Apoptosis and Inflammation Involved with Fluoride-Induced Bone Injuries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
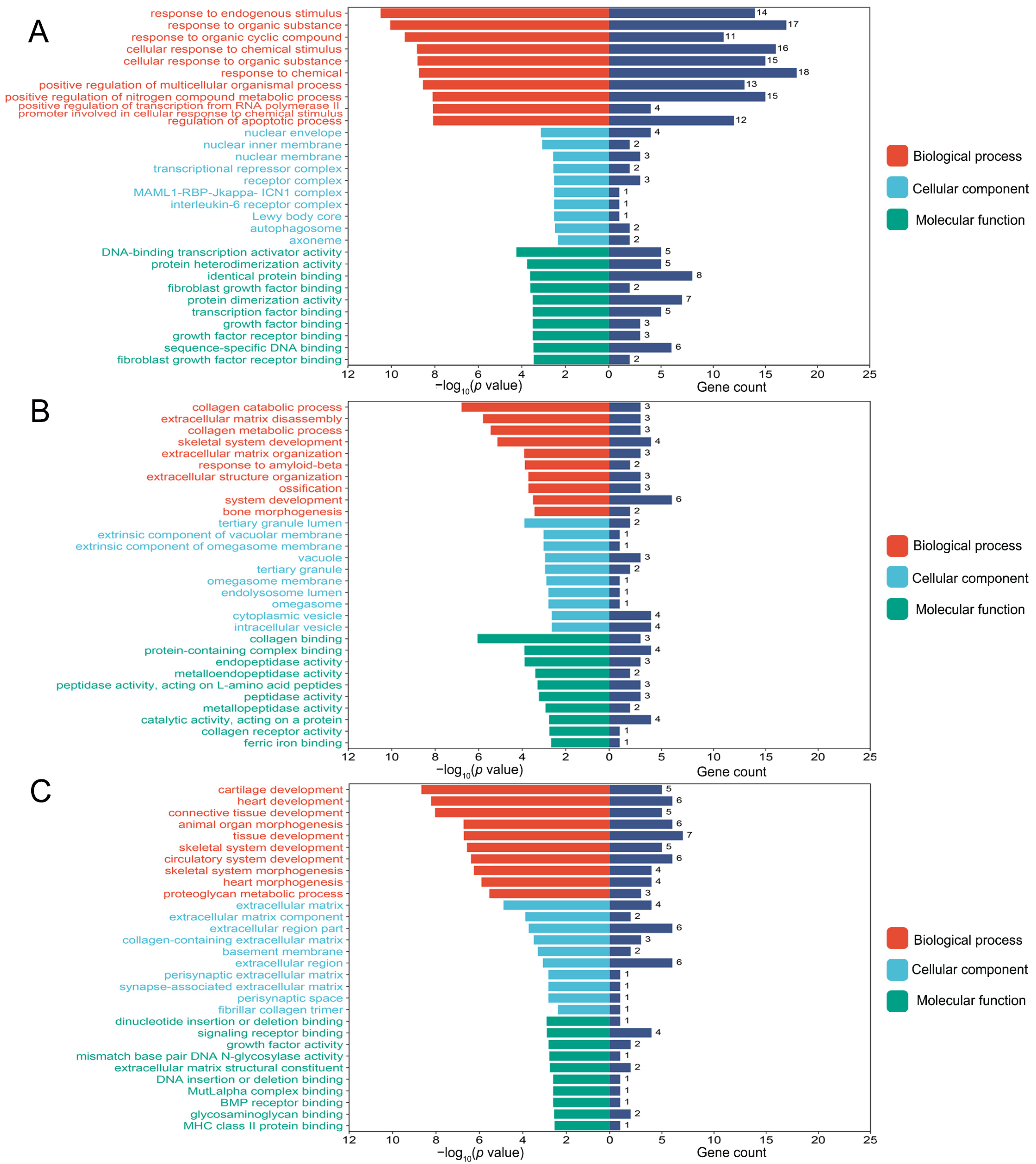
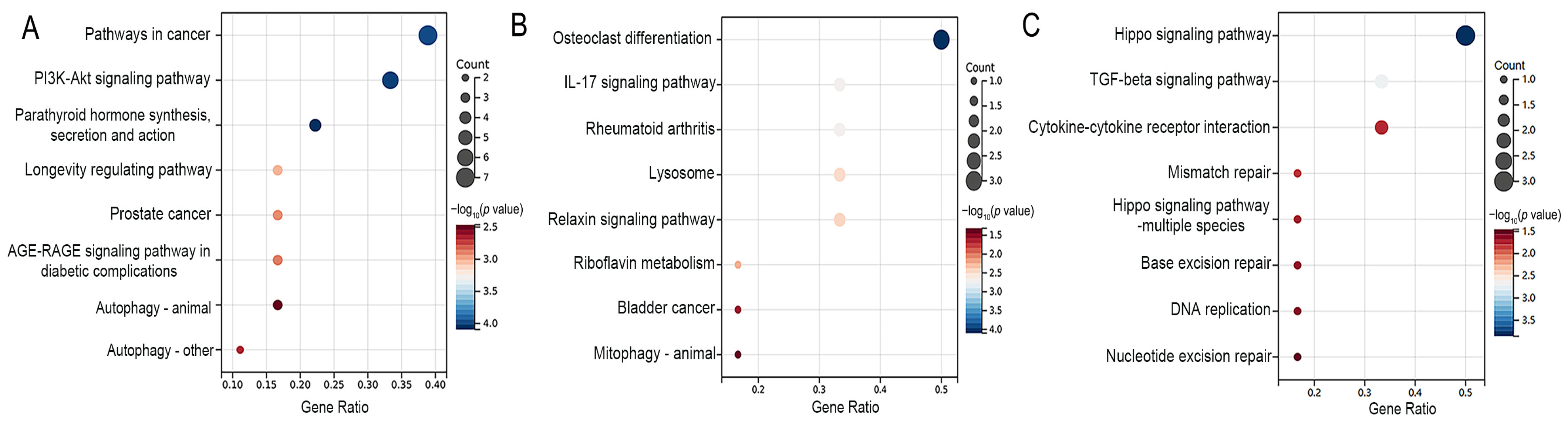
2.4. Gene Ontology and Kyoto Encyclopedia of Genes Genomes Pathway Enrichment Analyses
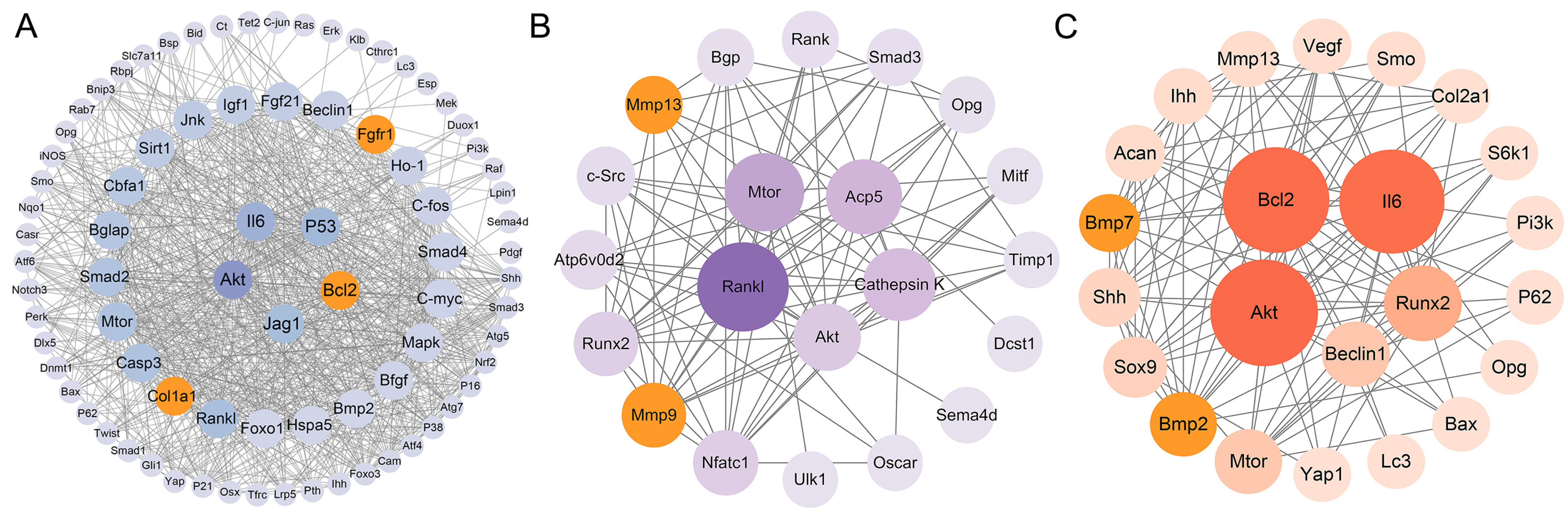
2.5. Protein–Protein Interaction Network Construction
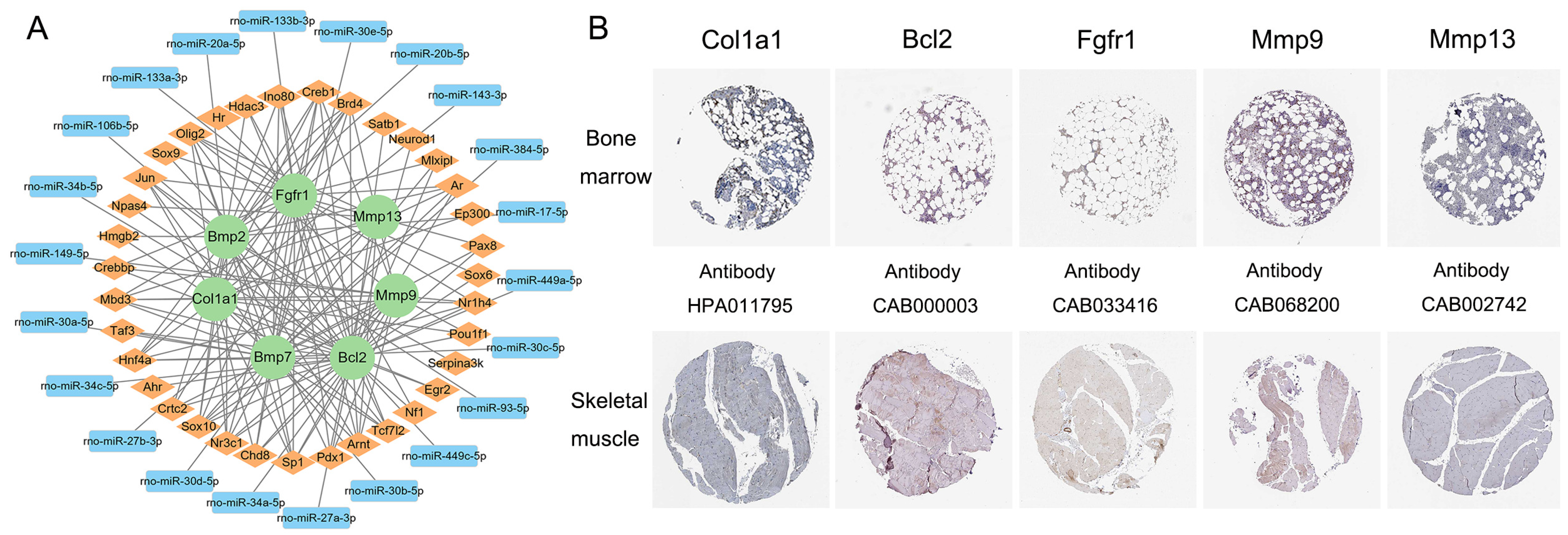
2.6. Transcription Factor-mRNA-miRNA Regulatory Network Construction and Key Gene Identification
2.7. Candidate Pharmacological Target Prediction
3. Results
3.1. General Characteristics
3.2. Identification and Classification of Differentially Expressed Genes
3.3. Functional Enrichment Analyses of Differentially Expressed Genes
3.4. Protein–Protein Interaction Network Analysis
3.5. Transcription Factor–mRNA–miRNA Regulatory Network Construction and Key Gene Identification
3.6. Prediction of Candidate Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, S.; Flora, S.J.S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Qin, M.; Gao, Y.; Zhang, M.; Wu, J.; Liu, Y.; Jiang, Y.; Zhang, X.; Wang, X.; Yang, Y.; Gao, Y. Association between ADAMTS14_rs4747096 gene polymorphism and bone mineral density of Chinese Han population residing in fluorine exposed areas in ShanXi province, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 106059–106067. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Li, M.; Zhao, T.; Zhang, W.; Wang, Y.; He, Y.; Zhao, H.; Li, H.; Wang, T.; et al. Calcium supplementation attenuates fluoride-induced bone injury via pink1/parkin-mediated mitophagy and mitochondrial apoptosis in mice. J. Hazard. Mater. 2024, 465, 133411. [Google Scholar] [CrossRef] [PubMed]
- Veneri, F.; Iamandii, I.; Vinceti, M.; Birnbaum, L.S.; Generali, L.; Consolo, U.; Filippini, T. Fluoride exposure and skeletal fluorosis: A systematic review and dose-response Meta-analysis. Curr. Environ. Health Rep. 2023, 10, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lv, M.; Zhang, Y.; Gao, Y.; Cai, Z.; Zhang, Y.; Song, J.; Liu, J.; Yin, H.; Shang, F. TDDFT study on the ESIPT properties of 2-(2′-hydroxyphenyl)-benzothiazole and sensing mechanism of a derived fluorescent probe for fluoride ion. Molecules 2024, 29, 1541. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.Y.; Parr, C.; Pesti, G.M. The effects of dietary fluoride on growth and bone mineralization in broiler chicks. Poult. Sci. 2011, 90, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, S.; Duan, L.; Yang, S.; Hou, X.; Du, Y.; Gao, M.; Zuo, J.; Sun, L.; Fu, X.; et al. Proteomics sequencing reveals the role of TGF-β signaling pathway in the peripheral blood of offspring rats exposed to fluoride. Biol. Trace Elem. Res. 2024, 202, 2100–2110. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Sivanesan, S.; Tarale, P.; Naoghare, P.K.; Bafana, A.; Parmar, D.; Kannan, K. Role of fluoride induced histone trimethylation in development of skeletal fluorosis. Environ. Toxicol. Pharmacol. 2018, 57, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 304, 119241. [Google Scholar] [CrossRef]
- Park, Y.A.; Plehwe, W.E.; Varatharajah, K.; Hale, S.; Christie, M.; Yates, C.J. Skeletal fluorosis secondary to methoxyflurane use for chronic pain. JBMR Plus 2024, 8, ziae032. [Google Scholar] [CrossRef]
- Shanthakumari, D.; Srinivasalu, S.; Subramanian, S. Effect of fluoride intoxication on lipidperoxidation and antioxidant status in experimental rats. Toxicology 2004, 204, 219–228. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Liu, J.; Jiang, M.; Yu, Y.; Zhou, Q.; Sun, L.; Zhang, Z.; Zhou, L. Protective effects of sodium butyrate on fluorosis in rats by regulating bone homeostasis and serum metabolism. Ecotoxicol. Environ. Saf. 2024, 276, 116284. [Google Scholar] [CrossRef]
- Collins, M.T.; Marcucci, G.; Anders, H.J.; Beltrami, G.; Cauley, J.A.; Ebeling, P.R.; Kumar, R.; Linglart, A.; Sangiorgi, L.; Towler, D.A.; et al. Skeletal and extraskeletal disorders of biomineralization. Nat. Rev. Endocrinol. 2022, 18, 473–489. [Google Scholar] [CrossRef]
- Pei, J.; Li, B.; Gao, Y.; Xu, J.; Darko, G.M.; Sun, D. Relationship between fluoride exposure and osteoclast markers during RANKL-induced osteoclast differentiation. Environ. Toxicol. Pharmacol. 2016, 46, 241–245. [Google Scholar]
- Bar-Shavit, Z. The osteoclast: A multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J. Cell. Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Huynh, N.C.; Okamoto, K.; Muro, R.; Terashima, A.; Kurikawa, Y.; Komatsu, N.; Pluemsakunthai, W.; Nitta, T.; Abe, T.; et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab. 2020, 2, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gui, F.; Li, D.; Zhang, R.; Sun, Q.; Guo, X. Fluoride regulates the expression of extracellular matrix HSPG and related signaling pathways FGFR3 and Ihh/PTHrP feedback loop during endochondral ossification. Environ. Toxicol. Pharmacol. 2020, 73, 103275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, A.; Mehmood, K.; Hussain, R.; Abbas, R.Z.; Javed, M.T.; Chang, Y.F.; Hu, L.; Pan, J.; Li, Y.; et al. Long-term exposure to the fluoride blocks the development of chondrocytes in the ducks: The molecular mechanism of fluoride regulating autophagy and apoptosis. Ecotoxicol. Environ. Saf. 2021, 217, 112225. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape string app: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Duan, M.; Zhang, C.; Wang, K.; Feng, L.; Song, L.; Wu, S.; Chen, X. Identification of potential biomarkers associated with acute myocardial infarction by weighted gene coexpression network analysis. Oxid. Med. Cell. Longev. 2021, 2021, 5553811. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, M.; Luo, K.; Sun, L.; Yu, S.; Zuo, J.; Wang, Y. Expression profiles of long non-coding RNAs in the articular cartilage of rats exposed to T-2 toxin. Int. J. Mol. Sci. 2023, 24, 13703. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Cao, Y. Effects of fluoride on bone morphogenetic protein expression in osteoblast. Chin. J. Public Health 2003, 19, 34–35. [Google Scholar]
- Zhang, W.; Cui, Y.; Gao, S.; Zhang, X.; Li, G. Expression of proto-oncogenes c-fos and c-jun in osteoblasts activated by excessive fluoride. Chin. J. Prev. Med. 2003, 37, 30–34+92. [Google Scholar]
- Xu, J.; Chen, J.; Cai, H.; Cao, Y. Influence of fluoride on bone morphogenetic protein expression and the antagonistic effect of zinc in rat osteoblasts: All in vitro study. Chin. J. Endem. 2004, 23, 20–22. [Google Scholar]
- Jing, L. Expression of Osteogenic Phenotype in Fibroblasts induced by Fluoride and its Role in the Development Ofextraperiosteal Ossification of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
- Jing, L.; Qi, L.; Li, T.; Li, G. Effect of fluoride on the expression of core-binding factor αl in fibroblast and osteoblast. Chin. J. Endem. 2006, 25, 629–632. [Google Scholar]
- Li, D. The role of Runx2 in the process of activating osteoblasts by excessive fluoride. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
- Wang, C. The effects of fluoride on marrow stromal cells, their products OPG, RANKL, and related cytokines. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
- Zhang, W.; Xue, L.; Cui, Y.; Li, G. The effect of different dosage of fluoride intake on activation of osteoblasts and the expression of Bmp-2, Bmp-4 and Smad-4. Chin. J. Endem. 2006, 25, 125–128. [Google Scholar]
- Zhao, Y.; Qi, L.; Xu, H.; Jing, L. Effects of fluoride on expressions of TGF-β and Smad2/3 in fibroblasts and osteoblasts. J. Jilin Univ. (Med.) 2006, 32, 956–959. [Google Scholar]
- Zhong, J. Effect of Proliferation and MCM3 Gene Expression in Osteoblasts Exposed to Fluoride. Ph.D. Thesis, Xinjiang Medical University, Urumqi, China, 2006. [Google Scholar]
- Liu, Y. Effect of Ras Gene Expression on Osteoblasts Cultured Invitro after Exposed to Fluoride. Master’s Thesis, Xinjiang Medical University, Urumqi, China, 2007. [Google Scholar]
- Wang, Y.; Tan, Q.; Yu, Y. Effect of fluoride on expression of calmodulin in rat osteoblasts in vitro. Chin. J. Public Health 2007, 23, 333–336. [Google Scholar]
- Wei, H. Expression and Effects of lGF-1 and bFGF in Fibroblast and Osteoblast Induced by Fluoride. Master’s Thesis, Jilin University, Changchun, China, 2007. [Google Scholar]
- Zhou, T. Effect on the Proliferation of Osteoblasts Cultured In Vitro and the Expression of TM9SF1 Gene after Exposed to Fluoride. Master’s Thesis, Xinjiang Medical University, Urumqi, China, 2007. [Google Scholar]
- Han, B. Influence of Subchronic Exposure to Excessive Fluoride on Level of Bone Sialoprotein Expression in Rat Bone Tissue. Master’s Thesis, China Medical University, Shenyang, China, 2008. [Google Scholar]
- Chi, G. Effects of fluoride on the gene expression of c-fos and c-jun in fibroblast and osteoblast. J. Inn. Mong. Univ. Natl. 2008, 23, 184–188. [Google Scholar]
- Liu, Y.; Liu, K.; Yang, X.; Liu, J.; Zhong, J.; Zhou, T. Effect of protein expression of Ras gene in osteoblasts cultured in vitro and exposed to fluoride. J. Xinjiang Med. Univ. 2008, 31, 940–942. [Google Scholar]
- Wang, M.; Yu, F.; Wu, P.; Zhao, D.; Su, J.; Han, B. Regulation of fluoride on osteocalcin gene expression in osteoblasts cultured in vitro. Sci. Agric. Sin. 2009, 42, 2629–2632. [Google Scholar]
- Dan, Y.; Yu, Y.; Deng, C. Effect of fluoride on expression of OSX mRNA and protein in bone tissue of rats. China Public Health 2011, 27, 1008–1009. [Google Scholar]
- Zhu, H.; Yu, Y.; Deng, C.; Yang, D. Effect of fluoride on expression of phosphoinositide 3-kinase, protein kinase bl mRNA and protein in bone tissue of rats. Chin. J. Endem. 2011, 30, 261–265. [Google Scholar]
- Guo, X.; Wu, S.; He, Y.; Zhang, Z.; Sun, G. Effect of subchronic fluoride exposure on pathologic change and β-catenin expression in rat bone tissue. J. Hyg. Res. 2011, 40, 304–307. [Google Scholar]
- Guo, X.; Cai, R.; Sun, G. Effects of fluoride and aluminum on mRNA expression of OPG and RANKL in MC3T3-El cells. Chin. J. Integr. Med. 2011, 24, 3–5+8. [Google Scholar]
- Zhong, Y. The Effects of Fluoride on Nrf2-ARE Signal Pathway in Rat Osteoblasts. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2011. [Google Scholar]
- Zhang, X.; Lv, P.; Zhang, J.; Zhao, Z.; Xu, H.; Li, G. Immunoglobulin binding protein gene and protein expression in femur tissue of fluorosis rats. Chin. J. Endem. 2011, 30, 502–505. [Google Scholar]
- Yang, S.; Wang, Z.; Farquharson, C.; Alkasir, R.; Zahra, M.; Ren, G.; Han, B. Sodium fluoride induces apoptosis and alters bcl-2 family protein expression in MC3T3-El osteoblastic cells. Biochem. Biophys. Res. Commun. 2011, 410, 910–915. [Google Scholar] [CrossRef]
- Wan, L.; Yu, Y.; Wan, C.; Xie, Y.; Chen, X. Effects of chronic fluorosis on expression of DLX5 protein and mRNA in bone tissue of rats. Chin. J. Prev. Med. 2012, 13, 641–644. [Google Scholar]
- Wan, W.; Wan, L.; Yu, Y.; Wan, C.; Guan, Z. Effect of fluoride on the expression of twist mRNA and protein in bone tissue of rats. J. GuiYang Med. Coll. 2012, 37, 221–224. [Google Scholar]
- Xie, Y.; Yu, Y.; Wan, L.; Chen, X. Effect of fluoride on expression of Can mRNA and protein in bone tissue of rats. Chin. J. Pathol. 2012, 41, 761–764. [Google Scholar]
- Yu, Y.; Yang, D.; Zhu, H.; Deng, C.; Guan, Z. Expression of mRNA and protein of p38, Osx, PI3K and Akt1 in rat bone with chronic fluorosis. Chin. J. Pathol. 2012, 41, 622–626. [Google Scholar]
- Guo, X.; Yang, M.; Liang, D.; Guo, B.; Yang, L.; Cao, J. Fluoride induces autophagy and apoptosis and the interaction in MC3T3-El cells. Chin. J. Endem. 2012, 27, 165–168. [Google Scholar]
- Hu, C.; Ren, L.; Li, X.; Wu, N.; Li, G.; Liu, Q.; Xu, H. Effect of fluoride on insulin level of rats and insulin receptor expression in the MC3T3-El cells. Biol. Trace Elem. Res. 2012, 150, 297–305. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.; Yi, W.; Wan, L.; Xie, Y. Effect of fluoride on expression of mRNA and protein of wnt3a and beta-catenin in osteoblast of rats. Chin. J. Endem. 2013, 32, 140–145. [Google Scholar]
- Li, X. Role of ER Stress and PERK Signaling Pathway in the Mechanism of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Deng, C.; Yu, Y.; Zhang, Y. Expressions of transforming growth factor-beta1 and interleukin 6 mRNA and protein in bone of rats with chronic fluorosis. Chin. J. Endem. 2014, 33, 609–614. [Google Scholar]
- Duan, X.; Xu, H.; Wang, Y.; Wang, H.; Li, G.; Jing, L. Expression of core-binding factor α1 and osteocalcin in fluoride-treated fibroblasts and osteoblasts. J. Trace Elem. Med. Biol. 2014, 28, 278–283. [Google Scholar] [CrossRef]
- Lv, P. Roles for Insulin and Insulin Receptor in the Mechanism Underlying the Process of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2015. [Google Scholar]
- Wang, Y. PTH, PTH-rp, CaSR Expression and Its Role in the pathogenesis of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2015. [Google Scholar]
- Yan, X.; Hao, X.; Nie, Q.; Feng, C.; Wang, H.; Sun, Z.; Niu, R.; Wang, J. Effects of fluoride on the ultrastructure and expression of type I collagen in rat hard tissue. Chemosphere 2015, 128, 36–41. [Google Scholar] [CrossRef]
- Liu, X.; Song, J.; Liu, K.; Wang, W.; Xu, C.; Zhang, Y.; Liu, Y. Role of inhibition of osteogenesis function by Sema4d/Plexin-B1 signaling pathway in skeletal fluorosis in vitro. J. Huazhong Univ. Sci. Technolog Med. Sci. 2015, 35, 712–715. [Google Scholar] [CrossRef]
- Chen, X.; Wan, C.; Xie, C.; Wei, Y.; Wu, Y.; Wan, W. Fluoride lnhibits Expressions of Notch3 and Jagl Proteins in Rat Bone Tissues. J. Environ. Occup. Med. 2016, 33, 494–498. [Google Scholar]
- Chen, X.; Wan, C.; Xie, C. Influence of fluoride on RBPJ and related genes in bone tissue of rats. China J. Public Health 2016, 32, 195–198. [Google Scholar]
- Yin, F. Effects of Exogenous Calcium on Proliferation and Wnt Signaling Pathway of Mouse Osteoblasts Exposed to Fluoride. Master’s Thesis, Shanxi Agricultural University, Taigu, China, 2016. [Google Scholar]
- Chen, S.; Zhang, A.; Pan, X. The effects of fluoride on hypermethylation, transcription and expression of p16 gene in osteoblasts of rats. Chin. J. Endem. 2016, 35, 89–93. [Google Scholar]
- Gu, X.; Han, D.; Chen, W.; Zhang, L.; Lin, Q.; Gao, J.; Fanning, S.; Han, B. Sirt1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-El cells exposed to sodium fluoride. Oncotarget 2016, 7, 65218–65230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Zhang, T.; Gao, X.; Yu, F.; Sun, B. Effect of inositol-requiring enzyme-1 signaling pathway on the differentiation of osteoblasts induced by fluoride. Chin. J. Endem. 2016, 35, 863–868. [Google Scholar]
- Li, Y.; Bian, S.; Wang, J.; Wang, J. Effects of fluoride and chitosan on the gene expressions of bone morphogenic protein 2 and collagen type-1 alpha 1 chain in the mouse femur. Fluoride 2016, 49, 47–55. [Google Scholar]
- Zhao, Y.; Huo, M.; Liu, Y.; Xie, Y.; Wang, J.; Li, Y.; Wang, J. Effects of fluoride on the expression of Bmp-2 and Smad 1 in rat osteoblasts in vitro. Fluoride 2016, 49, 13–22. [Google Scholar]
- Zhao, Y.; Li, Y.; Gao, Y.; Yuan, M.; Manthari, R.K.; Wang, J.; Wang, J. TGF-β1 acts as mediator in fluoride-induced autophagy in the mouse osteoblast cells. Food Chem. Toxicol. 2018, 115, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Effect of Combined Expose of Fluorine and Arsenic on Osteoclast Differentiation and the RANK/TRAF-6/NF-kB1 Pathway in a Co-culture System. Master’s Thesis, Guizhou Medical University, Guiyang, China, 2019. [Google Scholar]
- Wang, J.; Gao, Y.; Chen, X.; Yang, J.; Xu, H.; Zhao, Y.; Li, Y. Effects of GSTO1 gene silencing on autophagy and apoptosis of fluoride-induced osteoblasts. Acta Vet. Aootechnica Sin. 2019, 50, 183–192. [Google Scholar]
- Yang, X.; Hong, F.; Xie, W.; Zhang, J.; Jin, X.; Qin, Z. Effects of joint exposure to fluoride and arsenic on OPG/RANKL regulating osteoclast differentiation. J. Environ. Health 2019, 36, 305–310. [Google Scholar]
- Gu, X.; Wang, Z.; Gao, J.; Han, D.; Zhang, L.; Chen, P.; Luo, G.; Han, B. Sirt1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-El cells exposed to fluoride. Toxicol. Vitr. 2019, 57, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, N.; Yu, H.; Yu, X.; Guo, F.; Zhao, Z.; Xu, H. Requirement of TGF-β signaling for effect of fluoride on osteoblastic differentiation. Biol. Trace Elem. Res. 2019, 187, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yu, Y.; Xu, L.; Ming, P.; Shao, S.; Qiu, J. Regulation of osteoblast behaviors via cross-talk between Hippo/YAP and MAPK signaling pathway under fluoride exposure. J. Mol. Med. 2019, 97, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, Y.; Xu, L.; Zhao, L.; Ling, H.; Yu, Y. Expressions of Ihh, Shh and Smo mRNA and protein in rats’ bone exposed to different doses of fluoride and the significance. Chin. J. Endem. 2020, 39, 630–635. [Google Scholar]
- Deng, C.; Zhang, Y.; Xu, L.; Zhao, L.; Ling, H.; Yu, Y. Change and relationship between gli1 and beta-catenin on rats’ bone formation with chronic fluorosis. Chin. J. Pathol. 2020, 49, 168–173. [Google Scholar]
- Chen, L.; Zhang, M.; Ding, Y.; Li, M.; Zhong, J.; Feng, S. Fluoride induces hypomethylation of Bmp2 and activates osteoblasts through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2022, 356, 109870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xu, W.; Zhang, Z.; Jin, H.; Yang, Y.; Zhang, J.; Xu, H. Role of TGF-β1 in fluoride-treated osteoblasts at different stages. Biol. Trace Elem. Res. 2022, 200, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, W.; Liu, Y.; Gui, C.; Wang, L.; Chen, Y.; Deng, M.; Nan, N.; Duan, X.; Guan, Z. Effect of L-NMMA on NO/iNOS expression in MC3T3-El cells induced by excessive fluoride exposure. J. GuiZhou Med. Univ. 2023, 48, 266–271. [Google Scholar]
- Ding, H.; Yin, C.; Yang, M.; Zhou, R.; Wang, X.; Pan, X. Screening of differentially methylated genes in skeletal fluorosis of rats with different types and involvement of aberrant methylation of Cthrc1. Environ. Pollut. 2023, 332, 121931. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Gui, Y.; Zou, T.; Xi, S.; Guo, X. Fluoride regulates the differentiation and atrophy through FGF21/ERK signaling pathway in C2C12 cells. Ecotoxicol. Environ. Saf. 2023, 252, 114626. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Zhao, S.; Wu, J.; Lu, C.; Jiang, L.; Ran, S.; Wang, J.; Sun, F.; Liu, B. Fluoride resistance capacity in mammalian cells involves global gene expression changes associate with ferroptosis. Chem. Biol. Interact. 2023, 381, 110555. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Deng, C.; He, L.; Wu, Q.; Xu, L.; Yu, Y. Fluoride induces osteoblast autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Exp. Biol. Med. 2023, 248, 1159–1172. [Google Scholar]
- Sun, D.; Gao, Y.; Zhou, L.; Yu, J.; Li, Y.; Yu, W. Effects of sodium fluoride on matrix metal proteinases-13 mRNA and tissue inhibitor of metal protease-1 mRNA in rat bone tissue. Chin. J. Endem. 2008, 27, 364–367. [Google Scholar]
- Pei, J.; Gao, Y.; Li, B.; Zhou, L.; Zhang, Z.; Sun, D. Effect of fluoride on osteoclast formation at various levels of receptor activator of nuclear factor kappa-b ligand (RANKL). Fluoride 2012, 45, 86–93. [Google Scholar]
- Xie, Y.; Yu, Y.; Chen, X. Expression of NFAT mRNA and protein in osteoclasts of rats with chronic fluorosis. China Public Health 2013, 29, 530–533. [Google Scholar]
- Pei, J.; Yao, Y.; Li, B.; Wei, W.; Gao, Y.; Darko, G.M.; Sun, D. Excessive fluoride stimulated osteoclast formation through up-regulation of receptor activator for nuclear factor-κb ligand (RANKL) in C57bL/6 mice. Int. J. Clin. Exp. Med. 2017, 10, 15260–15268. [Google Scholar]
- Yu, H.; Jiang, N.; Yu, X.; Zhao, Z.; Zhang, X.; Xu, H. The role of TGFβ receptor1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 2018, 393, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Li, L.; Chai, B.; Xu, X.; Bai, S. Effect of fluoride on osteoclast autophagy through the AKT/mTOR/ULK1 signaling pathway. Acad. J. Chin. PLA Med. Sch. 2022, 43, 960–965. [Google Scholar]
- Ding, H. Screening of Differentially Methylated Genes in Skeletal Fluorosis of Rats with Different Types and Involvement of Aberrant Methylation of Cthrc1. Ph.D. Thesis, Guizhou Medical University, Guiyang, China, 2023. [Google Scholar]
- Pei, J.; Li, B.; Gao, Y.; Wei, Y.; Zhou, L.; Yao, H.; Wang, J.; Sun, D. Fluoride decreased osteoclastic bone resorption through the inhibition of NFATC1 gene expression. Environ. Toxicol. 2014, 29, 588–595. [Google Scholar] [CrossRef]
- Lv, Y.; Kang, L.; Wu, G. Fluorosis increases the risk of postmenopausal osteoporosis by stimulating interferon γ. Biochem. Biophys. Res. Commun. 2016, 479, 372–379. [Google Scholar] [CrossRef]
- Liu, B.-C.; Xu, Z.-L.; Miao, Q.; Xu, Y.-Y.; Xu, M.; Qian, X.-J.; You, B.-R.; Yuan, B.-H.; Kang, N. Expression of type II collagen gene and structural change in bone tissues of rats with experimental fluorosis. Chin. J. Prev. Med. 2003, 37, 243–245. [Google Scholar]
- Li, T.; Guo, Q.; Liao, L.; Li, Y.; Zhang, Y. Effect of Fluoride on the Expression of Caspasel4 in Human Osteoblasts and Bone Tissue in Mice. Prog. Mod. Blomedlcine 2017, 17, 1631–1634. [Google Scholar]
- Zhu, Z.; Yu, Y.; Tao, X. Role of Hh signaling pathway in fluoride-induced primary chondrocyte damage in rats. China J. Public Health. 2015, 31, 574–578. [Google Scholar]
- Wang, W.; Peng, H. Expression of bone morphogenetic protein-2 and bone morphogenetic protein-7 in the posterior longitudinal ligament of fluorosis rat and its effect on ossification. Chin. J. Exp. Surg. 2016, 33, 761–764. [Google Scholar]
- Li, T.; Bai, S.; Zhang, Y.; Liu, K.; Zhang, Y.; Zhong, J. Experimental study of cartilage lesions and COLIXA3 protein expression in rats cartilage with chronic fluorosis. Chin. J. Endem. 2011, 30, 389–392. [Google Scholar]
- Yang, Q.; Chu, Y.; Jiang, W.; Li, J.; Li, Y.; Bao, Y.; Chen, F.; Li, B.; Yang, Y.; Gao, Y. Effects of different doses of sodium fluoride on cartilage lesion and expression of interleukin-6 in Balb/c mice. Chin. J. Endem. 2017, 36, 408–413. [Google Scholar]
- Zhang, L. Effect of Fluoride on Endochondral Ossification in Rat Growth Plate Cartilage and the regulatory role of EGFR Signal Pathway. Master’s Thesis, China Medical University, Shenyang, China, 2018. [Google Scholar]
- Zhu, Z.; Yu, Y.; Chen, R. Role of hedgehog signaling pathway on cartilage tissue damage in chronic fluorosis rats. China J. Public Health 2018, 34, 241–245. [Google Scholar]
- Zhang, R. Fluoride Inhibits the Proliferation and Differentiation of ATDC5 Cells via the Pl3K/AKT/mTOR Signaling Pathway. Master’s Thesis, China Medical University, Shenyang, China, 2020. [Google Scholar]
- Ma, L.; Zhang, R.; Li, D.; Qiao, T.; Guo, X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem. Biol. Interact. 2021, 349, 109659. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zuo, J.; Fu, X.; Gao, M.; Sun, L.; Yu, S.; Li, Z.; Zhou, G.; Ba, Y. Role of the hippo signaling pathway in the extracellular matrix degradation of chondrocytes induced by fluoride exposure. Ecotoxicol. Environ. Saf. 2021, 225, 112796. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Zhao, J.; Zhou, B.; Zhang, Y.; Wang, H. Hif-1α-mediated autophagy and canonical wnt/β-catenin signaling activation are involved in fluoride-induced osteosclerosis in rats. Environ. Pollut. 2022, 315, 120396. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, T.; Liu, W.; Wang, H.; Wang, C.; Zhao, Z.; Liu, N.; Wang, W. Sodium fluoride induces apoptosis through the downregulation of hypoxia-inducible factor-1α in primary cultured rat chondrocytes. Int. J. Mol. Med. 2014, 33, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Guo, F.; Sun, B.; Zhang, X.; Xu, H. Different effects of fluoride exposure on the three major bone cell types. Biol. Trace Elem. Res. 2020, 193, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.S.; Silva, T.L.; Buzalaf, M.A.; Rodrigues, A.C.; de Oliveira, R.C. Differential effects of fluoride during osteoblasts mineralization in C57BL/6J and C3h/HeJ inbred strains of mice. Biol. Trace Elem. Res. 2014, 161, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, H.; Cheng, X.; Yang, J.; Yan, Z.; Ma, H.; Zhao, Y.; Ommati, M.M.; Manthari, R.K.; Wang, J. Calcium relieves fluoride-induced bone damage through the PI3K/AKT pathway. Food Funct. 2020, 11, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, P.; Gao, Y.; Ma, Z.; Wang, H.; Long, Y.; Ma, Z.; Liu, R. Effects of the combination of epimedii folium and ligustri lucidi fructus on apoptosis and autophagy in sop rats and osteoblasts via PI3K/AKT/mTOR pathway. Biomed. Pharmacother. 2024, 173, 116346. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xia, K.; Zheng, D.; Gong, C.; Guo, W. RILP inhibits tumor progression in osteosarcoma via Grb10-mediated inhibition of the PI3K/AKT/mTOR pathway. Mol. Med. 2023, 29, 133. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Huang, L.; Zhang, X.; Wang, N.; Wang, H.; Mu, Y.; Li, K.; Liu, Z. Molecular characteristics and promoter analysis of porcine COL1A1. Genes 2022, 13, 1971. [Google Scholar] [CrossRef] [PubMed]
- Corbeau, J.; Grohs, C.; Jourdain, J.; Boussaha, M.; Besnard, F.; Barbat, A.; Plassard, V.; Rivière, J.; Hamelin, C.; Mortier, J.; et al. A recurrent de novo missense mutation in COL1A1 causes osteogenesis imperfecta type II and preterm delivery in Normande cattle. Genet. Sel. Evol. 2024, 56, 39. [Google Scholar] [CrossRef]
- Moradifard, S.; Hoseinbeyki, M.; Emam, M.M.; Parchiniparchin, F.; Ebrahimi-Rad, M. Association of the Sp1 binding site and -1997 promoter variations in COL1A1 with osteoporosis risk: The application of meta-analysis and bioinformatics approaches offers a new perspective for future research. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108339. [Google Scholar] [CrossRef]
- Ding, D.; Xu, C.; Zhang, J.; Zhang, Y.; Xue, L.; Song, J.; Luo, Z.; Hong, X.; Wang, J.; Liang, W.; et al. Revealing underlying regulatory mechanisms of LINC00313 in Osimertinib-resistant LUAD cells by ceRNA network analysis. Transl. Oncol. 2024, 43, 101895. [Google Scholar] [CrossRef]
- Asada, H.; Tani, A.; Sakuma, H.; Hirabayashi, M.; Matsumoto, Y.; Watanabe, K.; Tsuboi, M.; Yoshida, S.; Harada, K.; Uchikai, T.; et al. Whole exome and transcriptome analysis revealed the activation of ERK and Akt signaling pathway in canine histiocytic sarcoma. Sci. Rep. 2023, 13, 8512. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, P.; Jin, M.; Huang, S.; Chen, H.; Chen, L.; Yang, J.; Su, N. Fgfr1 deficiency in osteocytes leads to increased bone mass by enhancing wnt/β-catenin signaling. Bone 2023, 174, 116817. [Google Scholar] [CrossRef]
- Xu, J.; Ze, X.; Zhao, L.; Sheng, L.; Ze, Y. Titanium dioxide nanoparticles oral exposure induce osteoblast apoptosis, inhibit osteogenic ability and increase lipogenesis in mouse. Ecotoxicol. Environ. Saf. 2024, 277, 116367. [Google Scholar] [CrossRef]
- Park, K.C.; Kwon, Y.; Lee, Y.; Kim, D.K.; Jang, Y.; Lee, S. Low selenium levels are associated with decreased bone mineral densities. J. Trace Elem. Med. Biol. 2020, 61, 126534. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, A.B.; Eren, B.; Sağir, D.; Yilmaz, B.D. Inhibition of acrolein-induced apoptosis by the antioxidant selenium. Toxicol. Ind. Health 2020, 36, 84–92. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Peng, R.; Dong, Y.; Zheng, M.; Kang, H.; Wang, P.; Zhu, M.; Song, K.; Wu, W.; Li, F. Il-17 promotes osteoclast-induced bone loss by regulating glutamine-dependent energy metabolism. Cell Death Dis. 2024, 15, 111. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, W.; Tang, C.Y.; McVicar, A.; Edwards, D.; Wang, J.; McConnell, M.; Yang, S.; Li, Y.; Chang, Z.; et al. Knockout and double knockout of cathepsin k and Mmp9 reveals a novel function of cathepsin k as a regulator of osteoclast gene expression and bone homeostasis. Int. J. Biol. Sci. 2022, 18, 5522–5538. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Scapolan, M.; Pecolo, M.; Wassermann, B.; Abu-Rumeileh, I.; Balestreri, L.; Borsatti, E.; Tripodo, C.; Colombatti, A.; Spessotto, P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011, 13, R105. [Google Scholar] [CrossRef]
- Behrends, M.; Wagner, S.; Kopka, K.; Schober, O.; Schäfers, M.; Kumbhar, S.; Waller, M.; Haufe, G. New matrix metalloproteinase inhibitors based on γ-fluorinated α-aminocarboxylic and α-aminohydroxamic acids. Bioorg Med. Chem. 2015, 23, 3809–3818. [Google Scholar] [CrossRef]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. Bmp signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, B. Chemokines in cartilage regeneration and degradation: New insights. Int. J. Mol. Sci. 2023, 25, 381. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shi, R.; Zhang, G.; Wang, Y.; Ye, L.; Peng, L.; Guo, S.; He, J.; Yang, H.; Jiang, Y. miR-539-5p targets BMP2 to regulate Treg activation in B-cell acute lymphoblastic leukemia through TGF-β/Smads/MAPK. Exp. Biol. Med. 2024, 249, 10111. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Habib, R.; Shafiq, S.; Abbas, S.S.; Khan, S.; Eqani, S.; Nepovimova, E.; Khan, M.S.; Kuca, K.; Nurulain, S.M. Influence of the chronic groundwater fluoride consumption on cholinergic enzymes, ACHE and BCHE gene SNPs and pro-inflammatory cytokines: A study with Pakistani population groups. Sci. Total Environ. 2023, 880, 163359. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ha, S.; Xu, L.; Liu, C.; Liu, Y.; Wu, X.; Li, Z.; Wu, S.; Yang, B.; Chen, Z. Fluorinated hydroxyapatite conditions a favorable osteo-immune microenvironment via triggering metabolic shift from glycolysis to oxidative phosphorylation. J. Transl. Med. 2024, 22, 437. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xia, B.; Mai, S.; Feng, Z.; Wang, X.; Liu, Y.; Liu, R.; Li, Z.; Xiao, Y.; Chen, Z.; et al. Sodium fluoride under dose range of 2.4-24 μM, a promising osteoimmunomodulatory agent for vascularized bone formation. ACS Biomater. Sci. Eng. 2019, 5, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Sun, K.Y.; Zhu, Y.; Zhang, X.; Zhou, Y.H.; Zou, X. Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt. Cell Death Dis. 2021, 12, 934. [Google Scholar] [CrossRef]
- Christodoulou, M.; Aspray, T.J.; Piec, I.; Fraser, W.D.; Schoenmakers, I. Alterations in regulators of the renal-bone axis, inflammation and iron status in older people with early renal impairment and the effect of vitamin d supplementation. Age Ageing 2024, 53, afae096. [Google Scholar] [CrossRef]
- Éva Sikura, K.; Combi, Z.; Potor, L.; Szerafin, T.; Hendrik, Z.; Méhes, G.; Gergely, P.; Whiteman, M.; Beke, L.; Fürtös, I.; et al. Hydrogen sulfide inhibits aortic valve calcification in heart via regulating runx2 by nf-κb, a link between inflammation and mineralization. J. Adv. Res. 2021, 27, 165–176. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, F.; Liu, K.; Xin, J.; Chen, J. HMGB1 could restrict 1,3-β-glucan induced mice lung inflammation by affecting Beclin1 and Bcl2 interaction and promoting the autophagy of epithelial cells. Ecotoxicol. Environ. Saf. 2021, 222, 112460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, T.; Luo, Q.; Chen, Y.; Leung, V.Y.; Wen, C.; Shah, M.F.; Pan, H.; Chiu, K.; Cao, X.; et al. Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-β signaling. J. Orthop. Res. 2016, 34, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.E.; Lee, S.B.; Lee, N.Y.; Park, S.Y. Gulp1 regulates chondrocyte growth arrest and differentiation via the TGF-β/Smad2/3 pathway. FEBS Lett. 2024, 598, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Gyemi, L.; Selznick, A.; Petrisor, B.; Ghert, M. Time to full weight-bearing with the use of a calcium sulfate-calcium phosphate bone substitute as a bone void filler following extended curettage in the treatment of primary benign bone tumours. J. Orthop. Surg. 2024, 32, 10225536241254200. [Google Scholar] [CrossRef] [PubMed]

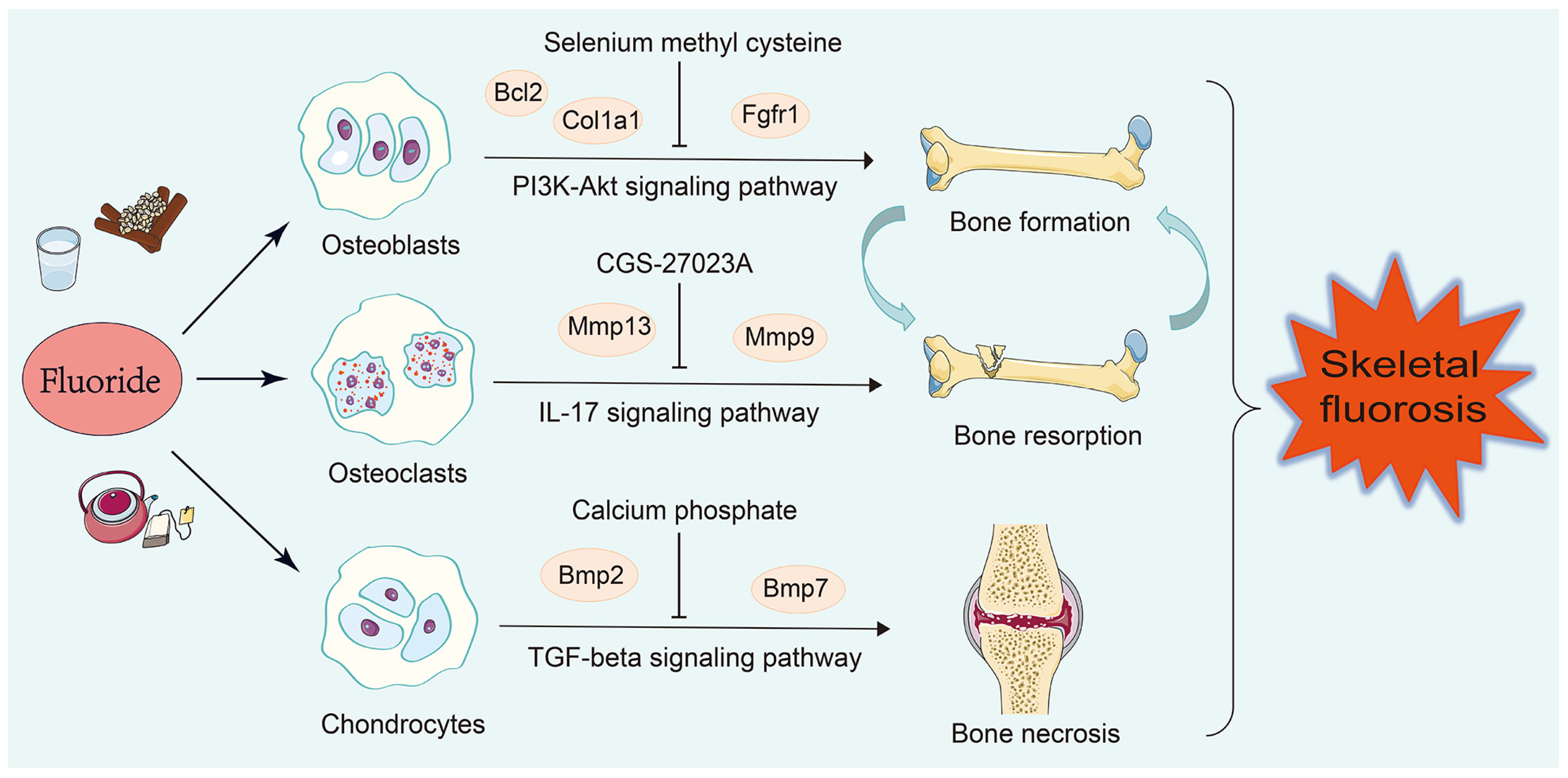
| Cell Type | Literature Number | Genes | Gene Number |
|---|---|---|---|
| Osteoblasts | 63 [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86] | Acetyl-p53, Acsl3, Akt, Akt1, Aloxe3, Alp, Atf4, Atf6, Atg5, Atg7, Bmp2, Bax, Bcl2, Beclin 1, Bfgf, Bgp, Bid, Bip, Bnip3, Bsp, CaM, CaN, Caspase-3, Casr, Cbfa1, Cdo1, c-fos, c-jun, C-myc, Col, Col I, Col1a1, Ct, Cthrc1, CyclinD1, Cyt C, Dlx5, Dnmt1, Duox1, Dv1, Enpp2, Erk, Esp, Fgf21, Fgfr1, Foxo1, Foxo3, Gli1, Gsk3β, Gsto1, Ho-1, Igf1, Ihh, Il6, iNOS, Insr, Ire1, Jag1, Jnk, Klb, Lc3, Lpin1, Lrp, Lrp5, Mapk, Mcm3, Mek, Mtor, Notch3, Nqo1, Nrf2, Ocn, Opg, Opn, Osx, p16, p21, p38, p53, p62, Pdgf, Perk, Pi3k, Pth, Pth-rp, Rab7, Raf, Rankl, Ras, Rbpj, Runx2, Sema4d, Shh, Sirt1, Slc7a11, Smad1, Smad2, Smad3, Smad4, Smo, Tet2, Tfrc, Tgf-β1, Tgf-β, Tm9sf1, Twist, Tβr2, Wnt10, Wnt3α, Xbp-1, Yap, β-catenin | 112 |
| Osteoclasts | 16 [44,56,59,62,72,74,83,87,88,89,90,91,92,93,94,95] | β-catenin, Acp5, Akt, Alp, Atp6v0d2, Bgp, Cathepsin K, Col1α1, c-Src, Ctsk, Dcst1, Erα, Ifnγ, Mitf, Mmp13, Mmp9, Mtor, Nfatc1, Nf-κb1, Opg, Oscar, Osterix, Rank, Rankl, Rcthrc1, Runx2, Sema4d, Smad3, Tgf-β1, Timp-1, Tracp, Traf-6, Trap, Tβr1, Ulk1 | 35 |
| Chondrocytes | 14 [44,96,97,98,99,100,101,102,103,104,105,106,107,108] | 4Ebp1, Acan, Aggrecan, Akt, Bax, Bcl2, Beclin1, Bmp2, Bmp7, Caspase14, Cleaved caspase 12, Cleaved caspase 3, Cleaved caspase 9, Col II, Col X, Col2a1, Colixa3, Hif-1α, Ihh, Il6, Lc3, Mig-6, Mmp13, Mtor, Opg, P62, Pcna, P-egfr, Pi3k, P-lats1/2, P-mst1/2, Ps6, P-thrp, Runx2, S6k1, Shh, Smo, Sox9, Vegf, Yap1, β-catenin | 41 |
| Cell Type | Candidate Drugs |
|---|---|
| Osteoblasts | Pamidronate (CTD 00000975), Geranylgeranyl pyrophosphate (CTD 00000102), Selenium methyl cysteine (CTD 00000103), Cyclohexanecarboxamide (CTD 00003513), Paricalcitol (CTD 00003033) |
| Osteoclasts | Ilomastat (TTD 00008545), CGS-27023A (TTD 00002801), CHEMBL475540 (TTD 00006054), doxycycline (CTD 00005875), Toluidine Blue O (BOSS) |
| Chondrocytes | Octreotide (CTD 00007059), Stannic fluoride (BOSS), Calcium phosphate (BOSS), Heparitin (BOSS), TITANIUM (BOSS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Luo, K.; Sha, T.; Li, Q.; Dong, Z.; Dou, Y.; Zhang, H.; Zhou, G.; Ba, Y.; Yu, F. Apoptosis and Inflammation Involved with Fluoride-Induced Bone Injuries. Nutrients 2024, 16, 2500. https://doi.org/10.3390/nu16152500
Wang M, Luo K, Sha T, Li Q, Dong Z, Dou Y, Zhang H, Zhou G, Ba Y, Yu F. Apoptosis and Inflammation Involved with Fluoride-Induced Bone Injuries. Nutrients. 2024; 16(15):2500. https://doi.org/10.3390/nu16152500
Chicago/Turabian StyleWang, Miao, Kangting Luo, Tongtong Sha, Qian Li, Zaichao Dong, Yanjie Dou, Huanxia Zhang, Guoyu Zhou, Yue Ba, and Fangfang Yu. 2024. "Apoptosis and Inflammation Involved with Fluoride-Induced Bone Injuries" Nutrients 16, no. 15: 2500. https://doi.org/10.3390/nu16152500





