Potential Human Health Benefits of Phaseolus vulgaris L. var Venanzio: Effects on Cancer Cell Growth and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of P. vulgaris var. Venanzio (FV) Extracts
2.2. Analysis of Polyphenolic
2.3. Cell Culture
2.4. MTT Assay
2.5. Western Blotting Analysis
2.6. Clonogenic Assay
2.7. Trypan Blue Assay
2.8. Immunofluorescence Assay
2.9. Annexin V-FITC Staining
2.10. Senescence Assay
2.11. Determination of Reactive Oxygen Species (ROS)
2.12. Determination of PGE2 by ELISA
2.13. RT-PCR
2.14. Statistical Analysis
3. Results
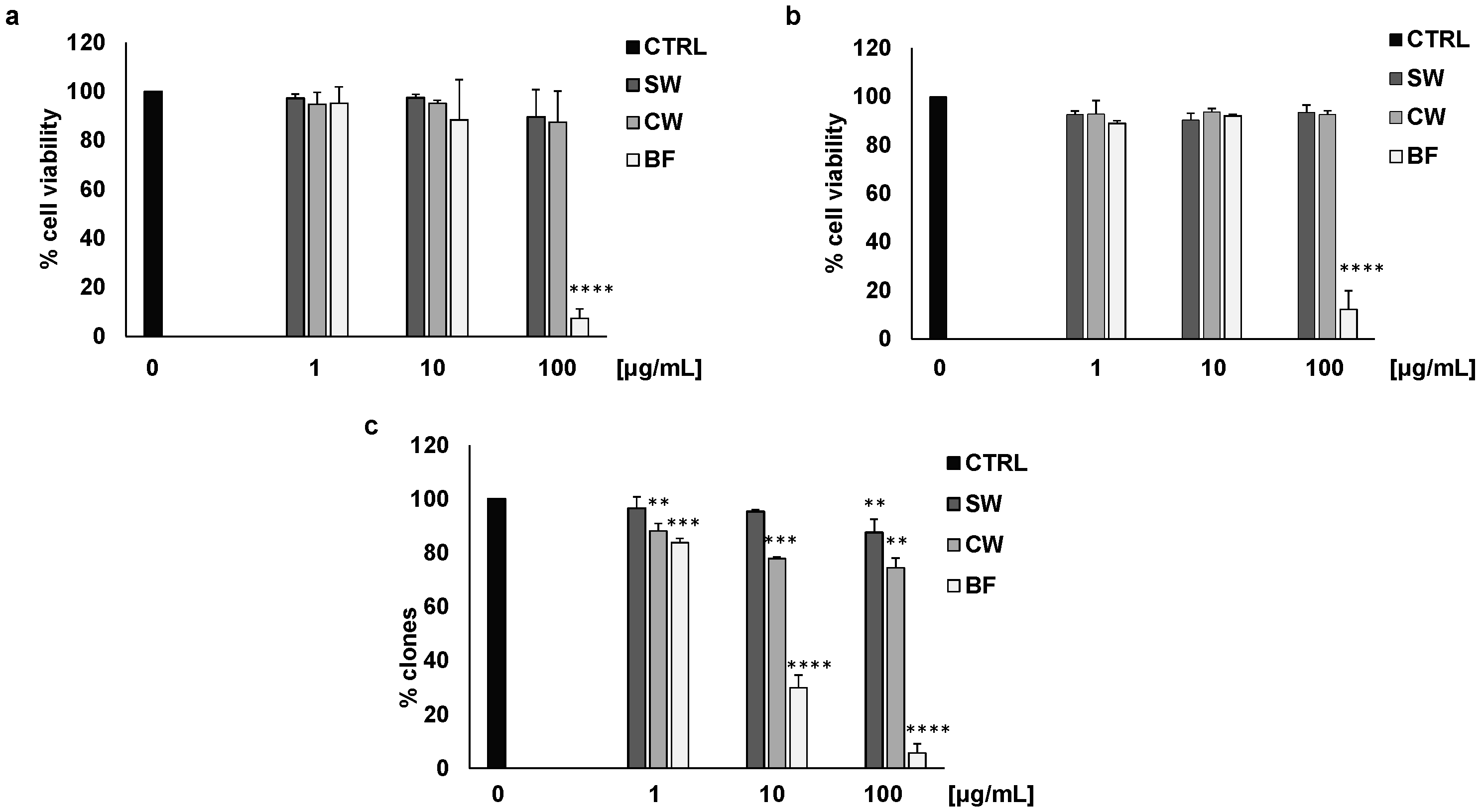
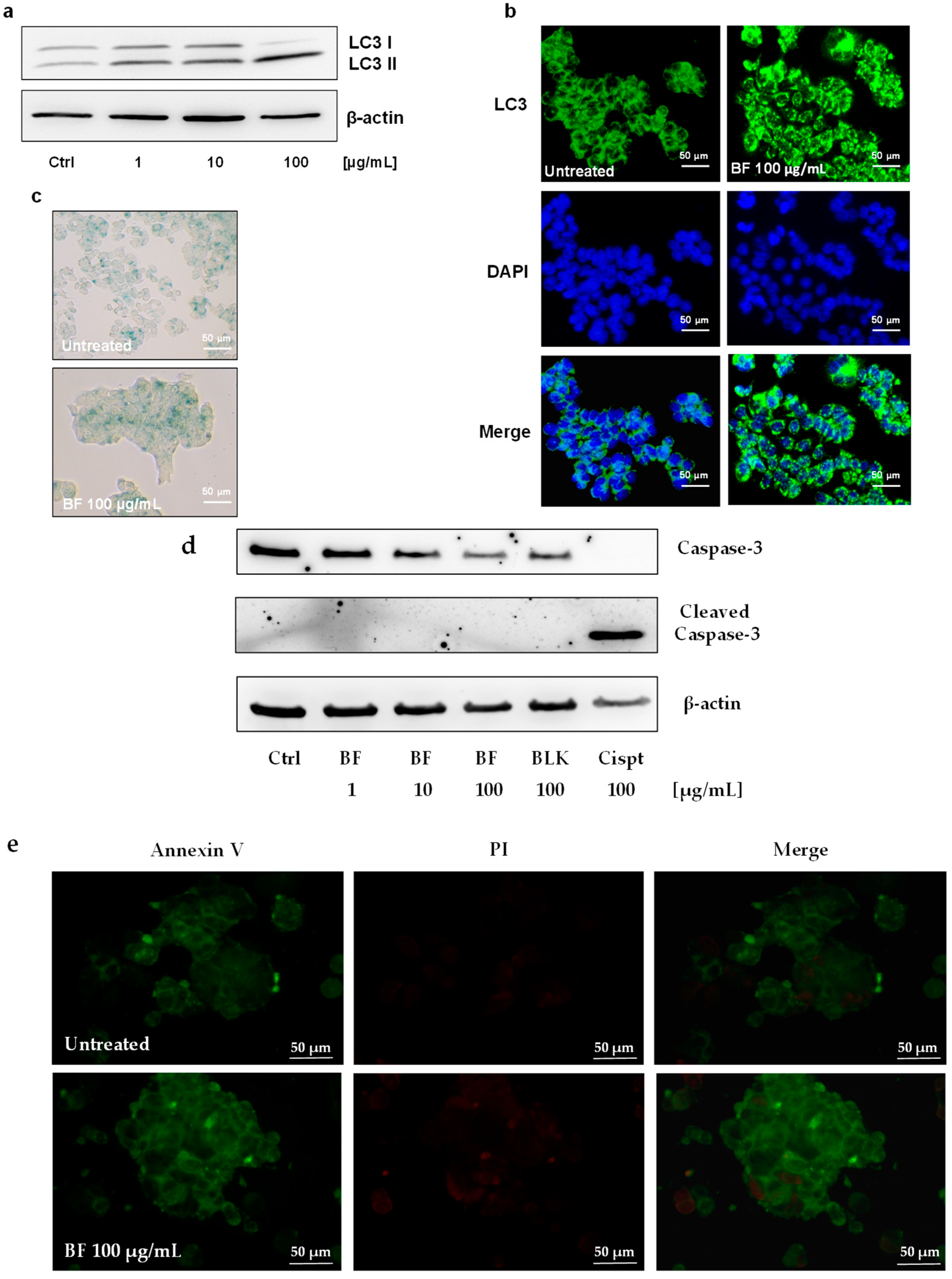
3.1. Activity of P. vulgaris Extracts on Colon Cancer Cells
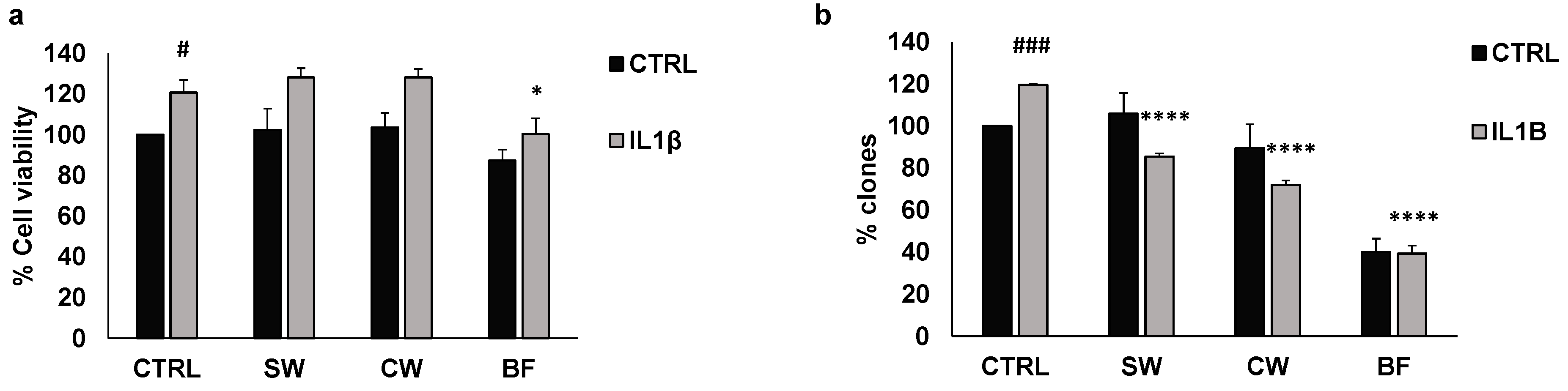
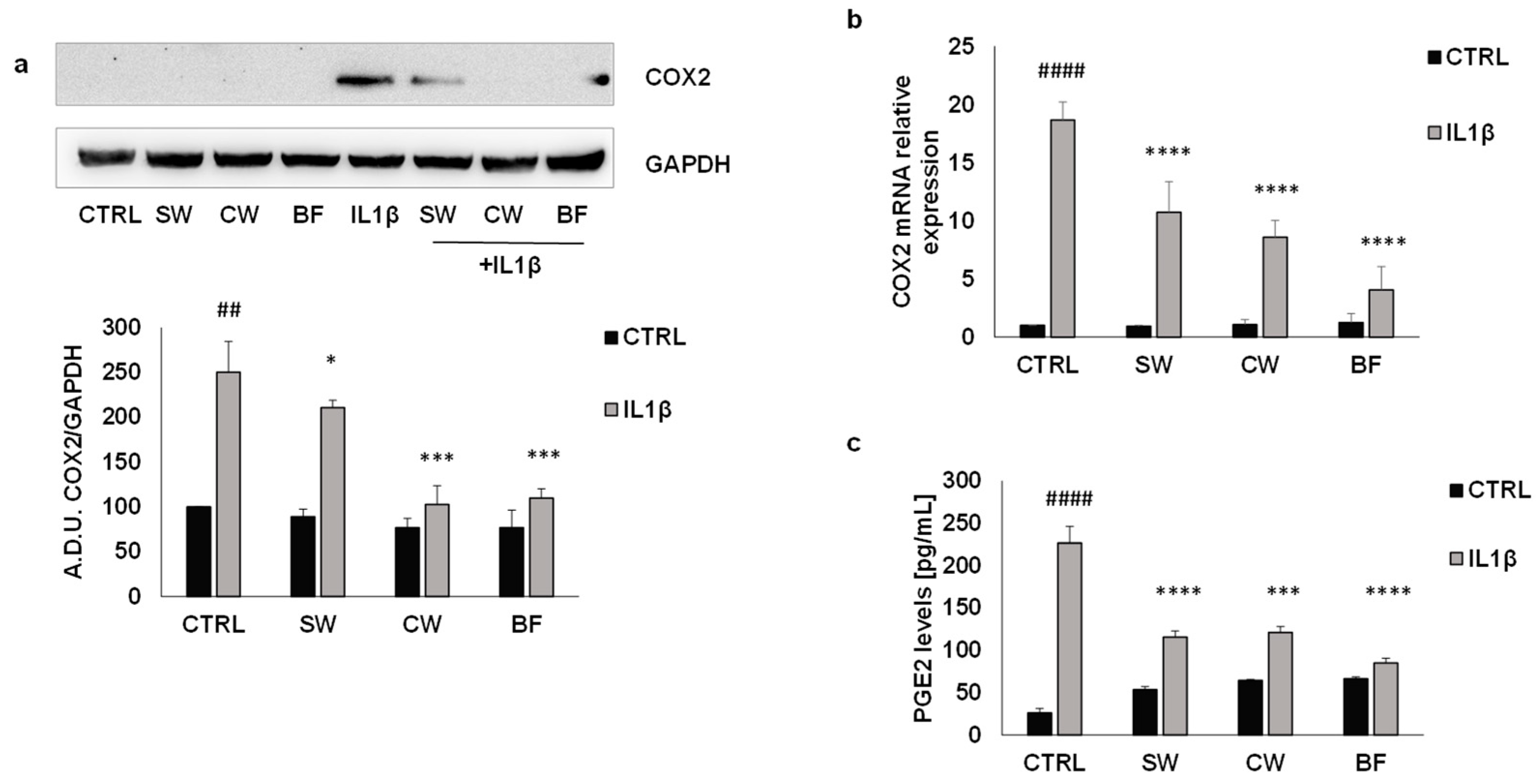
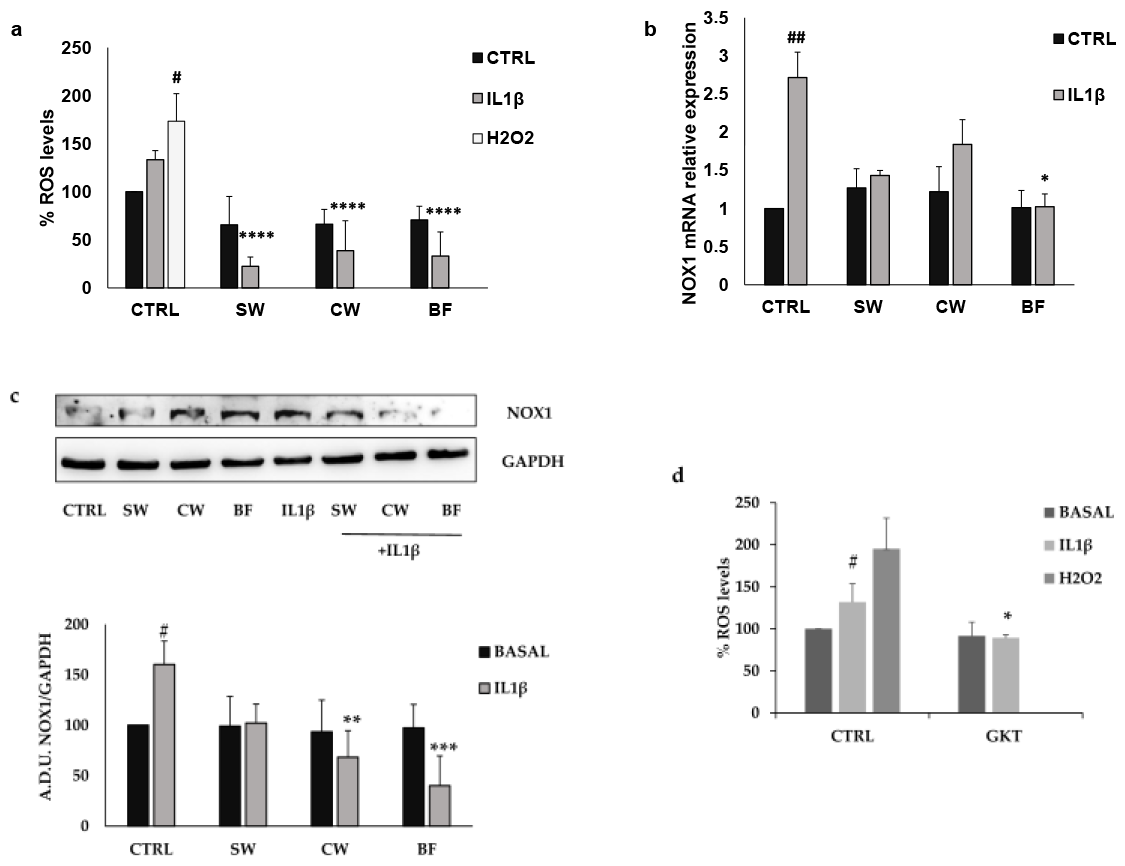
3.2. Effects of P. vulgaris Extracts on Inflammation and Oxidative Stress Promoted by Interleukin 1β
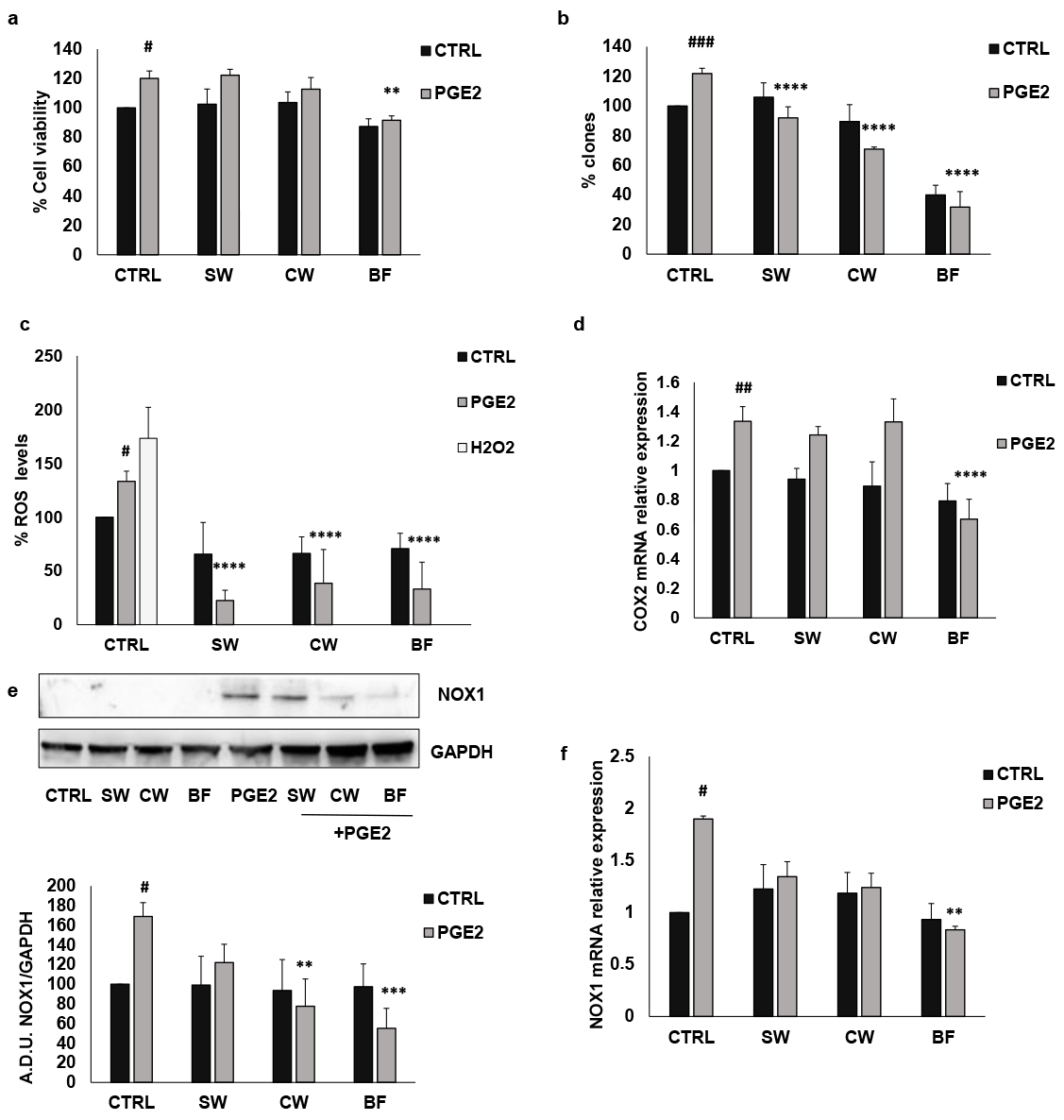
3.3. P. vulgaris Extracts Inhibit IL1β Effects in Colon Cancer Cells Amplified by PGE2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado, A.; Gonçalves, S.; Romano, A. Mediterranean Diet: The Role of Phenolic Compounds from Aromatic Plant Foods. Foods 2023, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Biagi, M.; Ercoli, J.; Macrì, G.; Miraldi, E.; Trabalzini, L. Phaseolus vulgaris L. var. Venanzio Grown in Tuscany: Chemical Composition and in Vitro Investigation of Potential Effects on Colorectal Cancer. Antioxidants 2020, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Han, W.; Yan, F.; Xu, D.; Chu, Q.; Zheng, X. Dietary Phaseolus vulgaris Extract Alleviated Diet-Induced Obesity, Insulin Resistance and Hepatic Steatosis and Alters Gut Microbiota Composition in Mice. J. Funct. Foods 2016, 20, 236–244. [Google Scholar] [CrossRef]
- Peddio, S.; Padiglia, A.; Cannea, F.B.; Crnjar, R.; Zam, W.; Sharifi-Rad, J.; Rescigno, A.; Zucca, P. Common Bean (Phaseolus vulgaris L.) α-Amylase Inhibitors as Safe Nutraceutical Strategy against Diabetes and Obesity: An Update Review. Phytother. Res. 2022, 36, 2803–2823. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, L.K.; Pushkarev, V.M. Prevention and Treatment of Diabetes Mellitus with Bioactive Preparations of Common Beans (Phaseolus vulgaris L.). Endokrynologia 2022, 27, 341–358. [Google Scholar] [CrossRef]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases—Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, C.; Macrì, G.; Biagi, M.; Miraldi, E.; Finetti, F.; Trabalzini, L. In Vitro Digestion of Phaseolus vulgaris L. Cooked Beans Induces Autophagy in Colon Cancer Cells. Foods 2023, 12, 839. [Google Scholar] [CrossRef]
- Correa, P. Epidemiological Correlations between Diet and Cancer Frequency. Cancer Res. 1981, 41, 3685–3689. [Google Scholar]
- Feregrino-Perez, A.A.; Piñol-Felis, C.; Gomez-Arbones, X.; Guevara-González, R.G.; Campos-Vega, R.; Acosta-Gallegos, J.; Loarca-Piña, G. A Non-Digestible Fraction of the Common Bean (Phaseolus vulgaris L.) Induces Cell Cycle Arrest and Apoptosis during Early Carcinogenesis. Plant Foods Hum. Nutr. 2014, 69, 248–254. [Google Scholar] [CrossRef]
- Vergara-Castañeda, H.A.; Guevara-González, R.G.; Ramos-Gómez, M.; Reynoso-Camacho, R.; Guzmán-Maldonado, H.; Feregrino-Pérez, A.A.; Oomah, B.D.; Loarca-Piña, G. Non-Digestible Fraction of Cooked Bean (Phaseolus vulgaris L.) Cultivar Bayo Madero Suppresses Colonic Aberrant Crypt Foci in Azoxymethane-Induced Rats. Food Funct. 2010, 1, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and Chemopreventive Effect of Polysaccharides from Common Beans (Phaseolus vulgaris L.) on Azoxymethane-Induced Colon Cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Thompson, H.J. Physiological Effects of Bean (Phaseolus vulgaris L.) Consumption on Cellular Signaling in Cancer. Cell Cycle 2012, 11, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, Å.M.; Margareta, E.; Nyman, G.L.; Björck, I.M.E. Content of Short-Chain Fatty Acids in the Hindgut of Rats Fed Processed Bean (Phaseolus vulgaris) Flours Varying in Distribution and Content of Indigestible Carbohydrates. Br. J. Nutr. 2001, 86, 379–389. [Google Scholar] [CrossRef]
- Finley, J.W.; Burrell, J.B.; Reeves, P.G. Pinto Bean Consumption Changes SCFA Profiles in Fecal Fermentations, Bacterial Populations of the Lower Bowel, and Lipid Profiles in Blood of Humans. J. Nutr. 2007, 137, 2391–2398. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Role of Flavonoids in Intestinal Tight Junction Regulation. J. Nutr. Biochem. 2011, 22, 401–408. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-Regulating the Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, and/or Fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Ji, G.E.; Sung, M.-K. Dietary Kaempferol Suppresses Inflammation of Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2012, 57, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Romier, B.; Schneider, Y.-J.; Larondelle, Y.; During, A. Dietary Polyphenols Can Modulate the Intestinal Inflammatory Response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The Beneficial Effect of Vanillic Acid on Ulcerative Colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Sung, M.K.; Park, M.Y. Nutritional Modulators of Ulcerative Colitis: Clinical Efficacies and Mechanistic View. World J. Gastroenterol. 2013, 19, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, J.-Y.; Liu, L.; Li, Y.-X.; Xun, A.-Y.; Zeng, W.-S.; Jia, C.-H.; Wei, X.-X.; Feng, J.-L.; Zhao, L.; et al. Anti-Oxidant Effects of Resveratrol on Mice with DSS-Induced Ulcerative Colitis. Arch. Med. Res. 2010, 41, 288–294. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Itzkowitz, S.H. Cancers Complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Grivennikov, S.I. Inflammation and Colorectal Cancer: Colitis-Associated Neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef]
- Bogweh, N.E.; Ageyo, O.C. Do Common Beans (Phaseolus vulgaris L.) Promote Good Health in Humans? A Systematic Review and Meta-Analysis of Clinical and Randomized Controlled Trials. Nutrients 2021, 13, 3701. [Google Scholar] [CrossRef]
- Deneo-Pellegrini, H.; Boffetta, P.; De Stefani, E.; Ronco, A.; Brennan, P.; Mendilaharsu, M. Plant Foods and Differences between Colon and Rectal Cancers. Eur. J. Cancer Prev. 2002, 11, 369–375. [Google Scholar] [CrossRef]
- Haydé, V.-C.; Ramón, G.-G.; Lorenzo, G.-O.; Dave, O.B.; Rosalía, R.-C.; Paul, W.; Guadalupe, L.-P. Non-Digestible Fraction of Beans (Phaseolus vulgaris L.) Modulates Signalling Pathway Genes at an Early Stage of Colon Cancer in Sprague–Dawley Rats. Br. J. Nutr. 2012, 108, S145–S154. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.S.; Ganthavorn, C.; Wilson-Sanders, S. Dry Beans Inhibit Azoxymethane-Induced Colon Carcinogenesis in F344 Rats. J. Nutr. 1997, 127, 2328–2333. [Google Scholar] [CrossRef]
- Wilson, J.C.; Anderson, L.A.; Murray, L.J.; Hughes, C.M. Non-Steroidal Anti-Inflammatory Drug and Aspirin Use and the Risk of Head and Neck Cancer: A Systematic Review. Cancer Causes Control 2011, 22, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; D’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic Composition and Antioxidant and Antiproliferative Activities of the Extracts of Twelve Common Bean (Phaseolus vulgaris L.) Endemic Ecotypes of Southern Italy before and after Cooking. Oxidative Med. Cell. Longev. 2016, 2016, 1398298. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Qin, X.; Li, Z. Revealing the Anti-Melanoma Mechanism of n-BuOH Fraction from the Red Kidney Bean Coat Extract Based on Network Pharmacology and Transcriptomic Approach. Food Res. Int. 2021, 140, 109880. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Celis, U.; López-Martínez, F.J.; Cervantes-Jiménez, R.; Ferríz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Tepary Bean (Phaseolus acutifolius) Lectins Induce Apoptosis and Cell Arrest in G0/G1 by P53(Ser46) Phosphorylation in Colon Cancer Cells. Molecules 2020, 25, 1021. [Google Scholar] [CrossRef]
- Moreno-Jiménez, M.R.; López-Barraza, R.; Cervantes-Cardoza, V.; Pérez-Ramírez, I.F.; Reyna-Rojas, J.A.; Gallegos-Infante, J.A.; Estrella, I.; Rojas-Contreras, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E. Mechanisms Associated to Apoptosis of Cancer Cells by Phenolic Extracts from Two Canned Common Beans Varieties (Phaseolus vulgaris L.). J. Food Biochem. 2019, 43, e12680. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M. Effects of in Vitro Simulated Digestion on the Antioxidant Activity of Different Camellia sinensis (L.) Kuntze Leaves Extracts. Eur. Food Res. Technol. 2022, 248, 119–128. [Google Scholar] [CrossRef]
- Finetti, F.; Moglia, A.; Schiavo, I.; Donnini, S.; Berta, G.N.; Di Scipio, F.; Perrelli, A.; Fornelli, C.; Trabalzini, L.; Retta, S.F. Yeast-Derived Recombinant Avenanthramides Inhibit Proliferation, Migration and Epithelial Mesenchymal Transition of Colon Cancer Cells. Nutrients 2018, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Trabalzini, L.; Ercoli, J.; Trezza, A.; Schiavo, I.; Macrì, G.; Moglia, A.; Spiga, O.; Finetti, F. Pharmacological and In Silico Analysis of Oat Avenanthramides as EGFR Inhibitors: Effects on EGF-Induced Lung Cancer Cell Growth and Migration. Int. J. Mol. Sci. 2022, 23, 8534. [Google Scholar] [CrossRef]
- Palumberi, D.; Aldi, S.; Ermini, L.; Ziche, M.; Finetti, F.; Donnini, S.; Rosati, F. RNA-Mediated Gene Silencing of FUT1 and FUT2 Influences Expression and Activities of Bovine and Human Fucosylated Nucleolin and Inhibits Cell Adhesion and Proliferation. J. Cell. Biochem. 2010, 111, 229–238. [Google Scholar] [CrossRef]
- Finetti, F.; Schiavo, I.; Ercoli, J.; Zotta, A.; Boda, E.; Retta, S.F.; Trabalzini, L. KRIT1 Loss-Mediated Upregulation of NOX1 in Stromal Cells Promotes Paracrine pro-Angiogenic Responses. Cell. Signal. 2020, 68, 109527. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin, X.; You, L.; Qiao, J.; Han, W.; Pan, H. Autophagy in Colitis-Associated Colon Cancer: Exploring Its Potential Role in Reducing Initiation and Preventing IBD-Related CAC Development. Autophagy 2023, 20, 242–258. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Tomic, I.J.; Krnic, M.; Ticinovic Kurir, T.; Bozic, J. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients 2022, 14, 757. [Google Scholar] [CrossRef]
- Gubatan, J.; Kulkarni, C.V.; Talamantes, S.M.; Temby, M.; Fardeen, T.; Sinha, S.R. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients 2023, 15, 579. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Paradisi, L.; Bernardi, C.; Pannini, M.; Trabalzini, L. Cooperation between Prostaglandin E2 and Epidermal Growth Factor Receptor in Cancer Progression: A Dual Target for Cancer Therapy. Cancers 2023, 15, 2374. [Google Scholar] [CrossRef]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dashwood, W.M.; Nian, H.; Löhr, C.V.; Fischer, K.A.; Tsuchiya, N.; Nakagama, H.; Ashktorab, H.; Dashwood, R.H. NADPH Oxidase Overexpression in Human Colon Cancers and Rat Colon Tumors Induced by 2-Amino-1-Methyl-6-Phenylimidazo[4,5-b]Pyridine (PhIP). Int. J. Cancer 2011, 128, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Konaté, M.M.; Antony, S.; Doroshow, J.H. Inhibiting the Activity of NADPH Oxidase in Cancer. Antioxid. Redox Signal. 2020, 33, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Echizen, K.; Oshima, H.; Nakayama, M.; Oshima, M. The Inflammatory Microenvironment That Promotes Gastrointestinal Cancer Development and Invasion. Adv. Biol. Regul. 2018, 68, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, M.; Abbasi, A.; Hassan, Z.M.; Pakravan, N. The Intrinsic and Extrinsic Elements Regulating Inflammation. Life Sci. 2020, 260, 118258. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yuan, Y.; Zhang, X.; Feng, Z.; Liu, C. Separation and Enrichment of Lectin from Zihua Snap-Bean (Phaseolus vulgaris) Seeds by PEG 600–Ammonium Sulfate Aqueous Two-Phase System. Molecules 2017, 22, 1596. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejía, E.G. Characterization and Comparison of Protein and Peptide Profiles and Their Biological Activities of Improved Common Bean Cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum. Nutr. 2015, 70, 105–112. [Google Scholar] [CrossRef]
- Mojica, L.; De Mejía, E.G. Optimization of Enzymatic Production of Anti-Diabetic Peptides from Black Bean (Phaseolus vulgaris L.) Proteins, Their Characterization and Biological Potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Luna-Vital, D.A.; González de Mejía, E. Characterization of Peptides from Common Bean Protein Isolates and Their Potential to Inhibit Markers of Type-2 Diabetes, Hypertension and Oxidative Stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef] [PubMed]

| Components | CW | BF |
|---|---|---|
| Total polyphenols | 0.84 ± 0.01 | n.d. |
| Total hydroxycinnamic derivatives | 0.24 ± 0.01 | 0.11 ± 0.01 |
| Gallic acid | 0.41 ± 0.02 | n.d. |
| Chlorogenic acid | 0.03 ± 0.01 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, C.; Cappellucci, G.; Baini, G.; Aloisi, A.M.; Finetti, F.; Trabalzini, L. Potential Human Health Benefits of Phaseolus vulgaris L. var Venanzio: Effects on Cancer Cell Growth and Inflammation. Nutrients 2024, 16, 2534. https://doi.org/10.3390/nu16152534
Bernardi C, Cappellucci G, Baini G, Aloisi AM, Finetti F, Trabalzini L. Potential Human Health Benefits of Phaseolus vulgaris L. var Venanzio: Effects on Cancer Cell Growth and Inflammation. Nutrients. 2024; 16(15):2534. https://doi.org/10.3390/nu16152534
Chicago/Turabian StyleBernardi, Clizia, Giorgio Cappellucci, Giulia Baini, Anna Maria Aloisi, Federica Finetti, and Lorenza Trabalzini. 2024. "Potential Human Health Benefits of Phaseolus vulgaris L. var Venanzio: Effects on Cancer Cell Growth and Inflammation" Nutrients 16, no. 15: 2534. https://doi.org/10.3390/nu16152534
APA StyleBernardi, C., Cappellucci, G., Baini, G., Aloisi, A. M., Finetti, F., & Trabalzini, L. (2024). Potential Human Health Benefits of Phaseolus vulgaris L. var Venanzio: Effects on Cancer Cell Growth and Inflammation. Nutrients, 16(15), 2534. https://doi.org/10.3390/nu16152534








