplifies Mortality Risk: A Prospective Study. Eur. J. Nutr. 2021, 60, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, M.; Hosoi, T.; Matsumoto, S.; Fujimori, K.; Tamaki, J.; Nakatoh, S.; Ishii, S.; Okimoto, N.; Kamiya, K.; Akishita, M.; et al. Prescription of Vitamin D Was Associated with a Lower Incidence of Hip Fractures. Sci. Rep. 2023, 13, 12889. [Google Scholar] [CrossRef] [PubMed]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Hu, H.; Zou, Y.; Li, D. The Role of Vitamin D in Menopausal Women’s Health. Front. Physiol. 2023, 14, 1211896. [Google Scholar] [CrossRef]
- Rokidi, S.; Paschalis, E.P.; Klaushofer, K.; Vennin, S.; Desyatova, A.; Turner, J.A.; Watson, P.; Lappe, J.; Akhter, M.P.; Recker, R.R. Organic Matrix Quality Discriminates between Age- and BMD-Matched Fracturing versus Non-Fracturing Post-Menopausal Women: A Pilot Study. Bone 2019, 127, 207–214. [Google Scholar] [CrossRef]
- Rizzo, S.; Farlay, D.; Akhter, M.; Boskey, A.; Recker, R.; Lappe, J.; Boivin, G. Variables Reflecting the Mineralization of Bone Tissue from Fracturing versus Nonfracturing Postmenopausal Nonosteoporotic Women. JBMR Plus 2018, 2, 323–327. [Google Scholar] [CrossRef]
- Ma, R.; Wu, M.; Li, Y.; Wang, J.; Yang, P.; Chen, Y.; Wang, W.; Song, J.; Wang, K. The Use of Bone Turnover Markers for Monitoring the Treatment of Osteoporosis in Postmenopausal Females Undergoing Total Knee Arthroplasty: A Prospective Randomized Study. J. Orthop. Surg. Res. 2021, 16, 195. [Google Scholar] [CrossRef]
- Schaafsma, A.; Muskiet, F.; Storm, H.; Hofstede, G.; Pakan, I.; Van Der Veer, E. Vitamin D3 and Vitamin K1 Supplementation of Dutch Postmenopausal Women with Normal and Low Bone Mineral Densities: Effects on Serum 25-Hydroxyvitamin D and Carboxylated Osteocalcin. Eur. J. Clin. Nutr. 2000, 54, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Braam, L.A.J.L.M.; Knapen, M.H.J.; Geusens, P.; Brouns, F.; Hamulyák, K.; Gerichhausen, M.J.W.; Vermeer, C. Vitamin K1 Supplementation Retards Bone Loss in Postmenopausal Women between 50 and 60 Years of Age. Calcif. Tissue Int. 2003, 73, 21–26. [Google Scholar] [CrossRef]
- Bolton-Smith, C.; McMurdo, M.E.T.; Paterson, C.R.; Mole, P.A.; Harvey, J.M.; Fenton, S.T.; Prynne, C.J.; Mishra, G.D.; Shearer, M.J. Two-Year Randomized Controlled Trial of Vitamin K1 (Phylloquinone) and Vitamin D3 plus Calcium on the Bone Health of Older Women. J. Bone Miner. Res. 2007, 22, 509–519. [Google Scholar] [CrossRef]
- Bügel, S.; Sørensen, A.D.; Hels, O.; Kristensen, M.; Vermeer, C.; Jakobsen, J.; Flynn, A.; Mølgaard, C.; Cashman, K.D. Effect of Phylloquinone Supplementation on Biochemical Markers of Vitamin K Status and Bone Turnover in Postmenopausal Women. Br. J. Nutr. 2007, 97, 373–380. [Google Scholar] [CrossRef]
- Booth, S.L.; Dallal, G.; Shea, M.K.; Gundberg, C.; Peterson, J.W.; Dawson-Hughes, B. Effect of Vitamin K Supplementation on Bone Loss in Elderly Men and Women. J. Clin. Endocrinol. Metab. 2008, 93, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Harke, J.; Krueger, D.; Engelke, J.; Vallarta-Ast, N.; Gemar, D.; Checovich, M.; Chappell, R.; Suttie, J. Vitamin K Treatment Reduces Undercarboxylated Osteocalcin but Does Not Alter Bone Turnover, Density, or Geometry in Healthy Postmenopausal North American Women. J. Bone Miner. Res. 2009, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Lamb, J.J.; Lerman, R.H.; Konda, V.R.; Darland, G.; Minich, D.M.; Desai, A.; Chen, T.C.; Austin, M.; Kornberg, J.; et al. Hop Rho Iso-Alpha Acids, Berberine, Vitamin D3 and Vitamin K1 Favorably Impact Biomarkers of Bone Turnover in Postmenopausal Women in a 14-Week Trial. J. Bone Min. Miner. Metab. 2010, 28, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Je, S.H.; Joo, N.S.; Choi, B.H.; Kim, K.M.; Kim, B.T.; Park, S.B.; Cho, D.Y.; Kim, K.N.; Lee, D.J. Vitamin K Supplement along with Vitamin D and Calcium Reduced Serum Concentration of Undercarboxylated Osteocalcin While Increasing Bone Mineral Density in Korean Postmenopausal Women over Sixty-Years-Old. J. Korean Med. Sci. 2011, 26, 1093–1098. [Google Scholar] [CrossRef]
- Lamb, J.J.; Holick, M.F.; Lerman, R.H.; Konda, V.R.; Minich, D.M.; Desai, A.; Chen, T.C.; Austin, M.; Kornberg, J.; Chang, J.L.; et al. Nutritional Supplementation of Hop Rho Iso-Alpha Acids, Berberine, Vitamin D3, and Vitamin K1 Produces a Favorable Bone Biomarker Profile Supporting Healthy Bone Metabolism in Postmenopausal Women with Metabolic Syndrome. Nutr. Res. 2011, 31, 347–355. [Google Scholar] [CrossRef]
- Moschonis, G.; Kanellakis, S.; Papaioannou, N.; Schaafsma, A.; Manios, Y. Possible Site-Specific Effect of an Intervention Combining Nutrition and Lifestyle Counselling with Consumption of Fortified Dairy Products on Bone Mass: The Postmenopausal Health Study II. J. Bone Min. Miner. Metab. 2011, 29, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kanellakis, S.; Moschonis, G.; Tenta, R.; Schaafsma, A.; Van Den Heuvel, E.G.H.M.; Papaioannou, N.; Lyritis, G.; Manios, Y. Changes in Parameters of Bone Metabolism in Postmenopausal Women Following a 12-Month Intervention Period Using Dairy Products Enriched with Calcium, Vitamin D, and Phylloquinone (Vitamin K1) or Menaquinone-7 (Vitamin K2): The Postmenopausal Health Study II. Calcif. Tissue Int. 2012, 90, 251–262. [Google Scholar] [CrossRef]
- Mazzanti, L.; Battino, M.; Nanetti, L.; Raffaelli, F.; Alidori, A.; Sforza, G.; Carle, F.; Quagliarini, V.; Cester, N.; Vignini, A. Effect of 1-Year Dietary Supplementation with Vitaminized Olive Oil on Markers of Bone Turnover and Oxidative Stress in Healthy Post-Menopausal Women. Endocrine 2015, 50, 326–334. [Google Scholar] [CrossRef]
- Graff, I.E.; Øyen, J.; Kjellevold, M.; Frøyland, L.; Gram Gjesdal, C.; Almås, B.; Rosenlund, G.; Lie, Ø. Reduced Bone Resorption by Intake of Dietary Vitamin D and K from Tailor-Made Atlantic Salmon: A Randomized Intervention Trial. Oncotarget 2016, 7, 69200–69215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Duan, L.; Ji, Y.; Yang, S.; Zhang, Y.; Li, H.; Wang, Y.; Wang, P.; Chen, J.; et al. Effect of Low-Dose Vitamin K2 Supplementation on Bone Mineral Density in Middle-Aged and Elderly Chinese: A Randomized Controlled Study. Calcif. Tissue Int. 2020, 106, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Takeda, T.; Ichimura, S. Effect of Combined Administration of Vitamin D3 and Vitamin K2 on Bone Mineral Density of the Lumbar Spine in Postmenopausal Women with Osteoporosis. J. Orthop. Sci. 2000, 5, 546–551. [Google Scholar] [CrossRef]
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Senior, P.A.; Jackson, S.T.; Ferland, G.; Presse, N.; Jindal, K.; Li, P.; Alzaben, A.S.; Mager, D.R. Vitamin d Status and Bone Mineral Density Is Influenced by Vitamin d Supplementation and Vitamin K1 Intake in Adults with Diabetes and Chronic Kidney Disease. Can. J. Diet. Pract. Res. 2017, 78, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Inaba, N.; Yamashita, T. MK-7 and Its Effects on Bone Quality and Strength. Nutrients 2020, 12, 965. [Google Scholar] [CrossRef]
- Douglas, A.S.; Robins, S.P.; Hutchison, J.D.; Porter, R.W.; Stewart, A.; Reid, D.M. Carboxylation of Osteocalcin in Post-Menopausal Osteoporotic Women Following Vitamin K and D Supplementation. Bone 1995, 17, 15–20. [Google Scholar] [CrossRef]
- Takahashi, M.; Naitou, K.; Ohishi, T.; Kushida, K.; Miura, M. Effect of Vitamin K and/or D on Undercarboxylated and Intact Osteocalcin in Osteoporotic Patients with Vertebral or Hip Fractures. Clin. Endocrinol. 2001, 54, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ushiroyama, T.; Ikeda, A.; Ueki, M. Effect of Continuous Combined Therapy with Vitamin K2 and Vitamin D3 on Bone Mineral Density and Coagulofibrinolysis Function in Postmenopausal Women. Maturitas 2002, 41, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Miyatani, Y.; Tomita, J.; Yamada, M.; Uemura, H.; Miura, M.; Irahara, M. Effect of Vitamin K2 Treatment on Carboxylation of Osteocalcin in Early Postmenopausal Women. Gynecol. Endocrinol. 2006, 22, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.M.; Tile, L.; Lee, Y.; Tomlinson, G.; Hawker, G.; Scher, J.; Hu, H.; Vieth, R.; Thompson, L.; Jamal, S.; et al. Vitamin K Supplementation in Postmenopausal Women with Osteopenia (ECKO Trial): A Randomized Controlled Trial. PLoS Med. 2008, 5, e196. [Google Scholar] [CrossRef]
- Rønn, S.; Harsløf, T.; Pedersen, S.; Langdahl, B. Vitamin K2 (Menaquinone-7) Prevents Age-Related Deterioration of Trabecular Bone Microarchitecture at the Tibia in Postmenopausal Women. Eur. J. Endocrinol. 2016, 175, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Swanson, M.H.; Enderby, L.T.; D’Amico, F.; Enderby, B.; Samsonraj, R.M.; Dudakovic, A.; van Wijnen, A.J.; Witt-Enderby, P.A. Melatonin-Micronutrients Osteopenia Treatment Study (MOTS): A Translational Study Assessing Melatonin, Strontium (Citrate), Vitamin D3 and Vitamin K2 (MK7) on Bone Density, Bone Marker Turnover and Health Related Quality of Life in Postmenopausal Osteopenic Women. Aging 2017, 9, 256–285. [Google Scholar]
- Morato-Martínez, M.; López-Plaza, B.; Santurino, C.; Palma-Milla, S.; Gómez-Candela, C. A Dairy Product to Reconstitute Enriched with Bioactive Nutrients Stops Bone Loss in High-Risk Menopausal Women without Pharmacological Treatment. Nutrients 2020, 12, 2203. [Google Scholar] [CrossRef]
- Rønn, S.H.; Harsløf, T.; Oei, L.; Pedersen, S.B.; Langdahl, B.L. The Effect of Vitamin MK-7 on Bone Mineral Density and Microarchitecture in Postmenopausal Women with Osteopenia, a 3-Year Randomized, Placebo-Controlled Clinical Trial. Osteoporos. Int. 2021, 32, 185–191. [Google Scholar] [CrossRef]
- Moore, A.E.; Dulnoan, D.; Voong, K.; Ayis, S.; Mangelis, A.; Gorska, R.; Harrington, D.J.; Tang, J.C.Y.; Fraser, W.D.; Hampson, G. The Additive Effect of Vitamin K Supplementation and Bisphosphonate on Fracture Risk in Post-Menopausal Osteoporosis: A Randomised Placebo Controlled Trial. Arch. Osteoporos. 2023, 18, 83. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Shi, J.; Liao, Z.; Zhang, Y.; Li, Y.; Jiang, M.; Liu, M. Distinct Metabolites in Osteopenia and Osteoporosis: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4895. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, C.; Guo, X.; Li, K.; Deng, Q.; Li, D. The Combination Effect of Vitamin K and Vitamin D on Human Bone Quality: A Meta-Analysis of Randomized Controlled Trials. Food Funct. 2020, 11, 3280–3297. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.M.; Moustafa, A.A. Osteoporosis and the Effect of Dysregulation of the Transsulfuration Pathway via Taurine on Intracellular Calcium Homeostasis, Vitamin D Absorption and Vitamin K Absorption. Clin. Nutr. ESPEN 2021, 43, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Earle-payne, K.; Kidd, M.T. Taurine as a Natural Antioxidant: From Direct Antioxidant Effects to Protective Action in Various Toxicological Models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, A.C.; Wasilewski, G.B.; Rapp, N.; Forin, F.; Singer, H.; Czogalla-Nitsche, K.J.; Schurgers, L.J. Menaquinone-7 Supplementation Improves Osteogenesis in Pluripotent Stem Cell Derived Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 8, 618760. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; La Manna, G.; et al. Vitamin K and Osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Olleros Rodríguez, C.; Díaz Curiel, M. Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. J. Osteoporos. 2019, 2019, 2069176. [Google Scholar] [CrossRef]
- Finnes, T.E.; Lofthus, C.M.; Meyer, H.E.; Søgaard, A.J.; Tell, G.S.; Apalset, E.M.; Gjesdal, C.; Grimnes, G.; Schei, B.; Blomhoff, R.; et al. A Combination of Low Serum Concentrations of Vitamins K1 and D Is Associated with Increased Risk of Hip Fractures in Elderly Norwegians: A NOREPOS Study. Osteoporos. Int. 2016, 27, 1645–1652. [Google Scholar] [CrossRef]
- Amstrup, A.K.; Sikjaer, T.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Melatonin Improves Bone Mineral Density at the Femoral Neck in Postmenopausal Women with Osteopenia: A Randomized Controlled Trial. J. Pineal Res. 2015, 59, 221–229. [Google Scholar] [CrossRef]
- Eiken, P.; Vestergaard, P. Treatment of Osteoporosis after Alendronate or Risedronate. Osteoporos. Int. 2016, 27, 1–12. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.H.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N. IOF Position Statement: Vitamin D Recommendations for Older Adults. Osteoporos. Int. 2010, 21, 1151–1154. [Google Scholar] [CrossRef]
- Kupisz-Urbańska, M.; Płudowski, P.; Marcinowska-Suchowierska, E. Vitamin d Deficiency in Older Patients—Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients 2021, 13, 1247. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbanska, M.; Lukaszkiewicz, J.; Pludowski, P.; Jones, G. Vitamin D Toxicity a Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Juszczak, A.B.; Kupczak, M.; Konecki, T. Does Vitamin Supplementation Play a Role in Chronic Kidney Disease? Nutrients 2023, 15, 2847. [Google Scholar] [CrossRef]
- Knapen, M.H.J.; Braam, L.A.J.L.M.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.G.; Vermeer, C. Menaquinone-7 Supplementation Improves Arterial Stiffness in Healthy Postmenopausal Women. A Double-Blind Randomised Clinical Trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Dalla Via, J.; Langley, C.; Smith, C.; Sale, C.; Sim, M. Nutritional Strategies to Optimise Musculoskeletal Health for Fall and Fracture Prevention: Looking beyond Calcium, Vitamin D and Protein. Bone Rep. 2023, 19, 101684. [Google Scholar] [CrossRef] [PubMed]
- AlHajri, L.; Ayoub, A.; Ahmed, H.; AlMulla, M. Effect of Vitamin K2 Alone or in Combination on Various Bone Turnover Markers amongst Postmenopausal Females. J. Bone Metab. 2021, 28, 11–26. [Google Scholar] [CrossRef]
- Erdélyi, A.; Pálfi, E.; Tűű, L.; Nas, K.; Szűcs, Z.; Török, M.; Jakab, A.; Várbíró, S. The Importance of Nutrition in Menopause and Perimenopause—A Review. Nutrients 2024, 16, 27. [Google Scholar] [CrossRef]
- Coppi, F.; Nasi, M.; Farinetti, A.; Manenti, A.; Gallina, S.; Mattioli, A.V. Physical Activity, Sedentary Behaviour, and Diet in Menopausal Women: Comparison between COVID19 “First Wave” and “Second Wave” of Pandemic in Italy. Prog. Progress. Nutr. 2021, 23, e2021194. [Google Scholar] [CrossRef]
- Hou, W.; Chen, S.; Zhu, C.; Gu, Y.; Zhu, L.; Zhou, Z. Associations between Smoke Exposure and Osteoporosis or Osteopenia in a US NHANES Population of Elderly Individuals. Front. Endocrinol. 2023, 14, 1074574. [Google Scholar] [CrossRef]
- Hayat, S.; Magrey, M.N. Glucocorticoid-Induced Osteoporosis: Insights for the Clinician. Cleve Clin. J. Med. 2020, 87, 417–426. [Google Scholar] [CrossRef]
- Singh, V.; Jain, S.; Prakash, S.; Thakur, M. Studies on the Synergistic Interplay of Vitamin D and K for Improving Bone and Cardiovascular Health. Curr. Res. Nutr. Food Sci. 2022, 10, 840–857. [Google Scholar] [CrossRef]
- Cancela, L.; Hsieh, C.L.; Francke, U.; Price, P.A. Molecular Structure, Chromosome Assignment, and Promoter Organization of the Human Matrix Gla Protein Gene. J. Biol. Chem. 1990, 265, 15040–15048. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-Dependent Carboxylation of Matrix Gla-Protein: A Crucial Switch to Control Ectopic Mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Liu, W.; Zhang, Y.; Wang, X.; Xu, C.; Li, Q.; Ji, P.; Wang, J.; Feng, P.; Wu, Y.; et al. Reduced APPL1 Impairs Osteogenic Differentiation of Mesenchymal Stem Cells by Facilitating MGP Expression to Disrupt the BMP2 Pathway in Osteoporosis. J. Biol. Chem. 2023, 299, 104823. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int. J. Mol. Sci. 2019, 20, 628. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, E.G.H.M.; Van Schoor, N.M.; Lips, P.; Magdeleyns, E.J.P.; Deeg, D.J.H.; Vermeer, C.; Heijer, M. Den Circulating Uncarboxylated Matrix Gla Protein, a Marker of Vitamin K Status, as a Risk Factor of Cardiovascular Disease. Maturitas 2014, 77, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K Status and Cardiovascular Events or Mortality: A Meta-Analysis. Eur. J. Prev. Cardiol. 2019, 26, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Pivin, E.; Ponte, B.; Pruijm, M.; Ackermann, D.; Guessous, I.; Ehret, G.; Liu, Y.P.; Drummen, N.E.A.; Knapen, M.H.J.; Pechere-Bertschi, A.; et al. Inactive Matrix Gla-Protein Is Associated with Arterial Stiffness in an Adult Population-Based Study. Hypertension 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Mayer, O.; Seidlerová, J.; Wohlfahrt, P.; Filipovský, J.; Vaněk, J.; Cífková, R.; Windrichová, J.; Topolčan, O.; Knapen, M.H.J.; Drummen, N.E.A.; et al. Desphospho-Uncarboxylated Matrix Gla Protein Is Associated with Increased Aortic Stiffness in a General Population. J. Hum. Hypertens. 2016, 30, 418–423. [Google Scholar] [CrossRef]
- Sardana, M.; Vasim, I.; Varakantam, S.; Kewan, U.; Tariq, A.; Koppula, M.R.; Syed, A.A.; Beraun, M.; Drummen, N.E.A.; Vermeer, C.; et al. Inactive Matrix Gla-Protein and Arterial Stiffness in Type 2 Diabetes Mellitus. Am. J. Hypertens. 2017, 30, 196–201. [Google Scholar] [CrossRef]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mazloomnejad, R.; Kasravi, M.; Gholamine, B.; Bahrami, S.; Sarzaeem, M.M.; Niknejad, H. Recent Advances on Small Molecules in Osteogenic Differentiation of Stem Cells and the Underlying Signaling Pathways. Stem Cell Res. Ther. 2022, 13, 518. [Google Scholar] [CrossRef]
- Huang, Z.B.; Wan, S.L.; Lu, Y.J.; Ning, L.; Liu, C.; Fan, S.W. Does Vitamin K2 Play a Role in the Prevention and Treatment of Osteoporosis for Postmenopausal Women: A Meta-Analysis of Randomized Controlled Trials. Osteoporos. Int. 2015, 26, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Gao, H.; Jin, J.S.; Ahn, B.Y. A Comparatively Study of Menaquinone-7 Isolated from Cheonggukjang with Vitamin K1 and Menaquinone-4 on Osteoblastic Cells Differentiation and Mineralization. Food Chem. Toxicol. 2019, 131, 110540. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Sugimoto, E.; Hachiya, S. Stimulatory Effect of Menaquinone-7 (Vitamin K 2) on Osteoblastic Bone Formation in Vitro. Mol. Cell Biochem. 2001, 223, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 Stimulates Osteoblastogenesis and Suppresses Osteoclastogenesis by Suppressing NF-ΚB Activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Zhang, N.; Ma, Y. Vitamin K2 Improves Osteogenic Differentiation by Inhibiting STAT1 via the Bcl-6 and IL-6/JAK in C3H10 T1/2 Clone 8 Cells. Nutrients 2022, 14, 2934. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Liu, J.; Liu, Y.; Liang, Q. Vitamin K2 Stimulates MC3T3-E1 Osteoblast Differentiation and Mineralization through Autophagy Induction. Mol. Med. Rep. 2019, 49, 3676–3684. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal Osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Gardinier, J.D.; Chougule, A.; Zhang, C. The Mechanotransduction of MLO-Y4 Cells Is Disrupted by the Senescence-Associated Secretory Phenotype of Neighboring Cells. J. Cell Physiol. 2022, 237, 2249–2257. [Google Scholar] [CrossRef]
- Al-Oanzi, Z.H.; Alenazy, F.O.; Alhassan, H.H.; Alruwaili, Y.; Alessa, A.I.; Alfarm, N.B.; Alanazi, M.O.; Alghofaili, S.I. The Role of Vitamin D in Reducing the Risk of Metabolic Disturbances That Cause Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2023, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Maresz, K.; Lee, P.S.; Wu, J.C.; Ho, C.T.; Popko, J.; Mehta, D.S.; Stohs, S.J.; Badmaev, V. Inhibition of TNF-α, IL-1α, and IL-1β by Pretreatment of Human Monocyte-Derived Macrophages with Menaquinone-7 and Cell Activation with TLR Agonists in Vitro. J. Med. Food 2016, 19, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Pillai, A.J.; Nair, N. Cardiovascular Changes in Menopause. Curr. Cardiol. Rev. 2020, 17, e230421187681. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.G.; Mohamed, R.H.; Shalaby, S.M.; Abd El Motteleb, D.M. Vitamin K2 Alleviates Type 2 Diabetes in Rats by Induction of Osteocalcin Gene Expression. Nutrition 2018, 47, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- Ruscica, M.; Busnelli, M.; Runfola, E.; Corsini, A.; Sirtori, C.R. Impact of PPAR-Alpha Polymorphisms-The Case of Metabolic Disorders and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 4378. [Google Scholar] [CrossRef] [PubMed]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of Vitamin D on Cardiovascular Risk and Oxidative Stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- Molinuevo, M.S.; Fernández, J.M.; Cortizo, A.M.; McCarthy, A.D.; Schurman, L.; Sedlinsky, C. Advanced Glycation End Products and Strontium Ranelate Promote Osteogenic Differentiation of Vascular Smooth Muscle Cells in Vitro: Preventive Role of Vitamin D. Mol. Cell Endocrinol. 2017, 450, 94–104. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Malka, G.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin in Vascular Calcification and Bone Metabolism: The Basis for Developing New Therapeutics. Calcif. Tissue Int. 2019, 105, 239–251. [Google Scholar] [CrossRef]
- Martin, T.; Gooi, J.; Sims, N. Molecular Mechanisms in Coupling of Bone Formation to Resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 73–88. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 910312. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Na, W.; Sohn, C. Vitamin K1 (Phylloquinone) and K2 (Menaquinonee4) Supplementation Improves Bone Formation in a Highhfat Diettinduced Obese Mice. J. Clin. Biochem. Nutr. 2013, 53, 108–113. [Google Scholar] [CrossRef]
- Lee, A.S.; Sung, M.J.; Son, S.J.; Han, A.-R.; Hong, S.-M.; Lee, S.-H. Effect of Menaquinone-4 on Receptor Activator of Nuclear Factor κB Ligand-Induced Osteoclast Differentiation and Ovariectomy-Induced Bone Loss. J. Med. Food 2023, 26, 128–134. [Google Scholar] [CrossRef]
- Matsunaga, S.; Ito, H.; Sakou, T. The Effect of Vitamin K and D Supplementation on Ovariectomy-Induced Bone Loss. Calcif. Tissue Int. 1999, 65, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, D.; Masaki, C.; Shibata, Y.; Watanabe, C.; Nodai, T.; Munemasa, T.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. Microstructural and Mechanical Recovery of Bone in Ovariectomized Rats: The Effects of Menaquinone-7. J. Mech. Behav. Biomed. Mater. 2021, 120, 104571. [Google Scholar] [CrossRef] [PubMed]
- Koshihara, Y.; Hoshi, K.; Okawara, R.; Ishibashi, H.; Yamamoto, S. Vitamin K Stimulates Osteoblastogenesis and Inhibits Osteoclastogenesis in Human Bone Marrow Cell Culture. J. Endocrinol. 2003, 176, 339–348. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Xin, N.; Yuan, Y.; Zhang, Q.; Gong, P.; Wu, Y. 1α,25-Dihydroxyvitamin D3 Promotes Osteogenesis by Promoting Wnt Signaling Pathway. J. Steroid Biochem. Mol. Biol. 2017, 174, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, J.; Zhang, J.; Zhang, M.; Zhang, S.; Bai, Y. Menaquinone-4 Modulates the Expression Levels of Calcification-Associated Factors to Inhibit Calcification of Rat Aortic Vascular Smooth Muscle Cells in a Dose-Dependent Manner. Exp. Ther. Med. 2018, 16, 3172–3178. [Google Scholar] [CrossRef]
- Cui, Q.; Li, N.; Nie, F.; Yang, F.; Li, H.; Zhang, J. Vitamin K2 Promotes the Osteogenic Differentiation of Periodontal Ligament Stem Cells via the Wnt/β-Catenin Signaling Pathway. Arch. Oral. Biol. 2021, 124, 105057. [Google Scholar] [CrossRef]
- Zhang, X.; Zanello, L.P. Vitamin D Receptor-Dependent 1α,25(OH)2 Vitamin D 3-Induced Anti-Apoptotic PI3K/AKT Signaling in Osteoblasts. J. Bone Miner. Res. 2008, 23, 1238–1248. [Google Scholar] [CrossRef]
- De Haes, P.; Garmyn, M.; Carmeliet, G.; Degreef, H.; Vantieghem, K.; Bouillon, R.; Segaert, S. Molecular Pathways Involved in the Anti-Apoptotic Effect of 1,25-Dihydroxyvitamin D3 in Primary Human Keratinocytes. J. Cell Biochem. 2004, 93, 951–967. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Ma, Y.; Wei, F. Vitamin D3 Contributes to Enhanced Osteogenic Differentiation of MSCs under Oxidative Stress Condition via Activating the Endogenous Antioxidant System. Osteoporos. Int. 2018, 29, 1917–1926. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Tsatsakis, A.; Rocha, J.B.T.; Santamaria, A.; Spandidos, D.A.; Martins, A.C.; Rongzhu, L.U.; Korobeinikova, T.V.; Chen, W.; et al. Role of Vitamins beyond Vitamin D3 in Bone Health and Osteoporosis (Review). Int. J. Mol. Med. 2024, 53, 9. [Google Scholar] [CrossRef]
- Gigante, A.; Brugè, F.; Cecconi, S.; Manzotti, S.; Littarru, G.P.; Tiano, L. Vitamin MK-7 Enhances Vitamin D3-Induced Osteogenesis in HMSCs: Modulation of Key Effectors in Mineralization and Vascularization. J. Tissue Eng. Regen. Med. 2015, 9, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Sukik, A.; Alalwani, J.; Ganji, V. Vitamin D, Gut Microbiota, and Cardiometabolic Diseases—A Possible Three-Way Axis. Int. J. Mol. Sci. 2023, 24, 940. [Google Scholar] [CrossRef]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of High Doses of Vitamin D3 on Mucosa-Associated Gut Microbiome Vary between Regions of the Human Gastrointestinal Tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, D.; Kubota, R.; Maeno, T.; Abdelhakim, M.; Hitosugi, N. Association between Gut Microbiota, Bone Metabolism, and Fracture Risk in Postmenopausal Japanese Women. Osteoporos. Int. 2021, 32, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Tangestani, H.; Boroujeni, H.K.; Djafarian, K.; Emamat, H.; Shab-Bidar, S. Vitamin D and The Gut Microbiota: A Narrative Literature Review. Clin. Nutr. Res. 2021, 10, 181. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pompili, M.; Di Stasio, E.; Zocco, M.A.; Gasbarrini, A.; Flore, R. Subclinical Atherosclerosis Is Linked to Small Intestinal Bacterial Overgrowth via Vitamin K2-Dependent Mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249. [Google Scholar] [CrossRef]

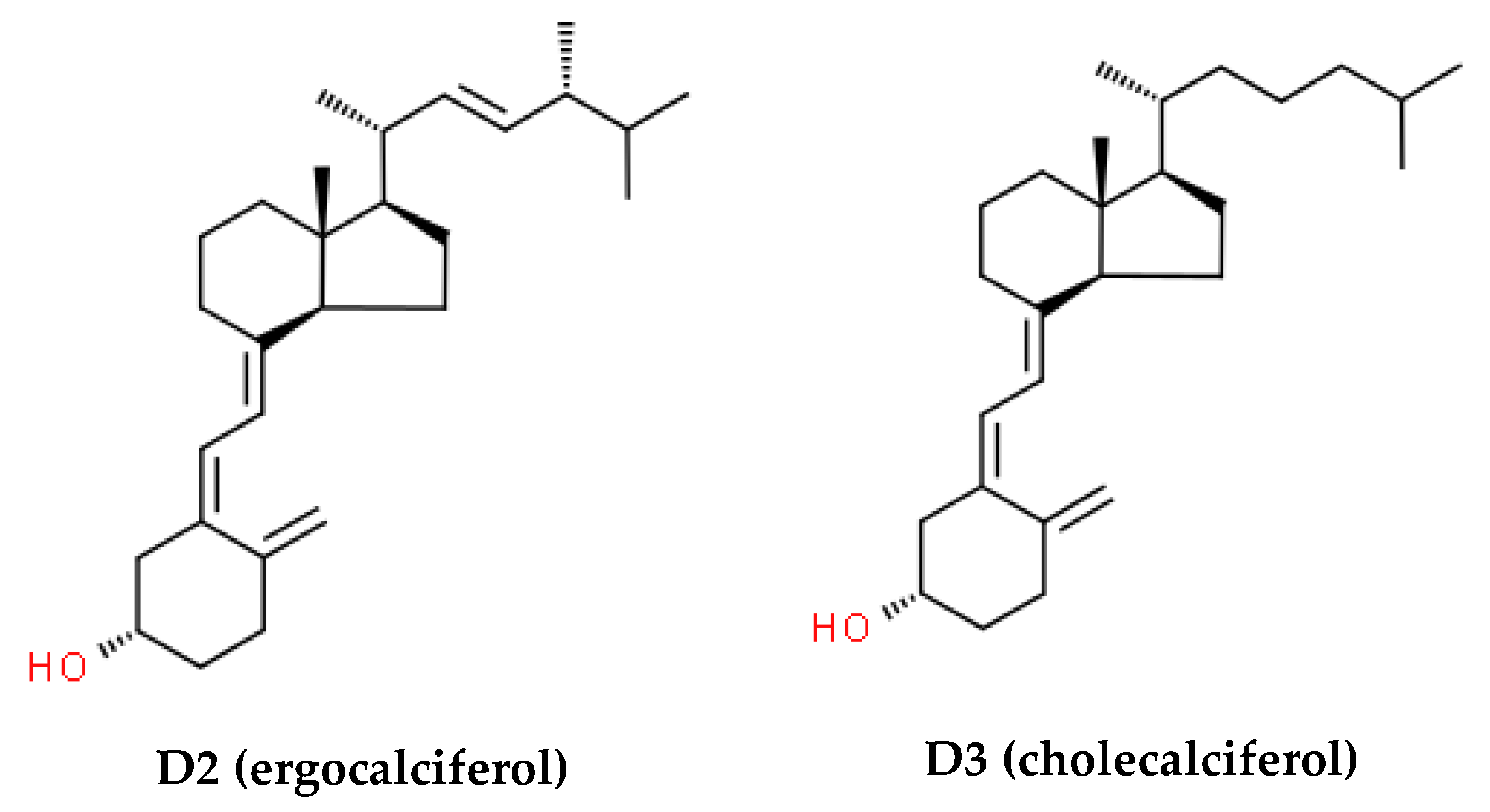
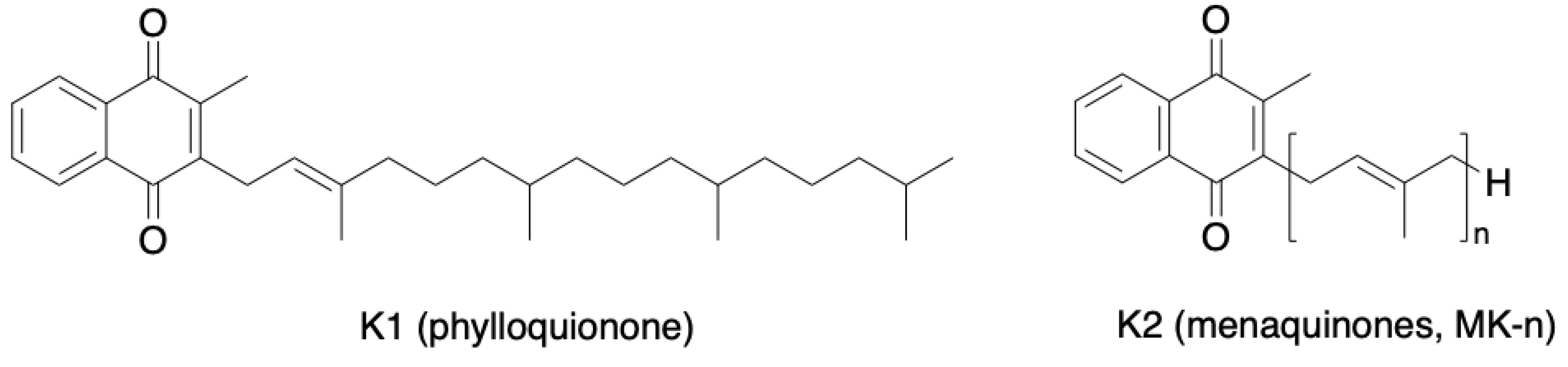
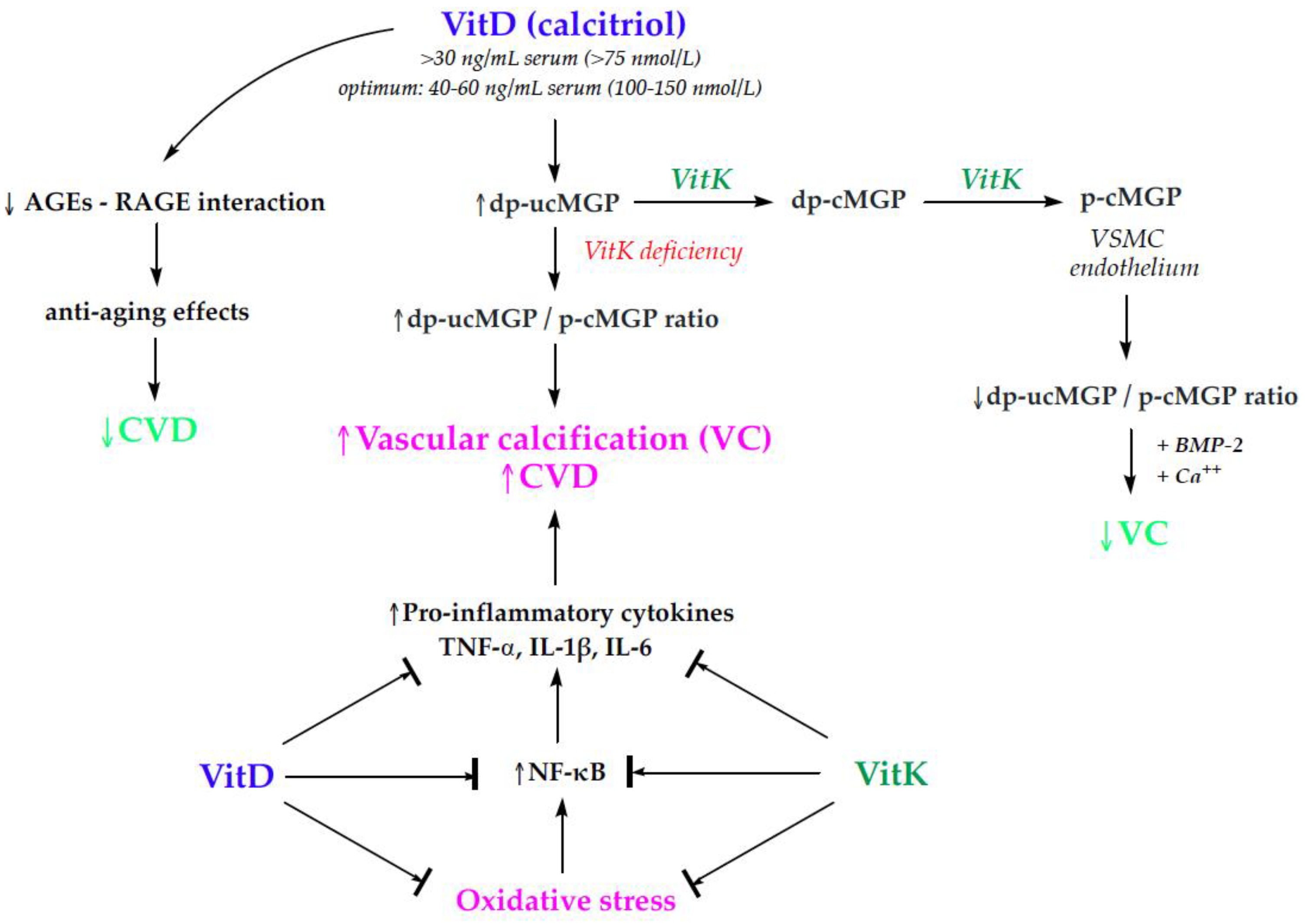
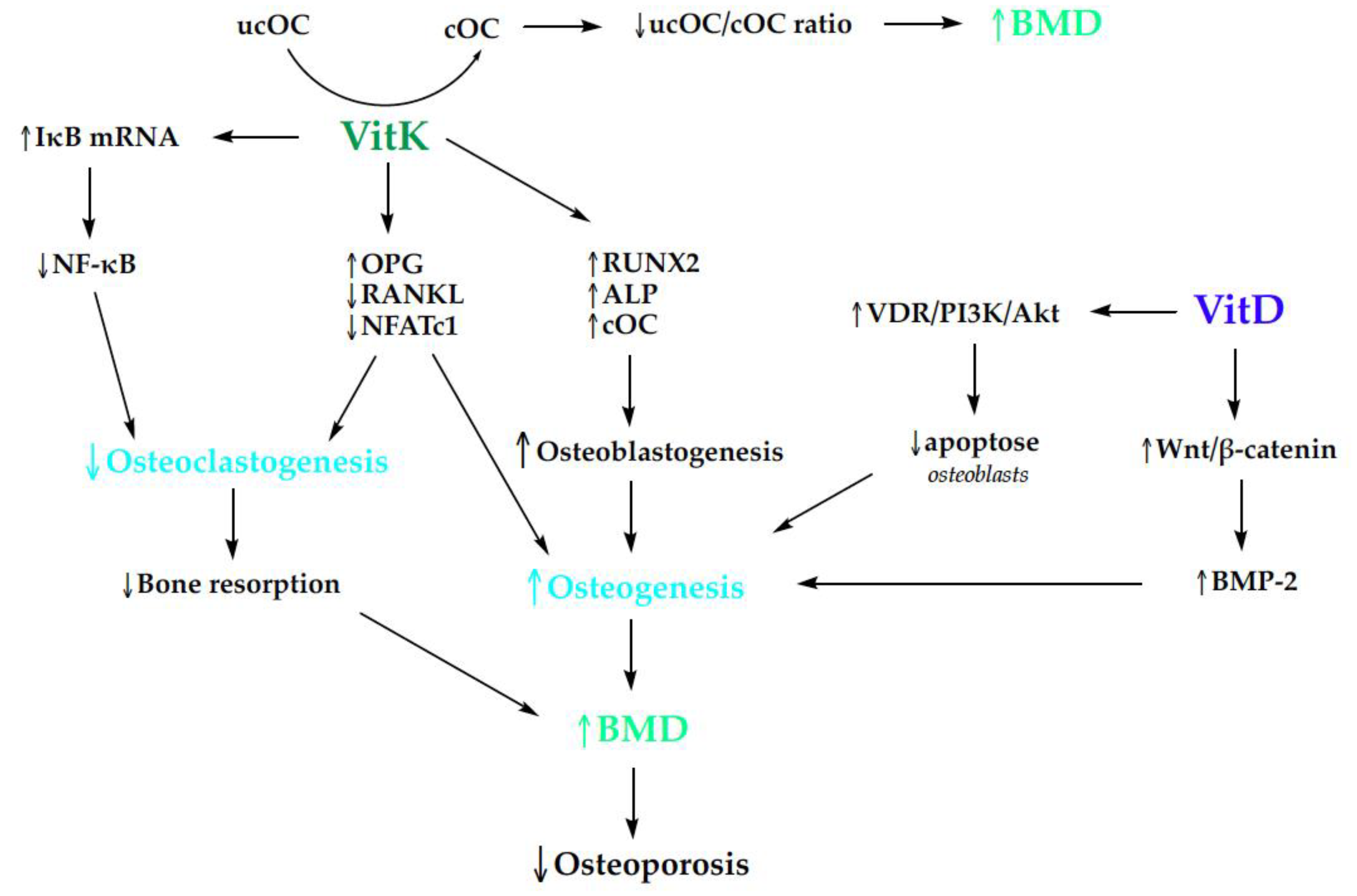
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| The study was published in a peer-reviewed scientific journal | The study was published in commentaries, case reports, books, dissertations, editorials, and conference proceedings |
| The study was a review | |
| The study utilized clinical trial study design | Not randomized clinical trial |
| The study aimed to examine the effects of vitamin D and vitamin K co-treatment | Not examining the co-treatment of vitamin D and vitamin K supplements |
| English (abstract) | Participants not experiencing menopause |
| Not Human | |
| Age group under 50 years |
| No. | Study, Year, Ref. | Country | Population Age | Duration Type of Study | Intervention | Comparison/Diets | Outcomes |
|---|---|---|---|---|---|---|---|
| 1 | Braam et al., 2004 [71] | The Netherlands | 181 PM women of which 150 completed the study; analysis performed on 108 participants | 3 years, double-blind, parallel, placebo-controlled intervention study | The participants were divided into 3 groups that received a daily supplement:
| The elastic properties of the common carotid artery were measured (including CC, DC, IMT, E) | After 3 years of supplementation and after adjustment for baseline heart rate, mean arterial pressure, age, weight and smoking: ↓ DC (8.8%, 95% CI: 1.9 to 21.4; p < 0.05) and CC (8.6%, 95% CI: 1.8 to 20.3; p < 0.05) in MDK group vs. placebo; ↑ Pulse pressure (6.3%, 95% CI: 0.7 to 17.1; p < 0.05) and E (13.2%, 95% CI: 5.3 to 35.8; p < 0.01) in MDK group vs. placebo; - no significant differences between the MD group and placebo for all parameters, as well as between the three groups for the change of IMT |
| 2 | Shea et al., 2009 [72] | USA | 388 healthy men and PM women (60–80 y) | 3 years, double-blind study |
| CAC progression | In a subgroup analysis of the participants who were adherent to supplementation (≥85%, n = 367): ↓ CAC progression in the phylloquinone group vs. control group (p = 0.03); In a subgroup analysis of the participants with preexisting CAC (Agatston score > 10 at baseline): ↓ CAC progression in the phylloquinone group at 6% vs. control group (p = 0.04); ↑ serum MGP in the phylloquinone group and ↓ in the control group (treatment effect: p ≤ 0.03 in all analysis) |
| 3 | Knapen et al., 2015 [73] | The Netherlands | 39 healthy men and 26 PM women, mean age 56 ± 5 y (45–65 y) | 12 weeks |
| Comparison between the groups at baseline and after 12 weeks | ↑ circulating MK-7 levels (from 0.28 to 1.94 ng/mL) (p = 0.004 vs. standard yogurt) ↓ serum ucOC levels (p = 0.001) and plasma dp-ucMGP levels (p < 0.0001) vs. standard yogurt |
| 4 | Knapen et al., 2016 [74] | The Netherlands | 43 healthy men and 64 PM women, 45–65 y | Intervention period of 42 days followed by a washout period of 2 weeks |
| The plasma MK-7, dp-ucMGP and ucOC were quantified and compared between the groups at baseline and after 14, 28, and 42 days - plasma MK-7 also determined at days 45, 49, and 56 (during the washout period) | Plasma MK-7 levels after 42 days: (A) 2.29 ± 0.08 ng/mL; (B) 2.17 ± 0.09 ng/mL; (C) 2.00 ± 0.09 ng/mL (p = 0.047 between the three groups) (mean ± SE) ↑ plasma MK-7 in (A) vs. (C) (p = 0.042) ↓ plasma MK-7 levels to 0.79 ± 0.05 ng/mL after 14 days of the washout period, being still higher compared with that at the start (p < 0.0001) ↓ plasma dp-ucMGP levels after 42 days: overall 445 ± 18 pmol/L (p = 0.005); (A) 485 ± 30 pmol/L; (B) 417 ± 33 pmol/L; (C) 434 ± 31 pmol/L (p = 0.019 between-group comparisons) ↓ ucOC levels after consumption of the yogurt products and the MK-7 capsules (p = 0.012), but w/o significant differences between groups |
| 5 | Vignini et al., 2017 [75] | Italy | 60 healthy white PM women, 50–61 y | 1 year, placebo-controlled trial | Oral supplementation with: (1) VitVOO group: 20 mL/d VOO fortified with D3 (50 μg/100 mL), K1 (0.70 mg/100 mL), and B6 (6.0 mg/100 mL); (2) PlaVOO group: only 20 mL/day VOO as placebo | Comparison between the groups at baseline and after 1 year | After 1 year of supplementation: ↓ NO levels in Vit VOO group vs. PlaVOO (37.20 ± 3.2 vs. 42.59 ± 4.31 nmol/mg protein; p < 0.001) ↓ hROS levels in Vit VOO group vs. PlaVOO (159.24 ± 15.3 vs. 226.23 ± 21.57; p < 0.05) ↑ plasma Na+/K+-ATPase activity in Vit VOO group vs. PlaVOO (0.650 ± 0.073 vs. 0.411 ± 0.043 µmol Pi/mg protein; p < 0.001) ↓ anisotropy in Vit VOO group vs. PlaVOO (0.152 ± 0.015 vs. 0.208 ± 0.025 arbitrary fluorescence numbers, p < 0.001); (0.204 ± 0.011 vs. 0.240 ± 0.014 arbitrary absorbance numbers, p < 0.001) |
| 6 | Rønn et al., 2021 [76] | Denmark | 142 PM women with osteopenia, 63–73 y | 12 months, placebo-controlled trial |
| Comparison between the groups at baseline and after 3, 6, and 12 months | ↓ ucOC in the MK-7 group (−70.3 (−75.6; −63.8)%) vs. placebo (−7.2 (−15.9; 2.0)%) after 12 months (p < 0.01) ↑ P-adiponectin in the MK-7 group (6.1 ± 20.1%) vs. placebo group (−0.7 ± 15.5%) after 12 months (p = 0.03) HOMA-IR and p-leptin—no changes |
| No. | Study, Year, Ref. | Country | Population Age | Duration Type of Study | Intervention | Comparison/Diets | Outcomes |
|---|---|---|---|---|---|---|---|
| 1 | Schaafsma et al., 2000 [92] | The Netherlands | Healthy PM Dutch women (>5 y PM) with documented normal (n = 96) and low BMD (n = 45), 50–77 y | 1 year | (1) Women with normal BMD (a double-blind study): Group A: 400 IU D3 and 80 μg K1; Group B: 80 μg K1; Placebo: 2.5 g of skimmed milk powder (2) Women with low BMD (an open study): Group C: 350 IU D3 and 80 μg K1; Group D: 400 IU D3 | - Comparison vs. baseline and between the groups, at 3, 6, and 12 months; - All women (except placebo group) received about 1000 mg of additional calcium (total daily calcium intake of 2200 ± 2300 mg) | ↑ 25(OH)D serum level after 3 months (p ≤ 0.0001), 6 months (p ≤ 0.0001), and 12 months (p < 0.001) in group A vs. group B + placebo, as well as vs. baseline (p < 0.005) ↑ 25(OH)D serum level after 1 y of supplementation with D3: 33 ± 29% (95% CI, 24.8–41.8%) and 68 ± 58% (95% CI, 50.1–84.6%) in women with normal and low BMD, respectively ↓ maximum 25(OH)D level: 29% in women with low BMD during supplementation with D3 in winter ↑ % cOC after 6 months (p = 0.009) and 12 months (p = 0.001) vs. placebo, and after 3, 6, 12 months (p ≤ 0.0001) vs. baseline in women with normal BMD after K1 supplementation (group A + B) ↓ % cOC at baseline for women with low BMD (of the lumbar spine and femoral neck, p < 0.005) vs. women with normal BMD; the difference disappeared after 1 y of supplementation with vitK1: 68 + 11% (95% CI, 64.5 ± 71.2%) vs. 72 + 6% (95% CI, 70.1 ± 72.9%), respectively |
| 2 | Braam et al., 2003 [93] | The Netherlands | 181 healthy PM women (50–60 y), 155 completed the study | 3 years, double-blind, parallel, placebo-controlled intervention study | The participants were divided into 3 groups that received a daily supplement:
| - Usual diets for all participants with supplements containing calcium, vitD, or vitK avoided throughout the study; - Comparison between the treated groups after 1, 2, and 3 years of supplementation | ↑ BMD in femoral neck in MDK group vs. placebo and MD group (p < 0.05) after 3 years of supplementation ↓ Bone loss of the femoral neck with 1.7% (95% Cl: 0.35–3.44) in the MDK group vs. placebo and with 1.3% (95% Cl: 0.10–3.41) vs. MD group, respectively, after 3 years of supplementation and adjustment for baseline BMD, age, BMI, and years since menopause - No significant differences in BMD at the site of the lumbar spine among the three groups after 3 years of supplementation |
| 3 | Bolton-Smith et al., 2007 [94] | UK | 244 healthy PM Scottish women, 60 y | 2 years, double-blind, placebo-controlled trial |
| - Comparison between the treated groups at baseline and after successive 6-month intervals of supplementation | ↑ BMD and BMC at the site of the ultradistal radius in combined group (4) vs. baseline (p < 0.01) after 2 years of supplementation ↑ serum vitK1 by 157% (95% CI, 101, 212) (p < 0.001) after 2 years of vitK supplementation ↓ ucOC by 51% (95% CI, −47.5, −54.0) (p < 0.001) after 2 years of vitK supplementation ↑ serum 25-hydroxyvitD [25(OH)D] by 17% (p < 0.001), and ↓ PTH by 11% (p = 0.049) after 2 years of vitD supplementation |
| 4 | Bügel et al., 2007 [95] | Denmark | 48 healthy PM women, 31 (62.5 ± 4.0 y) completed all three intervention periods | 3 × 6-week, cross-over study, with 3-week washout periods | Usual diet supplemented with 0 (placebo), 200, and 500 μg phylloquinone (vitK1)/day; - all volunteers received 10 μg D3/day throughout the study period | - Comparison between the treated groups after each 6-week period of K1 supplementation | ↑ daily K1 intake = ↑ cOC serum level and ↓ ucOC serum level, respectively, in a dose-dependent manner (p < 0.001) ↑ K1 serum level (p < 0.001) for 500 μg phylloquinone/day vs. placebo or 200 μg phylloquinone/day supplementation periods (which did not differ) (p = 0.15) ↑ serum total OC level (p < 0.001) for 500 (but not 200) μg phylloquinone vs. placebo |
| 5 | Booth et al., 2008 [96] | USA | 452 healthy, ambulatory men and PM women (60–80 y), which 401 participants completed the trial | 3 years, randomized, double-blind, parallel controlled trial | Diet supplemented with calcium (600 mg elemental calcium/day) and vitD (400 IU/day) containing either:
| - Comparison between treatment groups at baseline and after 3 years of supplemented diet | ↑ plasma phylloquinone concentrations (p < 0.0001) and ↓ % ucOC (p < 0.0001) after 3 years of vitK supplementation vs. the group that did not receive phylloquinone - overall increase in 25-hydroxyvitD plasma levels (p < 0.001) and a decrease of 1,25- dihydroxyvitD levels (p < 0.001) after 3 years of a daily supplement of 400 IU vitD, with the exception of women in the non-vitamin-K-supplemented group - No differences in lumbar spine or whole-body BMD between the two groups (p = 0.98 and 0.81, respectively) after 3 years of supplemented diet |
| 6 | Binkley et al., 2009 [97] | USA | 381 healthy PM women | 1 year, double-blind, placebo-controlled study | Phylloquinone (1) mg daily) (2) MK4 (45 mg daily), or (3) placebo - all participants received daily 315 mg calcium and 200 IU D3 supplementation. | - comparison between the groups at baseline and 1, 3, 6, and 12 months for biochemical parameters, and at baseline, 6, and 12 months for BMD, respectively | ↓ serum ucOC after both Phylloquinone (−61.1%, 95% CI: −65.5%, −56.1%; p < 0.0001) and MK4 (−60.7%, 95% CI: −65.1%, −55.8%; p < 0.0001) treatment after 1 year vs. placebo ↓ total OC after both Phylloquinone (−8.38%, 95% CI: −13.15%, −3.35%; p < 0.005) and MK4 (−5.65%, 95% CI: −10.5%, −0.54%; p < 0.005) treatment after 1 year vs. placebo - No effect of phylloquinone or MK4 on serum bone-specific alkaline phosphatase (BSALP) or n-telopeptide of type 1 collagen (NTX) as well as on lumbar spine or proximal femur BMD measurements |
| 7 | Holick et al., 2010 [98] | USA | 32 healthy PM women (50–70 y) | 14-week, single-blinded, 2-arm placebo-controlled pilot study |
| - all women consumed a modified Mediterranean -style, low-glycemic-load diet and limited aerobic exercise; - comparison between the groups at baseline and at 10 and 14 months | ↓ serum OC by 31% (p = 0.02) in the treatment group and ↑ by 19% (p = 0.03) in the placebo group vs. baseline at 14 weeks ↑ serum 25(OH)D by 13% (p = 0.24) in the treatment group and ↓ by 25% (p < 0.01) in the placebo group ↑ serum IGF-I in the treatment group at 14 weeks (p < 0.01) |
| 8 | Je et al., 2011 [99] | Korea | 78 healthy PM women (> 60 y), 45 completed the study | 6 months, randomized intervention study |
| - comparison between the groups after 6 months of treatment | ↑ L3 BMD in K group vs. contro group (0.01 ± 0.03 g/cm2 vs. −0.008 ± 0.04 g/cm2, p = 0.049) ↓ ucOC in K group vs. contro group (−1.6 ± 1.6 ng/dL vs. −0.4 ± 1.1 ng/dL, p = 0.008) |
| 9 | Lamb et al., 2011 [100] | USA | 51 PM women with the metabolic syndrome (no osteopenia/osteoporosis), 35–70 y, 45 completed the study | 14-week, single-blind, 2-arm placebo-controlled randomized trial |
| - all women consumed a modified Mediterranean -style, low-glycemic diet and limited aerobic exercise - comparison between the groups at baseline and at 10 and 14 months | ↓ serum OC (25%) to 2.28 ± 0.19 nmol/L and to 2.48 ± 0.19 after 10 and 14 weeks, respectively, vs. 3.31 ± 0.23 nmol/L at baseline (both p < 0.001) in the intervention arm ↑ serum OC (21%) to 2.96 ± 0.27 nmol/L and to 3.43 ± 0.28 (p < 0.001) after 10 and 14 weeks, respectively, vs. 2.84 ± 0.23 nmol/L at baseline in the placebo arm - Statistical changes in serum OC between arms after both 10 and 14 weeks (p < 0.001) ↑ serum 25(OH)D by 23% (139.95 ± 8.26 nmol/L vs. 113.69 ± 7.26 nmol/L, p = 0.001) in the intervention arm and ↓ by 12% (79.97 ± 5.27 nmol/L vs. 90.75 ± 6.46 nmol/L, p = 0.03) in the placebo arm after 14 weeks - Statistical changes in serum 25(OH)D between arms after both 10 and 14 weeks (p < 0.01) ↑ serum IGF-I in the intervention arm after 14 weeks (p < 0.01) |
| 10 | Moschonis et al., 2011 [101] | Greece | 115 healthy PM women | 12 months, RCT, with nutrition and lifestyle counseling |
| Comparison between the groups at baseline and after 12 months | ↑ BMD in all intervention groups (p < 0.001 vs. CO) after 12 months ↑ L2-L4 BMD in the CaDK1 and CaDK2 groups (p = 0.001 vs. CO) after 12 months |
| 11 | Kanellakis et al., 2012 [102] | Greece | 115 healthy PM women (55–65 y, mean of 62.0 ± 5.8 y) | 12 months, RCT, with nutrition and lifestyle counselling |
| All three intervention groups received calcium and vitamins in fortified dairy products; Comparison between the groups at baseline and after 12 months | ↑ serum 25(OH)D levels in all intervention groups vs. CG (p = 0.01) ↑ serum IGF-I levels in the CaDK2 group vs. CG (p < 0.05) ↓ serum ucOC/OC ratio and ↓ urine deoxypyridinoline (DPD) levels for both CaDK1 and CaDK2 groups vs. CaD and CG groups (p = 0.001 and p < 0.05, respectively) ↑ total-body BMD for both CaDK1 and CaDK2 groups vs. CG (p < 0.05) ↑ L2-L4 BMD in the CaDK1 and CaDK2 groups vs. CG (p < 0.01) |
| 12 | Mazzanti et al., 2015 [103] | Italy | 60 Caucasian healthy PM women, 50–61 y | 1 year, randomized, placebo-controlled trial | Oral supplementation with: (1) VitVOO group: 20 mL/day VOO fortified with D3 (50 mg/100 mL), K1 (0.70 mg/100 mL), and B6 (6.0 mg/100 mL); (2) PlaVOO group: only 20 mL/day VOO as placebo | - Comparison between the groups at baseline and after 1 year | ↓ ucOC levels in Vit VOO group vs. PlaVOO (2.60 ± 0.14 vs. 3.12 ± 0.19 ng/mL, p < 0.001) ↓ ucOC/carbOC ratio in Vit VOO group vs. PlaVOO (p < 0.05) ↑ BMD-T-score in Vit VOO group vs. PlaVOO after 1 year (−1.28 ± 0.18 vs. −2.43 ± 0.32, p < 0.05) ↓ oxidative stress biomarkers in VitVOO group vs. PlaVOO after 1 year: TBARS (12.10 ± 1.70 vs. 41.68 ± 3.68 nmol/mL, p < 0.001), lipid hydroperoxide (3.10 ± 0.30 vs. 8.8 ± 0.6 nmol/mg prot, p < 0.01) and conjugated diene (2.10 ± 0.30 vs. 4.5 ± 0.41, p < 0.01) plasma levels |
| 13 | Graff et al., 2016 [104] | Norway | 122 healthy PM women, 55 ± 5 y | 12 weeks, randomized intervention trial | Salmon groups (150 g/2x/week + 1000 mg Ca/day) fortified with:
| Comparison between the groups at baseline and after 12 weeks | ↓ serum ucOC within the HD/HK (p < 0.001) and LD/HK (p = 0.026) groups vs. baseline, and in all treated groups vs. the tablet group (HD/HK group: p = 0.004; LD/HK group: p = 0.035; HD/LK group: p = 0.020) ↓ serum cOC within the tablet (p < 0.05) and HD/LK (p < 0.001) groups vs. baseline ↓ GLU/GLA ratio decreased within the HD/HK group (p < 0.001) vs. baseline and in all treated groups vs. the tablet group (HD/HK group: p = 0.001; LD/HK group: p = 0.025; HD/LK group: p = 0.003) |
| 14 | Zhang et al., 2020 [105] | China | 311 healthy men and PM women, 50–75 y (59.78 ± 6.60 y) | 12 month, single-blind RCT |
| After 12 months of intervention: ↓ BMD in the placebo group (−0.006 g/cm2; 95% CI − 0.017, 0.004), but not in the 90-K2-plus group at lumbar spine (0.002 g/cm2; 95% CI − 0.005, 0.009) ↓ the bone loss of femoral neck in PM women in the 90-K2 and 90-K2-plus groups (treatment × time, p = 0.006) vs. placebo, but no effects in men ↑ serum vitK2 increased in all the three treatment groups (50-K2 group: + 0.43 nmol/L; 90-K2 group: + 0.22 nmol/L; 90-K2-plus group: + 0.40 nmol/L) (treatment × time, p = 0.015) ↑ cOC/ucOC ratio in the intervention groups (treatment × time, p < 0.001) ↑ serum 25(OH)D of 90-K2-plus group after additional vitD3 supplementation (treatment × time, p = 0.013) |
| No. | Study, Year, Ref. | Country | Population Age | Duration Type of Study | Intervention | Comparison/Diets | Outcomes |
|---|---|---|---|---|---|---|---|
| 1 | Douglas et al., 1995 [110] | UK | 20 osteoporotic PM women with previous Colles fractures, 52–73 y (mean of 61.7 y) | 2 weeks followed by 4 weeks without vitamins; Crossover study controlled with placebo, with a washout period of 3 months | Group 1 (n = 10): K1 Group 2 (n = 10): K1 + D2 Doses: 1 mg K1/day (in the morning); 200 IU D2/day (in the afternoon or evening) | - Each group was its own control (vs. placebo); - Comparison of OC level between the study’s PM women and younger pre-menopausal (PRE) women on the staff (n = 10, 22–39 y, mean of 29,6 y); - Comparison of bone mass measurements with 2 community controls of same age (who have no specific role in the study) | ↑ total serum OC after K1; higher level after K1 + D2 (p < 0.01) ↑ degree of carboxylation (p < 0.001 after K1; p < 0.01 after K1 + D2) at values comparable to those in PRE women (73% vs. 57%, the initial values) - The degree of carboxylation persists 4 weeks after K1 treatment (64%, p < 0.05), being completely lost at 14 weeks |
| 2 | Iwamoto et al., 2000 [106] | Japan | 92 osteoporotic PM women (>5 y PM), 55–81 y | 2 years, parallel study | D group (n = 29, mean of 63.4 y): D3 (1α hydroxyvitD3), 0.75 µg/day; K group (n = 22, mean of 65.8 y): K2 (MK-4), 45 mg/day; DK group (n = 21, mean of 63.6 y): D3 + K2; C group (n = 20, mean of 63.5 y): calcium (as calcium lactate), 2 g/day | - Comparison vs. baseline and between the groups, at 0, 1, and 2 years | ↓ BMD in C group (p < 0.001) vs. baseline ↑ BMD in D group (p < 0.05) and K group (p < 0.001) vs. C group ↑ BMD in DK group vs. C, D, and K groups (p < 0.0001, p < 0.05, and p < 0.01, respectively) |
| 3 | Takahashi et al., 2001 [111] | Japan | 43 PRE (22–52 y, 34.5 ± 10.3) and 48 PM (54–87 y, 74.4 ± 6.9) healthy females, 89 osteoporotic female patients (49–94 y, 73.5 ± 9.4) with vertebral fractures (VX), and 24 female patients (52–93 y, 79.9 ± 9.5) with hip fracture (HX) | 4 weeks | 56 of 89 VX patients were treated orally as follows: Group K2: 22 VX patients (56–81 y, 67.6 ± 6.8) received 45 mg K2(MK-4)/day; Group D3: 13 VX patients (61 ± 88 y, 72.1 ± 8.9) received 1 μg of 1α-hydroxyvitD3/day; Group K2 + D3: 21 VX patients (49 ± 88 y, 75 ± 9.2) received 45 mg K2 and 1 μg D3/day | - Comparison between the PRE, PM, VX, and HX groups; - comparison between the treated VX groups after 4 weeks of treatment and vs. baseline | ↑ OC in PM (p < 0.001 vs. PRE) and in VX (p < 0.001 vs. PRE and p < 0.05 vs. PM) ↓ OC in HX than in PM (p < 0.05) and VX (p < 0.001) - UcOC was higher in PM, VX, and HX than in PRE, but not significantly - The ucOC/OC ratio was higher in HX (p < 0.05) than in PM and in VX ↓ ucOC in the groups K2 and K2 + D3 after 4 weeks of treatment with K2, D3, and K2 + D3 vitamins, respectively, in the 56 VX patients ↓ ucOC/OC ratio to approximately 80% after 4 weeks of treatment with K2 and K2 + D3 (only vitD3 did not decrease this ratio) |
| 4 | Ushiroyama et al. 2002 [112] | Japan | 172 PM women with vertebral BMD of the lumbar spine < 0.98 g/cm2 (osteopenia and osteoporosis), of which 126 completed the study | 2 years | All patients were divided into 4 groups (n = 43 for each group at the start of the study): K2 group (n = 30, 54.1 ± 7.4 y): MK-4, 45 mg/day; D3 group (n = 32, 52.8 ± 5.6 y): 1 μg/day vit D3 (1α- hydroxyvitD3) K2 + D3 group (n = 31, 53.3 ± 4.5 y): combined therapy; Control group (n = 33, 53.5 ± 6.0 y) | - Control group received dietary therapy alone; - BMD and the bone markers were measured after 0, 6, 12, 18, and 24 months of treatment; - Comparison between the treated groups after 4 weeks of treatment and vs. baseline | ↑ BMD with 0.278 ± 6.55% and 0.135 ± 5.44% (both p < 0.05 vs. control) after 18 and 24 months of K2 treatment, respectively ↑ BMD with 4.10 ± 5.88%, 5.86 ± 6.85%, 5.01 ± 8.11%, and 4.92 ± 7.89% (p < 0.001 vs. control in all cases) after 6, 12, 18, and 24 months of combined therapy K2 + D3, respectively |
| 5 | Yasui et al. 2006 [113] | Japan | 34 PM women (mean of 53 y) with BMD at the lumbar spine < 0.809 g/cm2 (osteopenia and osteoporosis), which 30 completed the study | 2 years | Group K2: (n = 17, 52.9 ± 6.2 y) 45 mg K2/day, orally; Group K2 + D3: (n = 17, 54.9 ± 6.8 y) 45 mg K2 + vitD3 (1α-hydroxyvitD3) daily. One patient from group K2 and 3 patients from group K2 + D3 dropped out of the study | - Comparison between the treated groups before and at 1 and 2 years after the start of supplementation | ↓ ucOC serum levels in group K2 at 1 year and in group K2 + D3 at 1 and 2 years after the start of supplementation (p < 0.05) ↓ intact OC and BAP serum levels only in group K2 + D3 at 1 and 2 years after the start of supplementation (p < 0.05) ↓ BMD in group K2 at 1 and 2 years (p < 0.05 and p < 0.01, respectively) BMD was sustained in group K2 + D3 |
| 6 | Cheung et al., 2008 [114] | Canada | 440 PM women with osteopenia, with a mean serum 25-hydroxyvitD level of 77 nmol/L at baseline | 2 years, randomized, double-blind, placebo-controlled trial, extended to an additional 2 years for earlier participants | (1) 5 mg/day vitK1 (n = 217, 58,9 y (40.1–80.5), 198 completed the study at 2-y, 97 at 3-y, and 33 at 4-y) (2) placebo (n = 223, 59.2 y (46.1–82.3), 202 completed the study at 2-y, 107 at 3-y, and 40 at 4-y); - all participants received a daily diet supplemented with 1500 mg of calcium and 800 IU of vitD (diet plus supplements) | - Comparison between the two groups at baseline, 3, 12, 24, 36, and 48 months for serum parameters, and at baseline, 24, and 48 months, or at final visit, for BMD, respectively | ↓ BMD by −1.28% and −1.22% (p = 0.84) (difference of −0.06%; 95% CI, −0.67% to 0.54%) at the lumbar spine and −0.69% and −0.88% (p = 0.51) (difference of 0.19%; 95% CI, −0.37% to 0.75%) at the total hip in the vitK and placebo groups, respectively, over 2 years - No significant differences in changes in BMD at any site between the two groups over the 2- to 4-y period ↑ K1 serum levels in the K1 group vs. Placebo (22.6 nmol/L vs. 2.0 nmol/L, p < 0.0001) at 2 years ↓ ucOC and ↓ % ucOC in the K1 group vs. placebo (−52.8% vs. −3.5%, p < 0.0001, and −21.4% vs. −2.0%, p < 0.0001, respectively) at 2 years - Fewer women in the vitK group had clinical fractures (9 vs. 20, p = 0.04) and fewer had cancers (3 vs. 12, p = 0.02) |
| 7 | Rønn et al., 2016 [115] | Denmark | 142 osteopenic PM women, 60–80 y | 12 months, double-blind, randomized, placebo-controlled trial |
| - Comparison between the groups at baseline and after 3, 6, and 12 months | ↓ ucOC in the MK-7 group −65.6% (59.1; 71.0) vs. placebo −6.4%(−13.5; 1.2) after 3 months (p < 0.01), remaining low throughout the study (p < 0.05) ↓ ucOC in the MK-7 group (−65.2 ± 23.5%) vs. placebo (−0.03 ± 38.5%) after 1 year (p < 0.01) - Trabecular number in tibia (−0.1 ± 1.9%), trabecular spacing (+1.2 ± 8.0%), and trabecular thickness (+0.2 ± 1.7%) were unchanged in the MK-7 group (vs. −3.5 ± 2.2%, +4.5 ± 9.7%, and +4.0 ± 2.2%, respectively, in placebo group) (p < 0.05 between-groups) |
| 8 | Maria et al., 2017 [116] | USA | 23 osteopenic PM women, 49–75 y (mean of 58.6 ± 1.12 y), which 22 completed the study | 1-year, double-blind, randomized, placebo-controlled trial |
| - Comparison between the groups at baseline and after 12 weeks | ↑ BMD in lumbar spine L1-L4 (4.3%) and left femoral neck (2.2%) in the MSDK group vs. placebo, with an upward trend for total left hip (p = 0.069) ↑ serum P1NP levels in the MSDK group vs. placebo (p ≤ 0.05 after 6 months and p ≤ 0.01 after 1 year) ↓ bone turnover (CTX/P1NP) in the MSDK group in a time-dependent mode ↑ mood and sleep quality in the MSDK group ↑ urinary melatonin-sulfate levels in the MSDK group vs. placebo (p = 0.0463) - Correlation between melatonin levels and lumbar spine BMD (p = 0.029, r = 0.487; 95% CI = 0.0566 to 0.7648) |
| 9 | Morato-Martínez et al., 2020 [117] | Spain | 79 osteopenic PM women | 6 months, randomized, parallel, double-blind clinical trial |
| - Comparison between the groups at baseline and after 6 months | ↑ bone mass in the EG group vs. the CG group (0.01 ± 0.03 vs. −0.01 ± 0.03 kg; p < 0.05) ↑ serum P1NP levels in the EG group vs. the CG group (13.19 ± 25.17 vs. −4.21 ± 15.62 ng/mL; p < 0.05) ↓ CTX in the EG group vs. the CG group (−0.05 ± 0.19 vs. 0.04 ± 0.14 ng/mL; p < 0.05) ↓ systolic and diastolic blood pressure in the EG group vs. baseline |
| 10 | Rønn et al., 2021 [118] | Denmark | 119 PM women with osteopenia, 67.3 ± 4.4 y | 3 years |
| - Comparison between the groups at baseline and after 3, 6, and 12 months | ↓ total OC by 9.1 ± 25.6% (p < 0.01) in the MK-7 group (remaining unchanged 2.1 ± 19.6% (p = 0.11) in the placebo group) - Significant interaction between treatment group and time for total OC after 3 years (p <0.01) ↑ P1NP by 15.2 ± 39.4% (p < 0.05) in the MK-7 group (remaining unchanged in the placebo group) ↑ CTX and vitD in both groups (p < 0.03 for all) ↓ BAP in both groups (p < 0.01 for both) ↓ BMD at total hip (by 1.5 ± 2.5% for MK-7 vs. 2.4 ± 2.7% for placebo) and lumbar spine (by 1.8 ± 3.9% for MK-7 vs. 1.1 ± 3.1% for placebo) in both groups (p < 0.02 for all), and at the femoral neck only in the MK-7 group (p < 001) (by 1.5 ± 3.5% for MK-7 vs. 1.0 ± 5.0% for placebo) - Changes in microstructure were similar between groups over 3 years |
| 11 | Moore et al., 2023 [119] | UK | 105 PM women with osteoporosis and sub-optimum vitK status, 55–85 y (68.7 ± 12.3 y) | 18 months |
- all three groups: standard treatment of oral bisphosphonate and calcium (1 g/day) and/or vitD (800 IU/day) | - Comparison between the groups at baseline and after 3, 6, 12, and 18 months | ↑ trend in CTX and P1NP (p < 0.001) over time in all 3 arms Changes in HSA parameters at the intertrochanter (IT) and femoral shaft (FS):
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, M.E.; Bigman, G.; Ryan, A.S.; Popa, D.-S. Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies. Nutrients 2024, 16, 2356. https://doi.org/10.3390/nu16142356
Rusu ME, Bigman G, Ryan AS, Popa D-S. Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies. Nutrients. 2024; 16(14):2356. https://doi.org/10.3390/nu16142356
Chicago/Turabian StyleRusu, Marius Emil, Galya Bigman, Alice S. Ryan, and Daniela-Saveta Popa. 2024. "Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies" Nutrients 16, no. 14: 2356. https://doi.org/10.3390/nu16142356
APA StyleRusu, M. E., Bigman, G., Ryan, A. S., & Popa, D.-S. (2024). Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies. Nutrients, 16(14), 2356. https://doi.org/10.3390/nu16142356










