Evaluation of Sourdough Bread and Its Potential Use in Support of the Treatment of Chronic Non-Communicable Diseases
Abstract
:1. Introduction
2. Materials and Methods
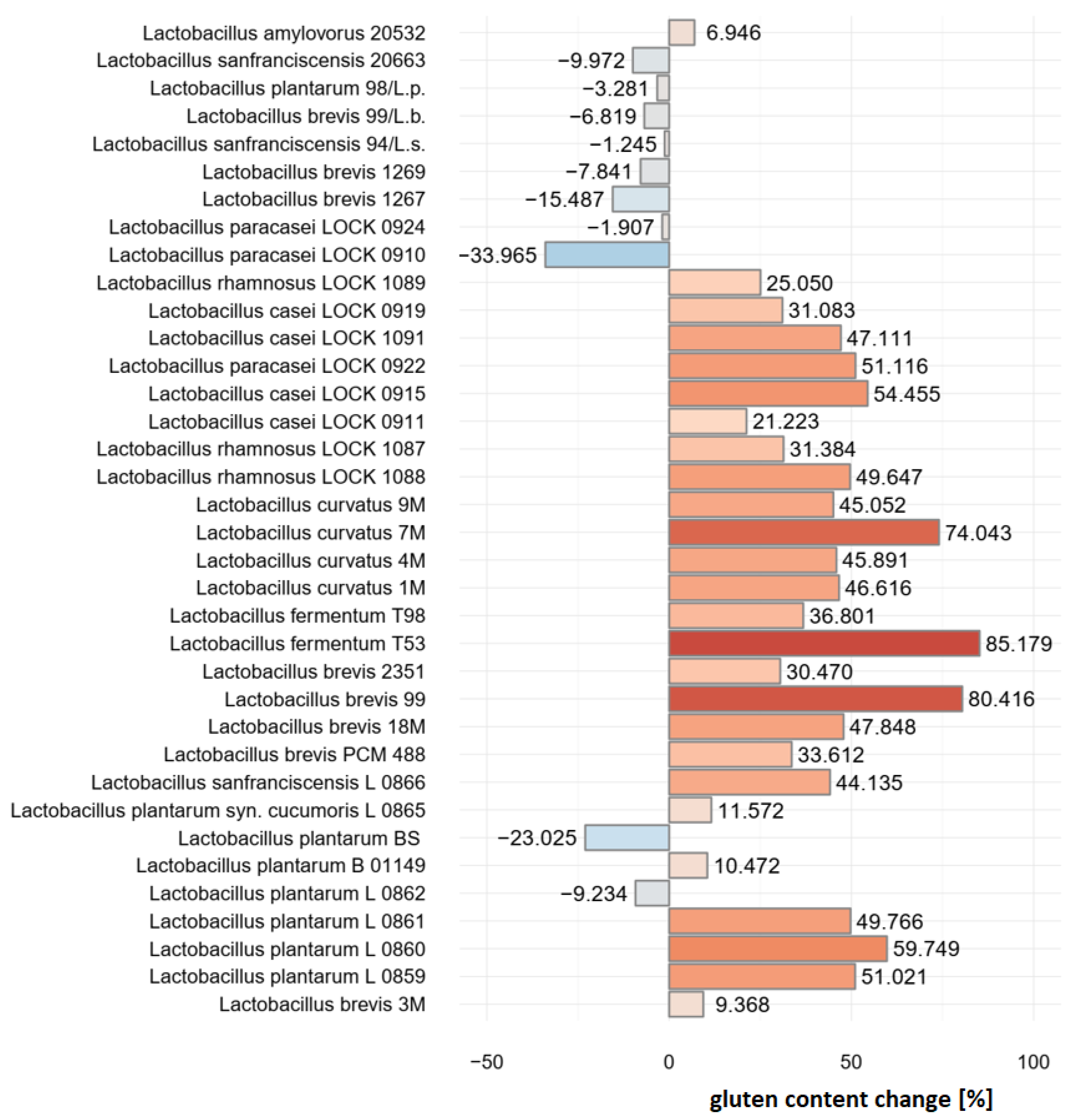
2.1. Pre-Selection of Bacterial Strains
2.2. Sample Preparation
- (A.)
- Reference bread (no sourdough)
- (B.)
- Wheat sourdough bread I
- (C.)
- Wheat–rye sourdough bread I
- (D.)
- Wheat sourdough bread II
- (E.)
- Wheat–rye sourdough bread II
2.3. Simulated Digestion
- pH = 1 (strong gastric hyperacidity);
- pH = 2 (gastric hyperacidity);
- pH = 3 (physiological conditions);
- pH = 4 (gastric hypoacidity / hypochlorhydria);
- pancreatin at 100% activity (physiological conditions);
- pancreatin at 75% activity (exocrine pancreatic insufficiency);
- pancreatin at 50% activity (strong exocrine pancreatic insufficiency).
- -
- 0.3 M calcium chloride;
- -
- α-amylase from porcine pancreas (EC 3.2.1.1), type Vi-B, ≥10 units/mg solid;
- -
- Pepsin from porcine gastric mucosa, powder, ≥250 units/mg solid (Sigma-Aldrich, St. Louis, MO, USA);
- -
- Bile bovine, dried Ox gall powder (Sigma-Aldrich);
- -
- Pancreatin (Kreon travix, 10000, Abbott Products GMBH, Wiesbaden, Germany);
- -
- 1 M hydrochloric acid;
- -
- 1 M sodium hydroxide.
- (a.)
- Salivary phase: First, 0.7 mL SSF, prepared as described in Table 1, was mixed with 295 µL H2O and 5 µL 0.3 M CaCl2. The solution was then used to dissolve 4.69 mg α-amylase, which was followed by the addition of 1 g sample, which was prepared as described in Section 2.2. The mixture was incubated for 2 min at 37 °C.
- (b.)
- Gastric phase: Afterwards, 1.5 mL SGF, prepared as described in Table 2, was mixed in a fresh test tube with 0.459 µL H2O, 1 µL 0.3 M CaCl2 and 40 µL 1 M HCl. The solution was then used to dissolve 12 mg pepsin, which was followed by addition of the analytical test sample pre-treated as described above. Four individual samples were prepared where the pH value was set to 1, 2 (both to simulate gastric hyperacidity), 3 (physiological pH upon food consumption) and 4 (to simulate gastric hypoacidity), respectively, using 6 M HCl. The samples were incubated for 2 h at 37 °C.
- (c.)
- Intestinal phase: In a fresh test tube, 2.2 mL SIF, prepared as described in Table 3, was mixed with 0.5 mL bile, 1.262 mL H2O, 8 µL 0.3 M CaCl2, 30 µL 1 M NaOH and 200 mg pancreatin. Analytical test samples, each at a distinct pH value, prepared and pre-treated as described above, were added to the solution, and the pH was set to 7 using 1 M NaOH in every sample. The samples were incubated for 2 h at 37 °C. In addition, an analytical test sample previously digested at physiological pH = 3 was not only subjected to 200 mg pancreatin (100% activity) but also to lower doses of the enzyme, which was thought to mimic the treatment with 75% and 50% pancreatin activity.
2.4. QQQPP Peptide Determination with ELISA
- -
- PBS buffer (0.7551 g KH2PO4; 11.466 g Na2HPO4 and 18 g NaCl dissolved in 2 L distilled water);
- -
- Carbonate-bicarbonate buffer;
- -
- PBS-T buffer (1 mL Tween per every 1 L PBS);
- -
- Milk powder 3% solution in PBS (3 g milk powder in 1 mL PBS);
- -
- Rabbit serum with anti-QQQPP antibodies;
- -
- Anti-Rabbit antibodies (Anti-Rabbit IgG (whole molecule)–peroxidase, Sigma-Aldrich);
- -
- TMB (3,3′,5,5′-tetramethylbenzidine) Liquid Substrate System for ELISA, Sigma-Aldrich);
- -
- 1 M sulfuric acid.
2.5. Inhibition of Enzymatic Conversion of Angiotensin
- -
- Sodium-potassium buffer at pH 8.3 (0.2 M K3PO4 and 0.3 M NaCl);
- -
- N-Hippuryl-His-Leu hydrate, HHL (Sigma-Aldrich);
- -
- Angiotensin-converting enzyme from rabbit lung, ACE (Sigma-Aldrich);
- -
- 1 M hydrochloric acid;
- -
- Ethyl acetate.
- -
- Negative control: 15 µL extract sample was replaced with 15 µL water to prevent enzymatic reaction due to the lack of substrate;
- -
- Reaction blank: HCl was added before ACE so that a 15 µL extract sample could not be converted enzymatically;
- -
- Sample blank: 15 µL extract sample was replaced with 15 µL water; HCl was added before ACE.
- A—negative control;
- B—reaction blank;
- C—analytical sample;
- D—sample blank.
2.6. Inhibition of α-Amylase
- -
- Phosphate buffer pH 7.0, 0.1 M (61.5 mL 1 M K2HPO4 solution mixed with 38.5 mL 1 M KH2PO4 solution and filled up with water to final volume 200 mL);
- -
- α-amylase solution: 100 mg α-amylase from porcine pancreas, EC 3.2.1.1 (type Vi-B, ≥10 units/mg solid) dissolved in 400 mL distilled water;
- -
- Starch solution: 0.125 g starch dissolved in 25 mL phosphate buffer pH 7.0 and incubated for 20 min at 65 °C;
- -
- DNS solution: 1 g 3,5-dinitrosalicylic acid and 30 g potassium sodium tartrate were dissolved in 20 mL 2 M NaOH. The solution was filled up to 100 mL with distilled water.
2.7. Statistical Analysis
3. Results
3.1. Immunoreactivity
- (a.)
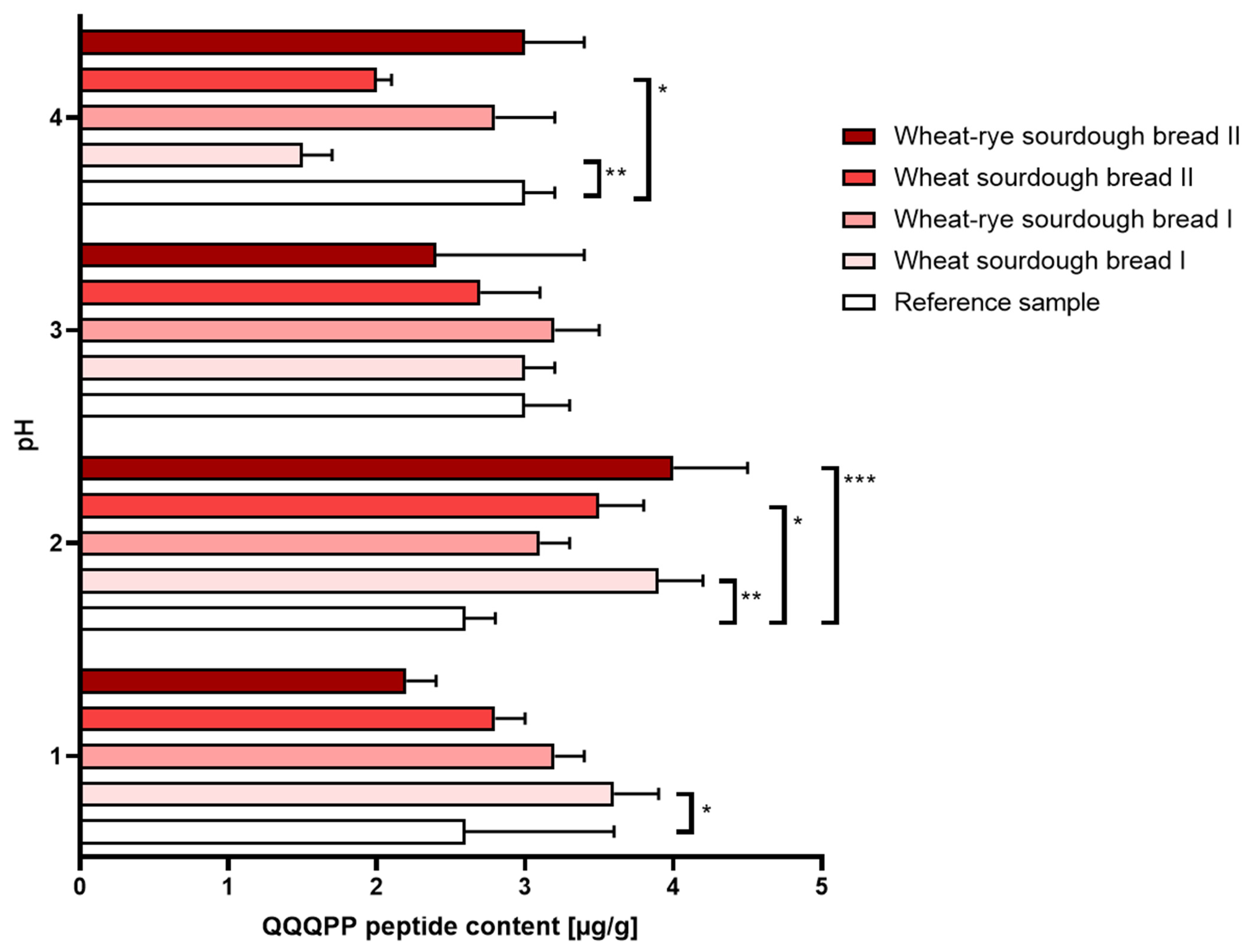
- Gastric disorders: Digestion performed under the conditions mimicking gastric hypoacidity resulted in a decreased immunoreactivity reported in wheat bread regardless of the sourdough used for manufacturing. Gastric hyperacidity, on the other hand, was mostly characterized by an increase in immunoreactivity reported in in all types of sourdough bread relative to the reference bread sample. The most amount of QQQPP allergen was determined at pH = 2 in wheat bread whether the sourdough was commercial or laboratory. The results were shown in Figure 2.
- (b.)
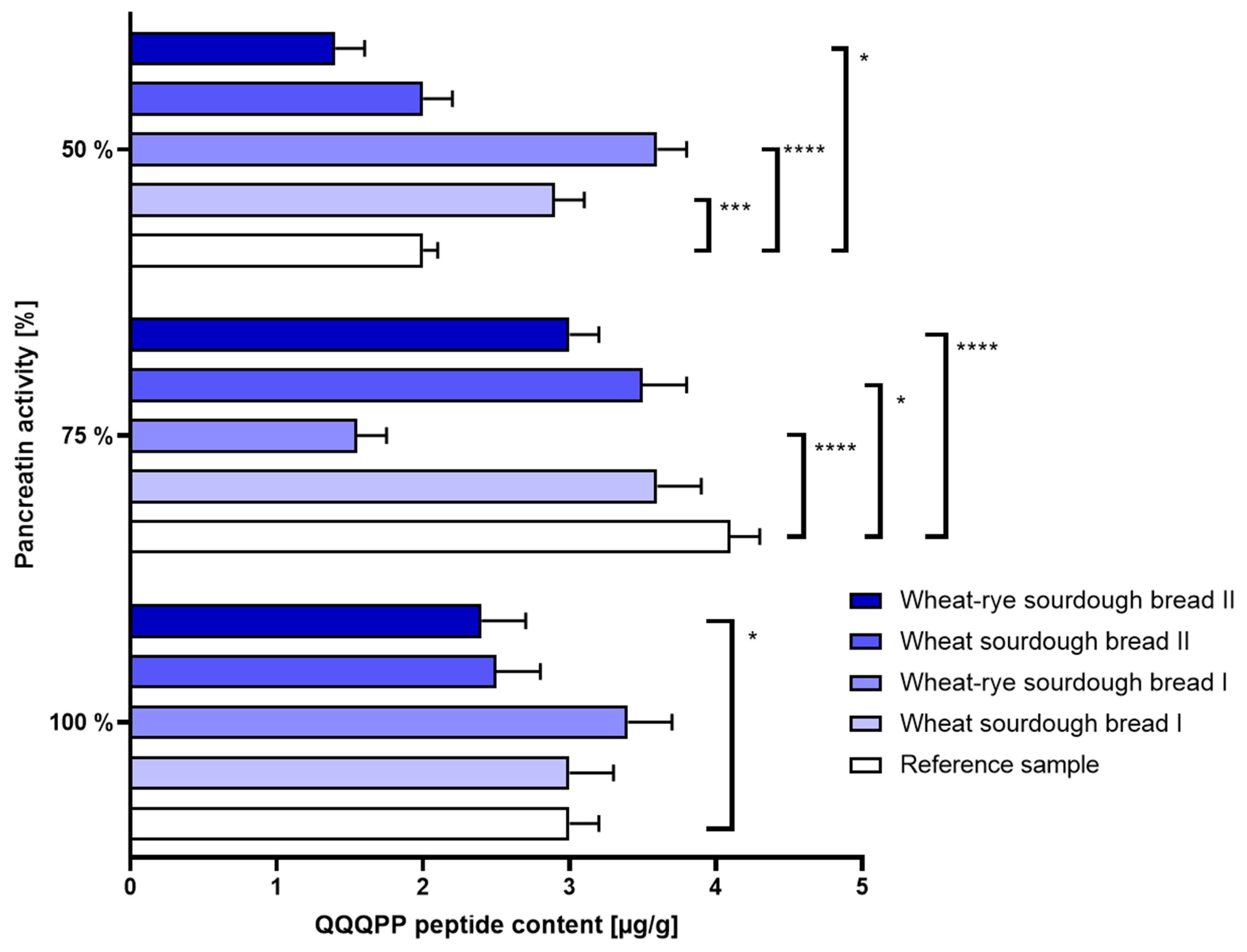
- Intestinal disorders: When only half a physiological dose of pancreatin was used, the immunoreactivity of bread made with commercial sourdough (samples B and C) was slightly increased. The use of laboratory sourdough, however, did not elevate bread allergenicity beyond the level set by reference bread sample. The results were shown in Figure 3.
3.2. Inhibition of Enzymatic Conversion of Angiotensin
- (a.)
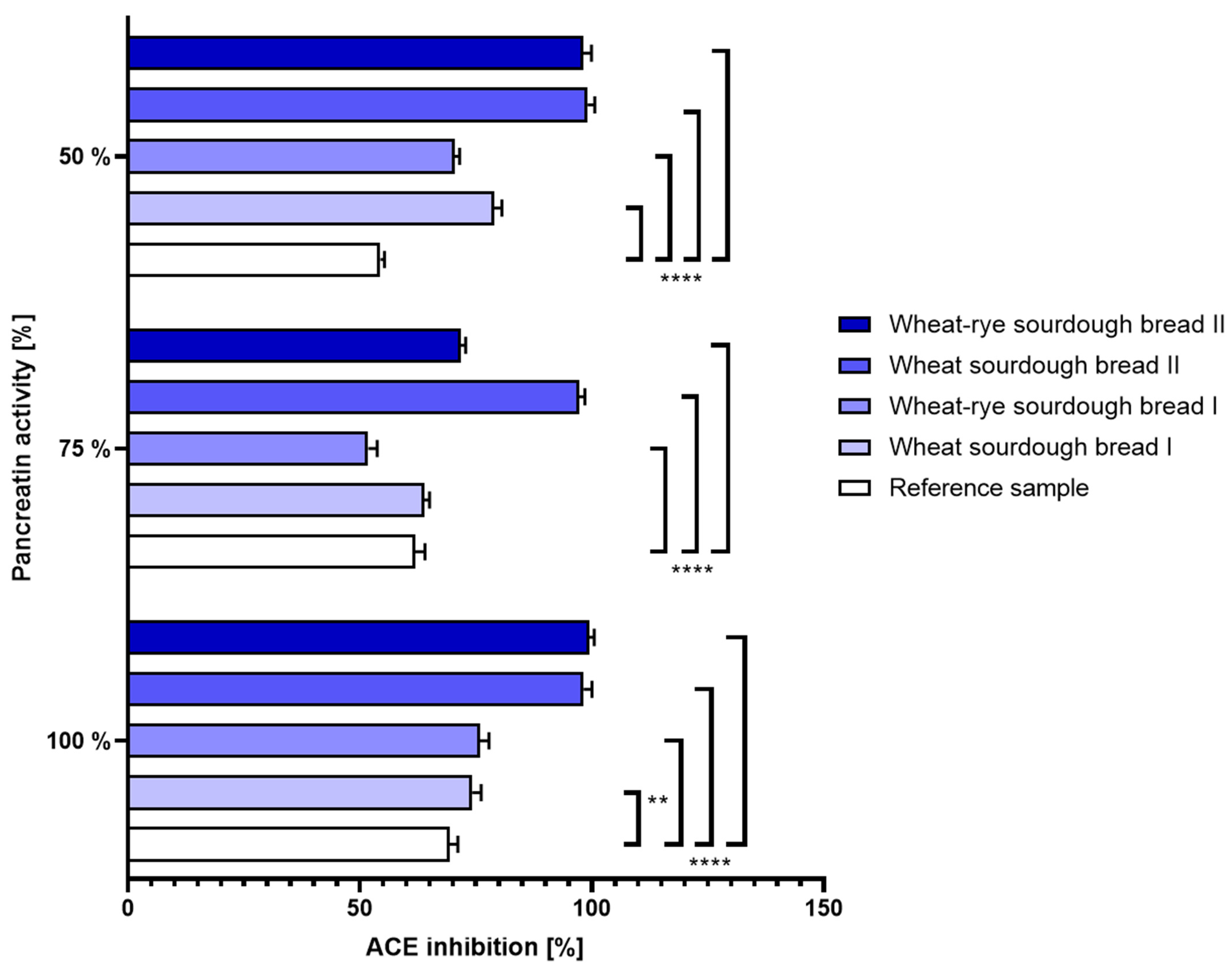
- Gastric disorders: In the reference bread sample, digestion performed under different pH conditions did not translate into noticeable changes determined in the level of angiotensin conversion. Bread samples manufactured with laboratory sourdough (D and E) were marked by a significantly enhanced inhibition of ACE relative to reference bread as well as to the samples made with commercial sourdough. The reported effect was robust and not affected by either hypo- or hyperacidity conditions. The most pronounced inhibitory properties were seen to occur consistently under non-disturbed, physiological pH conditions. The results were shown in Figure 4.
- (b.)
- Intestinal disorders: The same can be said about the ACE inhibition determined in bread samples when variable pancreatin activity was used during simulated digestion. At all tested enzyme doses, wheat and wheat–rye bread produced with laboratory sourdough displayed superior characteristics to its counterparts. The results were shown in Figure 5.
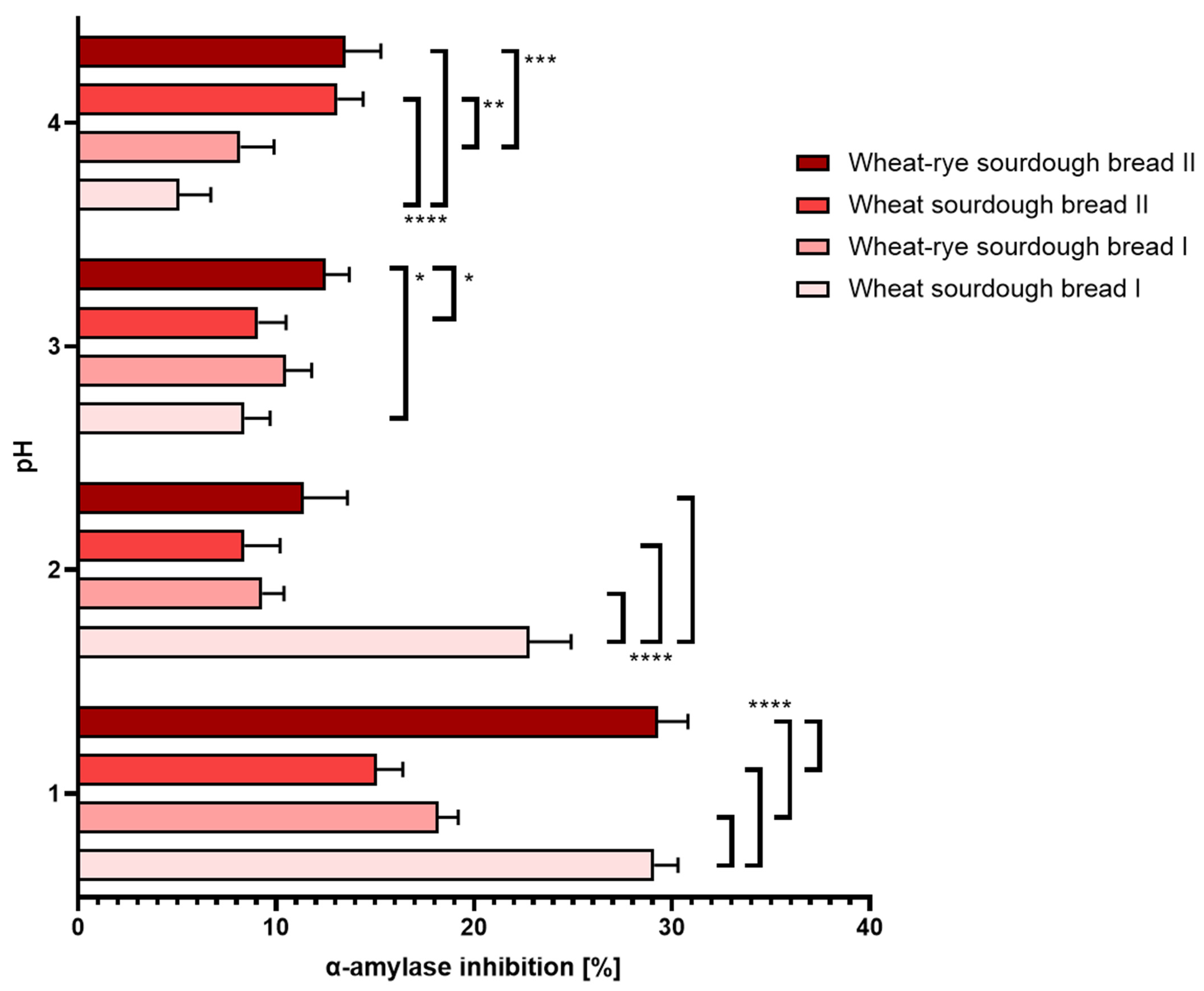
3.3. Inhibition of α-Amylase
- (a.)
- Gastric disorders: At physiological pH and above, all tested sourdough bread samples exhibited similar level of α-amylase inhibition. Overly acidic pH conditions amplified differences between the consecutive samples, but in every case, it largely elevated the extent of the inhibition. The results were shown in Figure 6.
- (b.)
- Intestinal disorders: The mostly stable level of ACE inhibition at 100% and 75% pancreatin activity is thought to indicate no clear link between pancreatin and α-amylase activities. Changes in α-amylase activity reported at the lowest pancreatin concentration tested were not conclusive. The results were shown in Figure 7.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorrian, K.; Mkhabela, M.; Sapirstein, H.; Bullock, P. Effects of delayed harvest on wheat quality, gluten strength, and protein composition of hard red spring wheat. Cereal Chem. 2023, 100, 196–212. [Google Scholar] [CrossRef]
- Xhaferaj, M.; Muskovics, G.; Schall, E.; Bugyi, Z.; Tömösközi, S.; Scherf, K.A. Development of a barley reference material for gluten analysis. Food Chem. 2023, 424, 136414. [Google Scholar] [CrossRef] [PubMed]
- Muskovics, G.; Tömösközi, S.; Bugyi, Z. Investigation of the effects of sample preparation on gluten quantitation in rye and barley flours. Acta Aliment. 2023, 52, 73–81. [Google Scholar] [CrossRef]
- Kamal, G.M.; Noreen, A.; Tahir, M.S.; Zahra, S.M.; Uddin, J.; Liaquat, A.; Khalid, M.; Goksen, G.; Sabir, A.; Ramniwas, S.; et al. Chapter 11—Isolation of gluten from wheat flour and its structural analysis. In Handbook of Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 275–292. [Google Scholar]
- Shewry, P. Wheat grain proteins: Past, present, and future. Cereal Chem. 2023, 100, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sharma, P.; Kaushik, M.; Divte, P.R.; Mandal, P.K.; Jain, N.; Anand, A. Gluten subfractions of wheat storage proteins are affected by high night temperature during grain formation. J. Agron. Crop. Sci. 2023, 209, 854–863. [Google Scholar] [CrossRef]
- Raj, R.; Shams, R.; Pandey, V.K.; Dash, K.K.; Singh, P.; Bashir, O. Barley phytochemicals and health promoting benefits: A comprehensive review. J. Agric. Food Res. 2023, 14, 100677. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health benefits of cereal grain- and pulse-derived proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Saeed, F.; Noor, R.A.; Imran, A.; Afzaal, M.; Rasheed, A.; Islam, F.; Iqbal, A.; Zahoor, T.; Naz, S.; et al. A comprehensive review on biochemical and technological properties of rye (Secale cereale L.). Int. J. Food Prop. 2023, 26, 2212–2228. [Google Scholar] [CrossRef]
- Morita, E.; Matsuo, H.; Kohno, K.; Yokooji, T.; Yano, H.; Endo, T. A narrative mni review on current status of hypoallergenic wheat development for IgE-mediated wheat allergy, wheat-dependent exercise-induced anaphylaxis. Foods 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Tsurunaga, Y.; Arima, S.; Kumagai, S.; Morita, E. Low allergenicity in processed wheat flour products using tannins from agri-food wastes. Foods 2023, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Elbek-Cubukcu, C.; Arsoy, H.; Ozkaya, G. Assessment of oral manifestations in pediatric patients with celiac disease in relation to marsh types. Med. Oral Patol. Oral Cirugia Bucal 2023, 28, e9–e15. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.; Villanueva, M.; Caballero, P.A.; Lazaridou, A.; Biliaderis, C.G.; Ronda, F. Flours from microwave-treated buckwheat grains improve the physical properties and nutritional quality of gluten-free bread. Food Hydrocoll. 2024, 149, 109644. [Google Scholar] [CrossRef]
- Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough fermentation as a tool to improve the nutritional and health-promoting properties of its derived-products. Fermentation 2021, 7, 246. [Google Scholar] [CrossRef]
- Scricciolo, A.; Lombardo, V.; Elli, L.; Bascuñán, K.; Doneda, L.; Rinaldi, F.; Pinto, D.; Araya, M.; Costantino, A.; Vecchi, M.; et al. Use of a proline-specific endopeptidase to reintroduce gluten in patients with non-coeliac gluten sensitivity: A randomized trial. Clin. Nutr. 2022, 41, 2025–2030. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, I. Comparison of elimination diets: Minerals in gluten-free, dairy-free, egg-free and low-protein breads. J. Food Compos. Anal. 2023, 118, 105204. [Google Scholar] [CrossRef]
- Parsons, K.; Brown, L.; Clark, H.; Allen, E.; McCammon, E.; Clark, G.; Oblad, R.; Kenealey, J. Gluten cross-contact from common food practices and preparations. Clin. Nutr. 2021, 40, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.; Heynen, L.; Vreugdenhil, A. Nutrient deficiencies in children with celiac disease during long term follow-up. Clin. Nutr. 2023, 42, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, L.; Mackinder, M.; Stefanowicz, F.; Aroutiounova, M.; Catchpole, A.; Wadsworth, J.; Buchanan, E.; Cardigan, T.; Duncan, H.; Hansen, R.; et al. Micronutrient deficiencies in children with coeliac disease; a double-edged sword of both untreated disease and treatment with gluten-free diet. Clin. Nutr. 2021, 40, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Fraberger, V.; Ladurner, M.; Nemec, A.; Grunwald-Gruber, C.; Call, L.M.; Hochegger, R.; Domig, K.J.; D’amico, S. Insights into the Potential of Sourdough-Related Lactic Acid Bacteria to Degrade Proteins in Wheat. Microorganisms 2020, 8, 1689. [Google Scholar] [CrossRef]
- Menard, O.; Lesmes, U.; Shani-Levi, C.S.; Calahorra, A.A.; Lavoisier, A.; Morzel, M.; Rieder, A.; Feron, G.; Nebbia, S.; Mashiah, L.; et al. Static in vitro digestion model adapted to the general older adult population: An INFOGEST international consensus. Food Funct. 2023, 14, 4569–4582. [Google Scholar] [CrossRef]
- Nagy, A.; Szűcs, K.F.; Grosz, G.; Süle, M.; Fekete, F.; Karoliny, A.; Borsos, M.; Papp, Z.; Vigh, D.; Gáspár, R. Prediction of gastroesophageal reflux episodes by smooth muscle electromyography: A translational study in rats and adolescents. Heliyon 2023, 9, e18859. [Google Scholar] [CrossRef] [PubMed]
- Braham, D.; Mills, M.; Lee, M.; Brown, E.; Gordon-Strachan, G. prevalence of gastroesophageal reflux disease symptoms in a jamaican population. West Indian Med. J. 2023, 70, 32–35. [Google Scholar] [CrossRef]
- Chen, J.W.; Vela, M.F.; Peterson, K.A.; Carlson, D.A. AGA clinical practice update on the diagnosis and management of extraesophageal gastroesophageal reflux disease: Expert review. Clin. Gastroenterol. Hepatol. 2023, 21, 1414–1421.e3. [Google Scholar] [CrossRef]
- Wang, X.; Wright, Z.; Patton-Tackett, E.D.; Song, G. The relationship between gastroesophageal reflux disease and chronic kidney disease. J. Pers. Med. 2023, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Campbell-McBride, N.; Rice, S.M.; Murphy, T. Chapter 8—Gastrointestinal health and therapeutic carbohydrate restriction. In Ketogenic; Academic Press: Cambridge, MA, USA, 2023; pp. 383–413. [Google Scholar]
- Huang, H.; Zhong, W.; Wang, X.; Yang, Y.; Wu, T.; Chen, R.; Liu, Y.; He, F.; Li, J. The role of gastric microecological dysbiosis in gastric carcinogenesis. Front. Microbiol. 2023, 14, 1218395. [Google Scholar] [CrossRef] [PubMed]
- Kiecka, A.; Szczepanik, M. Proton pump inhibitor-induced gut dysbiosis and immunomodulation: Current knowledge and potential restoration by probiotics. Pharmacol. Rep. 2023, 75, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Scalese, G.; Virili, C.; Pontone, S.; Di Pietro, M.; Covelli, A.; Bedetti, G.; Marinelli, P.; Bruno, G.; Stramazzo, I.; et al. The potential role of hypochlorhydria in the development of duodenal dysbiosis: A preliminary report. Front. Cell. Infect. Microbiol. 2022, 12, 854904. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagiotou, O.; Schizas, D.; Vailas, M.; Tsoli, M.; Sakarellos, P.; Sotiropoulou, M.; Papalambros, A.; Felekouras, E. All you need to know about gastrinoma today | Gastrinoma and Zollinger-Ellison syndrome: A thorough update. J. Neuroendocr. 2023, 35, e13267. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Buchner, A.M.; Forsmark, C.E. AGA clinical practice update on the epidemiology, evaluation, and management of exocrine pancreatic insufficiency: Expert review. Gastroenterology 2023, 165, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.M.; Dominguez-Munoz, J.E. Differential diagnosis of chronic diarrhea: An algorithm to distinguish irritable bowel syndrome with diarrhea from other organic gastrointestinal diseases, with special focus on exocrine pancreatic insufficiency. J. Clin. Gastroenterol. 2023, 57, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Boland, A.; Carroll, A.; O’connor, A. Concurrent functional gastrointestinal disorders in patients with inflammatory bowel disease. Front. Gastroenterol. 2022, 1, 959082. [Google Scholar] [CrossRef]
- Diowksz, A.; Malik, A.; Jaśniewska, A.; Leszczyńska, J. The inhibition of amylase and ACE enzyme and the reduction of immunoreactivity of sourdough bread. Foods 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Y.; Wang, J.; Wang, Z.; Gao, J.; Yuan, J.; Chen, H. A Meta-analysis of the prevalence of wheat allergy worldwide. Nutrients 2023, 15, 1564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ma, S.; Liu, X.; Yin, X.; Liu, S.; Zhou, J.; Du, G. Protein-glutaminase: Research progress and prospect in food manufacturing. Food Biosci. 2021, 43, 101314. [Google Scholar] [CrossRef]
- Cutrell, S.; Alhomoud, I.S.; Mehta, A.; Talasaz, A.H.; Van Tassell, B.; Dixon, D.L. ACE-inhibitors in hypertension: A historical perspective and current insights. Curr. Hypertens. Rep. 2023, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Arouna, N.; Árvay, J.; Longo, V.; Pucci, L. Sourdough fermentation improves the antioxidant, antihypertensive, and anti-inflammatory properties of Triticum dicoccum. Int. J. Mol. Sci. 2023, 24, 6283. [Google Scholar] [CrossRef] [PubMed]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’hocine, L. Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activity of Faba Bean-Derived Peptides After In Vitro Gastrointestinal Digestion: Insight into Their Mechanism of Action. J. Agric. Food Chem. 2024, 72, 6432–6443. [Google Scholar] [CrossRef] [PubMed]
- Adanaş, R.; Türkoğlu, V.; Baş, Z. Inhibition effect of Gly-Arg-Gly-Asp-Ser (GRGDS) and Arg-Gly-Asp (RGD) Bioactive Peptides on Angiotensin-Converting Enzyme Activity Purified from Human Serum. J. Inst. Sci. Technol. 2023, 13, 2847–2860. [Google Scholar] [CrossRef]
- Pratiwi, A.; Hakim, T.R.; Abidin, M.Z.; Fitriyanto, N.A.; Jamhari, J.; Rusman, R.; Erwanto, Y. Angiotensin-converting enzyme inhibitor activity of peptides derived from Kacang goat skin collagen through thermolysin hydrolysis. Vet. World 2021, 14, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Nuchprapha, A.; Paisansak, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. Two novel ACE inhibitory peptides isolated from longan seeds: Purification, inhibitory kinetics and mechanisms. RSC Adv. 2020, 10, 12711–12720. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, N.S.; Yanni, A.E.; Karathanos, V.T. Bread making technology influences postprandial glucose response: A review of the clinical evidence. Br. J. Nutr. 2017, 117, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Calazans, A.P.C.T.; Milani, T.M.S.; Prata, A.S.; Clerici, M.T.P.S.; Nicoli, J.R.; Martins, F.S.; Borges, M.C. A Functional Bread Fermented with Saccharomyces cerevisiae UFMG A-905 Prevents Allergic Asthma in Mice. Curr. Dev. Nutr. 2024, 8, 102142. [Google Scholar] [CrossRef] [PubMed]
- Kaim, U.; Goluch, Z.S. Health Benefits of Bread Fortification: A Systematic Review of Clinical Trials according to the PRISMA Statement. Nutrients 2023, 15, 4459. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Shiri, A.; Tahmouzi, S.; Mollakhalili-Meybodi, N.; Nematollahi, A. Application of inulin in bread: A review of technological properties and factors affecting its stability. Food Sci. Nutr. 2023, 11, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Gachons, C.P.D.; Breslin, P.A.S. Salivary amylase: Digestion and metabolic syndrome. Curr. Diabetes Rep. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.E.J.; Langsted, A.; Varbo, A.; Madsen, C.M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Low and high pancreatic amylase is associated with pancreatic cancer and chronic pancreatitis. Eur. J. Epidemiol. 2021, 36, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schuppan, D.; Rojas Tovar, L.E.; Zevallos, V.F.; Loponen, J.; Gänzle, M. Sourdough fermentation degrades wheat alpha-amylase/trypsin inhibitor (ATI) and reduces pro-inflammatory activity. Foods 2020, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.G.; Kougblenou, I.; Murai, T.; Okyere, A.Y.; Anderson, J.; Bajgain, P.; Philipp, B.; LaPlante, B.; Schlecht, S.; Vogel, C.; et al. Impact of sourdough fermentation on FODMAPs and amylase-trypsin inhibitor levels in wheat dough. J. Cereal Sci. 2022, 108, 103574. [Google Scholar] [CrossRef]


| Reagent | Concentration [mol/dm3] | Volume [mL] |
|---|---|---|
| KCl | 0.5 | 15.1 |
| KH2PO4 | 0.5 | 3.7 |
| NaHCO3 | 1 | 6.8 |
| MgCl2(H2O)6 | 0.15 | 0.5 |
| (NH4)2CO3 | 0.5 | 0.06 |
| Distilled water | Up to 400 |
| Reagent | Concentration [mol/dm3] | Volume [mL] |
|---|---|---|
| KCl | 0.5 | 6.9 |
| KH2PO4 | 0.5 | 0.9 |
| NaHCO3 | 1 | 12.5 |
| NaCl | 2 | 11.8 |
| MgCl2(H2O)6 | 0.15 | 0.4 |
| (NH4)2CO3 | 0.5 | 0.5 |
| Distilled water | Up to 400 |
| Reagent | Concentration [mol/dm3] | Volume [mL] |
|---|---|---|
| KCl | 0.5 | 6.8 |
| KH2PO4 | 0.5 | 0.8 |
| NaHCO3 | 1 | 42.5 |
| MgCl2(H2O)6 | 0.15 | 1.1 |
| NaCl | 2 | 9.6 |
| Distilled water | Up to 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartos, A.; Malik, A.; Diowksz, A.; Podolska, G.; Leszczyńska, J. Evaluation of Sourdough Bread and Its Potential Use in Support of the Treatment of Chronic Non-Communicable Diseases. Nutrients 2024, 16, 2485. https://doi.org/10.3390/nu16152485
Bartos A, Malik A, Diowksz A, Podolska G, Leszczyńska J. Evaluation of Sourdough Bread and Its Potential Use in Support of the Treatment of Chronic Non-Communicable Diseases. Nutrients. 2024; 16(15):2485. https://doi.org/10.3390/nu16152485
Chicago/Turabian StyleBartos, Adrian, Alicja Malik, Anna Diowksz, Grażyna Podolska, and Joanna Leszczyńska. 2024. "Evaluation of Sourdough Bread and Its Potential Use in Support of the Treatment of Chronic Non-Communicable Diseases" Nutrients 16, no. 15: 2485. https://doi.org/10.3390/nu16152485
APA StyleBartos, A., Malik, A., Diowksz, A., Podolska, G., & Leszczyńska, J. (2024). Evaluation of Sourdough Bread and Its Potential Use in Support of the Treatment of Chronic Non-Communicable Diseases. Nutrients, 16(15), 2485. https://doi.org/10.3390/nu16152485







