Oral Supplementation of L-Carnosine Attenuates Acute-Stress-Induced Corticosterone Release and Mitigates Anxiety in CD157 Knockout Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Acute Stress
2.3. Plasma Sampling and Enzymatic Detection of Corticosterone
2.4. Enzyme Immunoassay of Corticosterone
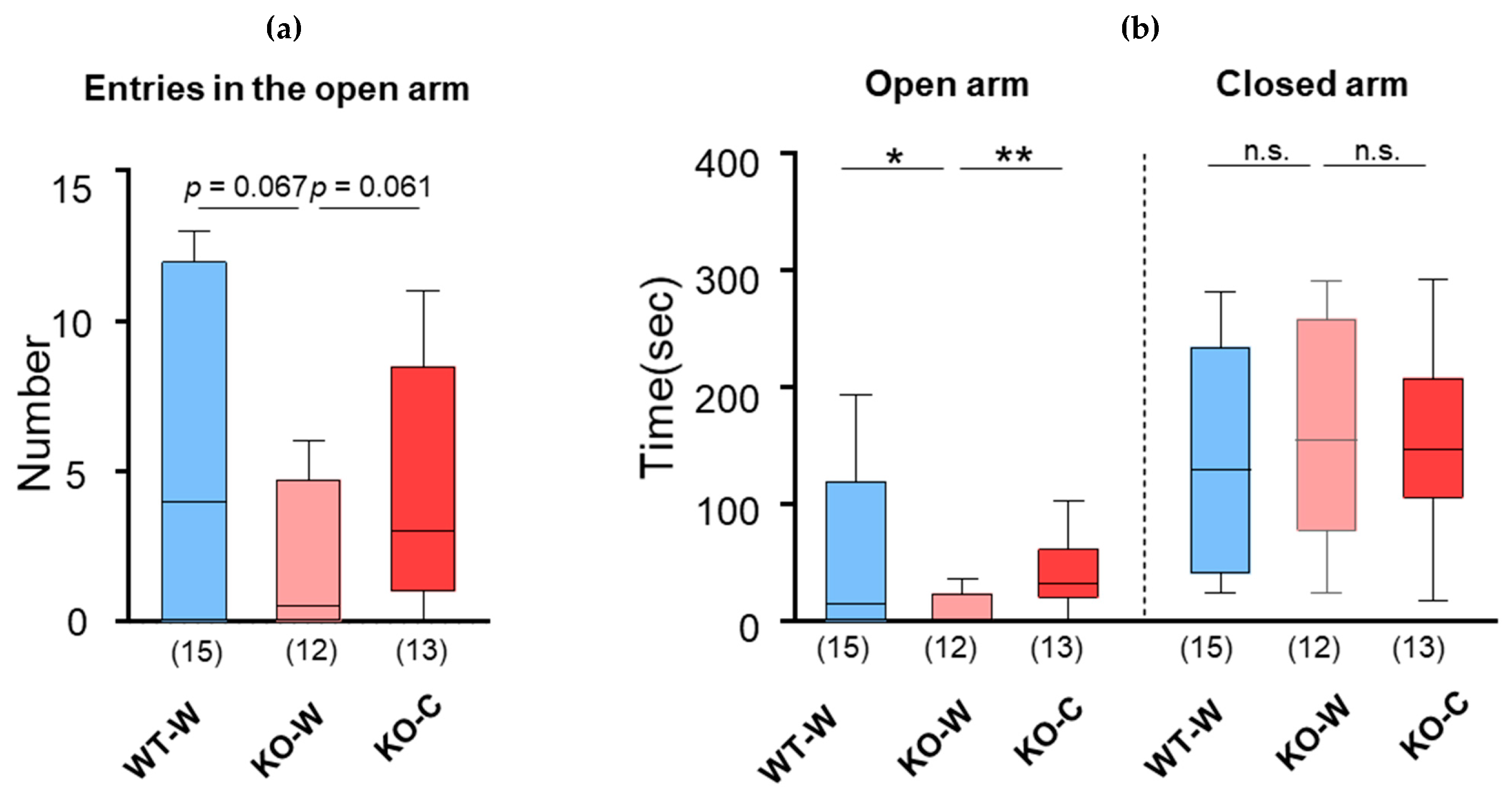
2.5. Elevated Plus Maze
2.6. Statistical Analysis
3. Results
L-Carnosine Mitigated Forced Swimming or Restraint-Stress-Induced Elevation in Plasma Corticosterone Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, V.K.; Vaishnaw, A.; Shirbhate, E.; Kore, R.; Singh, V.; Veerasamy, R.; Rajak, H. Cortisol as a Target for Treating Mental Disorders: A Promising Avenue for Therapy. Mini Rev. Med. Chem. 2024, 24, 588–600. [Google Scholar] [CrossRef]
- Toyoda, A. Nutritional interventions for promoting stress resilience: Recent progress using psychosocial stress models of rodents. Anim. Sci. J. 2020, 91, e13478. [Google Scholar] [CrossRef]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef]
- Davey, C.L. The significance of carnosine and anserine in striated skeletal muscle. Arch. Biochem. Biophys. 1960, 89, 303–308. [Google Scholar] [CrossRef]
- Baek, S.H.; Noh, A.R.; Kim, K.A.; Akram, M.; Shin, Y.J.; Kim, E.S.; Yu, S.W.; Majid, A.; Bae, O.N. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 2014, 45, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef]
- Abraham, D.A.; Undela, K.; Narasimhan, U.; Rajanandh, M.G. Effect of L-Carnosine in children with autism spectrum disorders: A systematic review and meta-analysis of randomised controlled trials. Amino Acids 2021, 53, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Khoaie-Ardakani, M.R.; Shahmoradi, Z.; Alavi, A.R.; Afarideh, M.; Shalbafan, M.R.; Ghazizadeh-Hashemi, M.; Akhondzadeh, S. L-carnosine as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: A double-blind, randomized placebo-controlled trial. Psychiatry Res. 2018, 262, 94–101. [Google Scholar] [CrossRef]
- Tharoor, H.; Maran, S.; Chandan, A.K.; Pari, M.; Rao, S.; Durairaj, J. Cognitive and negative symptoms in schizophrenia with L-Carnosine adjuvant therapy—A randomized double-blind placebo-controlled study. Pharmacol. Res. Perspect. 2023, 11, e01074. [Google Scholar] [CrossRef]
- Ding, Q.; Tanigawa, K.; Kaneko, J.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Preserves Blood Flow in the Prefrontal Brain of Elderly People Carrying APOE e4. Aging Dis. 2018, 9, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Ghajar, A.; Aghajan-Nashtaei, F.; Afarideh, M.; Mohammadi, M.R.; Akhondzadeh, S. l-Carnosine as Adjunctive Therapy in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Child. Adolesc. Psychopharmacol. 2018, 28, 331–338. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine treatment for gulf war illness: A randomized controlled trial. Glob. J. Health Sci. 2013, 5, 69–81. [Google Scholar] [CrossRef]
- Li, Y.F.; He, R.R.; Tsoi, B.; Li, X.D.; Li, W.X.; Abe, K.; Kurihara, H. Anti-stress effects of carnosine on restraint-evoked immunocompromise in mice through spleen lymphocyte number maintenance. PLoS ONE 2012, 7, e33190. [Google Scholar] [CrossRef]
- Tsoi, B.; He, R.R.; Yang, D.H.; Li, Y.F.; Li, X.D.; Li, W.X.; Abe, K.; Kurihara, H. Carnosine ameliorates stress-induced glucose metabolism disorder in restrained mice. J. Pharmacol. Sci. 2011, 117, 223–229. [Google Scholar] [CrossRef]
- Kaisho, T.; Ishikawa, J.; Oritani, K.; Inazawa, J.; Tomizawa, H.; Muraoka, O.; Ochi, T.; Hirano, T. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc. Natl. Acad. Sci. USA 1994, 91, 5325–5329. [Google Scholar] [CrossRef]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef]
- Ishihara, K.; Hirano, T. BST-1/CD157 regulates the humoral immune responses in vivo. Chem. Immunol. 2000, 75, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S. Genetic polymorphisms of bone marrow stromal cell antigen-1 (BST-1/CD157): Implications for immune/inflammatory dysfunction in neuropsychiatric disorders. Front. Immunol. 2023, 14, 1197265. [Google Scholar] [CrossRef]
- Yokoyama, S.; Al Mahmuda, N.; Munesue, T.; Hayashi, K.; Yagi, K.; Yamagishi, M.; Higashida, H. Association Study between the CD157/BST1 Gene and Autism Spectrum Disorders in a Japanese Population. Brain Sci. 2015, 5, 188–200. [Google Scholar] [CrossRef]
- Mo, W.; Liu, J.; Zhang, Z.; Yu, H.; Yang, A.; Qu, F.; Hu, P.; Liu, Z.; Hu, F. A study of single nucleotide polymorphisms in CD157, AIM2 and JARID2 genes in Han Chinese children with autism spectrum disorder. Nord. J. Psychiatry 2018, 72, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Yoshihara, T.; Nishimura, T.; Zhong, J.; Akther, S.; Fakhrul, A.A.; Liang, M.; Higashida, C.; Sumi, K.; Furuhara, K.; et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front. Behav. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef]
- Mizuno, A.; Cherepanov, S.M.; Kikuchi, Y.; Fakhrul, A.A.; Akther, S.; Deguchi, K.; Yoshihara, T.; Ishihara, K.; Shuto, S.; Higashida, H. Lipo-oxytocin-1, a Novel Oxytocin Analog Conjugated with Two Palmitoyl Groups, Has Long-Lasting Effects on Anxiety-Related Behavior and Social Avoidance in CD157 Knockout Mice. Brain Sci. 2015, 5, 3–13. [Google Scholar] [CrossRef]
- Kasai, S.; Yoshihara, T.; Lopatina, O.; Ishihara, K.; Higashida, H. Selegiline Ameliorates Depression-Like Behavior in Mice Lacking the CD157/BST1 Gene, a Risk Factor for Parkinson's Disease. Front. Behav. Neurosci. 2017, 11, 75. [Google Scholar] [CrossRef]
- Tsuji, T.; Furuhara, K.; Gerasimenko, M.; Shabalova, A.; Cherepanov, S.M.; Minami, K.; Higashida, H.; Tsuji, C. Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release. Nutrients 2022, 14, 803. [Google Scholar] [CrossRef]
- Engelmann, M.; Ebner, K.; Landgraf, R.; Holsboer, F.; Wotjak, C.T. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J. Neuroendocrinol. 1999, 11, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jurek, B. The interplay between oxytocin and the CRF system: Regulation of the stress response. Cell Tissue Res. 2019, 375, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Windle, R.J.; Shanks, N.; Lightman, S.L.; Ingram, C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology 1997, 138, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.J.; Buckmaster, C.L.; Schatzberg, A.F.; Lyons, D.M. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 2005, 30, 924–929. [Google Scholar] [CrossRef]
- Windle, R.J.; Kershaw, Y.M.; Shanks, N.; Wood, S.A.; Lightman, S.L.; Ingram, C.D. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 2004, 24, 2974–2982. [Google Scholar] [CrossRef]
- Karelina, K.; Norman, G.J. Oxytocin Influence on NTS: Beyond Homeostatic Regulation. J. Neurosci. 2009, 29, 4687–4689. [Google Scholar] [CrossRef] [PubMed]
- van Steensel, F.J.; Bogels, S.M.; Perrin, S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clin. Child. Fam. Psychol. Rev. 2011, 14, 302–317. [Google Scholar] [CrossRef]
- van Steensel, F.J.A.; Heeman, E.J. Anxiety Levels in Children with Autism Spectrum Disorder: A Meta-Analysis. J. Child Fam. Stud. 2017, 26, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Hanzu, F.A. Cortisol Measurements in Cushing's Syndrome: Immunoassay or Mass Spectrometry? Ann. Lab. Med. 2020, 40, 285–296. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, T.; Furuhara, K.; Guo, E.; Wu, Y.; Zhong, J.; Higashida, H.; Yamamoto, Y.; Tsuji, C. Oral Supplementation of L-Carnosine Attenuates Acute-Stress-Induced Corticosterone Release and Mitigates Anxiety in CD157 Knockout Mice. Nutrients 2024, 16, 2821. https://doi.org/10.3390/nu16172821
Tsuji T, Furuhara K, Guo E, Wu Y, Zhong J, Higashida H, Yamamoto Y, Tsuji C. Oral Supplementation of L-Carnosine Attenuates Acute-Stress-Induced Corticosterone Release and Mitigates Anxiety in CD157 Knockout Mice. Nutrients. 2024; 16(17):2821. https://doi.org/10.3390/nu16172821
Chicago/Turabian StyleTsuji, Takahiro, Kazumi Furuhara, Erchu Guo, Yijing Wu, Jing Zhong, Haruhiro Higashida, Yasuhiko Yamamoto, and Chiharu Tsuji. 2024. "Oral Supplementation of L-Carnosine Attenuates Acute-Stress-Induced Corticosterone Release and Mitigates Anxiety in CD157 Knockout Mice" Nutrients 16, no. 17: 2821. https://doi.org/10.3390/nu16172821
APA StyleTsuji, T., Furuhara, K., Guo, E., Wu, Y., Zhong, J., Higashida, H., Yamamoto, Y., & Tsuji, C. (2024). Oral Supplementation of L-Carnosine Attenuates Acute-Stress-Induced Corticosterone Release and Mitigates Anxiety in CD157 Knockout Mice. Nutrients, 16(17), 2821. https://doi.org/10.3390/nu16172821







