Neuronutrition and Its Impact on Post-Stroke Neurorehabilitation: Modulating Plasticity Through Diet
Abstract
1. Introduction
2. General Overview on Functional Recovery Mechanism after Stroke
3. Neuronutrition as an Adjuvant Strategy in Post-Stroke Neurorehabilitation
4. Neuronutrition and Crosstalk Between Oxidative/Nitrosative Stress and Inflammation
4.1. Oxidative/Nitrosative Stress and Inflammation
4.2. Role of Minerals in the Oxidative/Nitrosative Stress and Inflammation
4.3. Effects of Antioxidant and Anti-Inflammatory Diets: The Mediterranean Diet and Ketogenic Diet
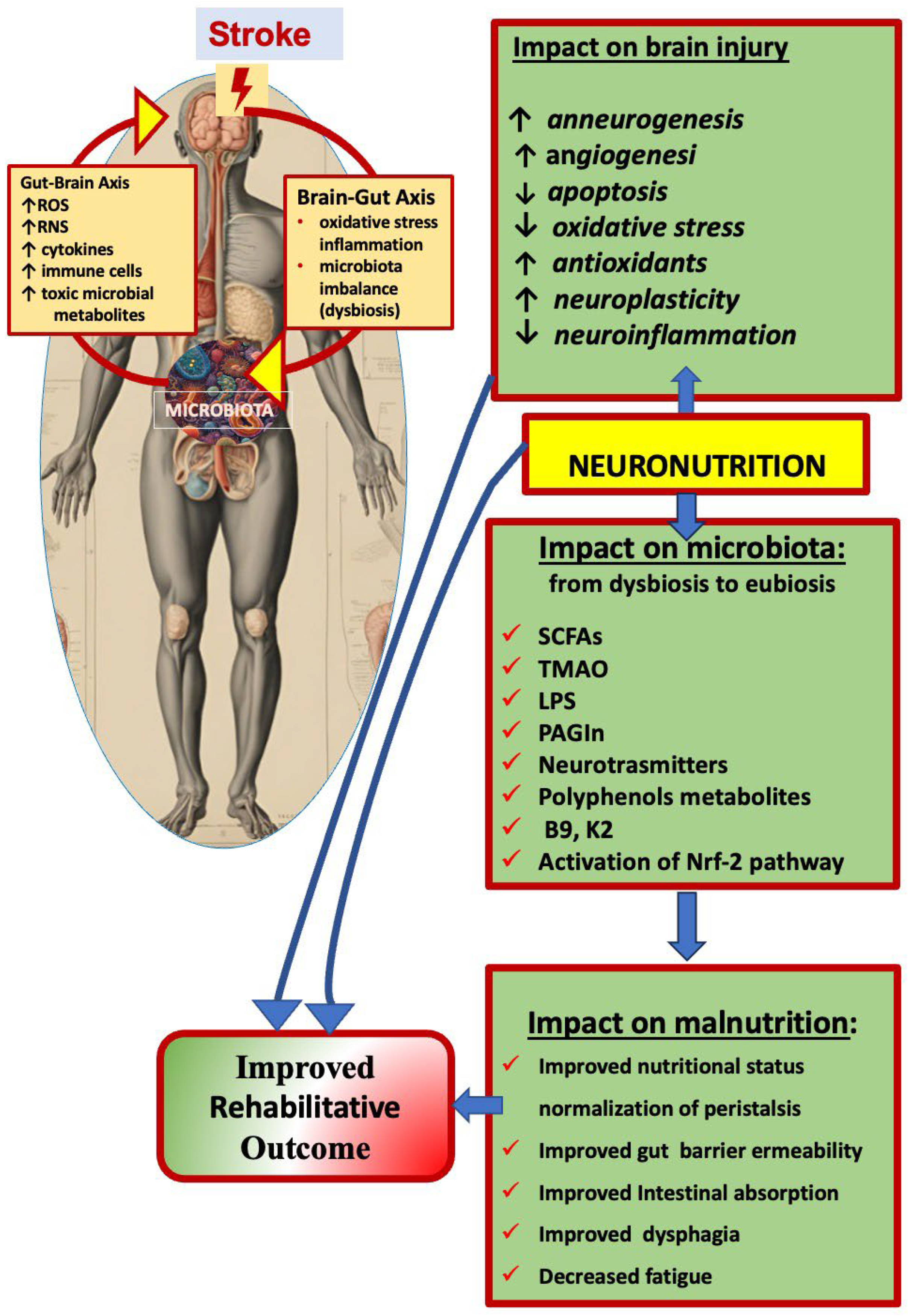
5. Neuronutrition and Stroke-Induced Gut–Brain Axis Disturbance
5.1. The Gut–Brain Axis, the Gut Microbiota, and Eubiosis
5.2. Stroke-Induced Modifications of the Gut–Brain Axis and Dysbiosis
5.3. The Gut Microbiota as a Potential Target for Post-Stroke Functional Recovery
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheatwood, J.L.; Emerick, A.J.; Kartje, G.L. Neuronal plasticity and functional recovery after ischemic stroke. Top. Stroke Rehabil. 2008, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.T.; Carmichael, S.T. Encouraging an excitable brain state: Mechanisms of brain repair in stroke. Nat. Rev. Neurosci. 2021, 22, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.; Czajka, A.; Zielińska-Turek, J.; Jaroszyński, J.; Furtak-Niczyporuk, M.; Mela, A.; Poniatowski, Ł.A.; Drop, B.; Dorobek, M.; Barcikowska-Kotowicz, M.; et al. Brain Functional Reserve in the Context of Neuroplasticity after Stroke. Neural Plast. 2019, 2019, 9708905. [Google Scholar] [CrossRef] [PubMed]
- Warraich, Z.; Kleim, J.A. Neural plasticity: The biological substrate for neurorehabilitation. PM&R J. Inj. Funct. Rehabil. 2010, 2 (Suppl. 2), S208–S219. [Google Scholar] [CrossRef]
- Ciancarelli, I.; Morone, G.; Iosa, M.; Cerasa, A.; Calabrò, R.S.; Iolascon, G.; Gimigliano, F.; Tonin, P.; Tozzi Ciancarelli, M.G. Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients 2022, 15, 108. [Google Scholar] [CrossRef]
- Malik, A.N.; Tariq, H.; Afridi, A.; Rathore, F.A. Technological advancements in stroke rehabilitation. J. Pak. Med. Assoc. 2022, 72, 1672–1674. [Google Scholar] [CrossRef] [PubMed]
- Platz, T. Evidence-based guidelines and clinical pathways in stroke rehabilitation—An international perspective. Front. Neurol. 2019, 10, 200. [Google Scholar] [CrossRef]
- Prabhakaran, S.; Zarahn, E.; Riley, C.; Speizer, A.; Chong, J.Y.; Lazar, R.M.; Marshall, R.S.; Krakauer, J.W. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil. Neural Repair. 2008, 22, 64–71. [Google Scholar] [CrossRef]
- Carnwath, T.P.; Demel, S.L.; Prestigiacomo, C.J. Genetics of ischemic stroke functional outcome. J. Neurol. 2024, 271, 2345–2369. [Google Scholar] [CrossRef]
- Feys, H.; De Weerdt, W.; Nuyens, G.; van de Winckel, A.; Selz, B.; Kiekens, C. Predicting motor recovery of the upper limb after stroke rehabilitation: Value of a clinical examination. Physiother. Res. Int. 2000, 5, 1–18. [Google Scholar] [CrossRef]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Le, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of Stroke Recovery: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 480–493. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Qi, X.; Xia, X.; Wang, L.; Li, X. Malnutrition on admission increases the in-hospital mortality and length of stay in elder adults with acute ischemic stroke. J. Clin. Lab. Anal. 2021, 36, e24132. [Google Scholar] [CrossRef] [PubMed]
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocrit. Care 2017, 29, 374–384. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Kim, J.S.; Kwon, S.U.; Yun, S.-C.; Koh, J.-Y.; Kang, D.-W. Undernutrition as a Predictor of Poor Clinical Outcomes in Acute Ischemic Stroke Patients. Arch. Neurol. 2008, 65, 39–43. [Google Scholar] [CrossRef]
- Ko, S.H.; Shin, Y.I. Nutritional Supplementation in Stroke Rehabilitation: A Narrative Review. Brain Neurorehabil. 2022, 15, e3. [Google Scholar] [CrossRef]
- Dennis, M.; Lewis, S.; Cranswick, G.; Forbes, J.; FOOD Trial Collaboration. FOOD: A multicenter randomized animals trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol. Assess. 2006, 10, iii–iv; ix–x; 1–120. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Lanzillo, B.; Guida, P.; Passantino, A.; Spaccavento, S.; Battista, P. Association Between Malnutrition and Outcomes in Patients with Severe Ischemic Stroke Undergoing Rehabilitation. Arch. Phys. Med. Rehabil. 2020, 101, 852–860. [Google Scholar] [CrossRef]
- Liu, J.; Dong, J.; Guo, J. The effects of nutrition supplement on rehabilitation for patients with stroke: Analysis based on 16 randomized controlled trials. Medicine 2022, 101, e29651. [Google Scholar] [CrossRef]
- Huppertz, V.; Guida, S.; Holdoway, A.; Strilciuc, S.; Baijens, L.; Schols, J.M.G.A.; van Helvoort, A.; Lansink, M.; Muresanu, D.F. Impaired Nutritional Condition After Stroke from the Hyperacute to the Chronic Phase: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 12, 780080. [Google Scholar] [CrossRef] [PubMed]
- Rabadi, M.H.; Coar, P.L.; Lukin, M.; Lesser, M.; Blass, J.P. Intensive nutritional supplements can improve outcomes in stroke rehabilitation. Neurology 2008, 71, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, H.; Yozu, A.; Khono, Y.; Oose, H. Nutritional improvement is associated with better functional outcome in stroke rehabilitation: A cross-sectional study using controlling in nutritional status. J. Rehabil. Med. 2020, 52, jrm00028. [Google Scholar] [CrossRef] [PubMed]
- Lieber, A.C.; Hong, E.; Putrino, D.; Nistal, D.A.; Pan, J.S.; Kellner, C.P. Nutrition, Energy Expenditure, Dysphagia, and Self-Efficacy in Stroke Rehabilitation: A Review of the Literature. Brain Sci. 2018, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass; muscle strength; and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2018, 58, 1–6. [Google Scholar] [CrossRef]
- Aquilani, R.; Scocchi, M.; Iadarola, P.; Franciscone, P.; Verri, M.; Boschi, F.; Pasini, E.; Viglio, S. Protein supplementation may enhance the spontaneous recovery of neurological alterations in patients with ischaemic stroke. Clin. Rehabil. 2008, 22, 1042–1050. [Google Scholar] [CrossRef]
- Zielinska-Nowak, E.; Cichon, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. Nutritional Supplements and Neuroprotective Diets and Their Potential Clinical Significance in Post-Stroke Rehabilitation. Nutrients 2021, 13, 2704. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briones-Valdivieso, C.; Briones, F.; Orellana-Urzúa, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Novel Multi-Antioxidant Approach for Ischemic Stroke Therapy Targeting the Role of Oxidative Stress. Biomedicines 2024, 12, 501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Gomes, F.; Emery, P.W.; Weekes, C.E. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.A.; Dinan, M.A.; Robinson, T.J.; Snyderman, R. Personalized medicine is more than genomic medicine: Confusion over terminology impedes progress towards personalized healthcare. Pers. Med. 2012, 9, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Badaeva, A.V.; Danilov, A.B.; Clayton, P.; Moskalev, A.A.; Karasev, A.V.; Tarasevich, A.F.; Vorobyeva, Y.D.; Novikov, V.N. Perspectives on Neuronutrition in Prevention and Treatment of Neurological Disorders. Nutrients 2023, 15, 2505. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Anthony, J.C.; Carvajal, R.; Chae, L.; Khoo, C.S.H.; Latulippe, M.E.; Matusheski, N.V.; McClung, H.L.; Rozga, M.; Schmid, C.H.; et al. Perspective: Guiding Principles for the Implementation of Personalized Nutrition Approaches That Benefit Health and Function. Adv. Nutr. 2020, 11, 25–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020, 158, 104877. [Google Scholar] [CrossRef]
- Pires, I.M.; Denysyuk, H.V.; Villasana, M.V.; Sá, J.; Lameski, P.; Chorbev, I.; Zdravevski, E.; Trajkovik, V.; Morgado, J.F.; Garcia, N.M. Mobile 5P-Medicine Approach for Cardiovascular Patients. Sensors 2021, 21, 6986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tozzi Ciancarelli, M.G.; Di Massimo, C.; De Amicis, D.; Ciancarelli, I. Mediterranean Diet and Health Promotion: Evidence and Current Concerns. Med. Res. Arch. 2017, 5, 1–16. Available online: https://esmed.org/MRA/mra/article/view/1385 (accessed on 1 October 2024). [CrossRef][Green Version]
- Gantenbein, K.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanchez-Moreno, C.; Dashe, J.F.; Scott, T.; Thaler, D.; Folstein, M.F.; Martin, A. Decreased Levels of Plasma Vitamin C and Increased Concentrations of Inflammatory and Oxidative Stress Markers After Stroke. Stroke 2004, 35, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, F.C.; van Rensburg, S.J.; Hon, D.; Emsley, R.A.; Moodie, I.M.; Erasmus, R.T. Oral zinc augmentation with vitamins A and D increases plasma zinc concentration: Implications for burden of disease. Metab. Brain Dis. 2006, 21, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Miro’nczuk, A.; Kapica-Topczewska, K.; Socha, K.; Soroczy’nska, J.; Jamiołkowski, J.; Kułakowska, A.; Kochanowicz, J. Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland-A New Insight into Stroke Pathophysiology. Nutrients 2021, 13, 2139. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R. Leptin and zinc relation: In regulation of food intake and immunity. Indian J. Endocrinol. Metab. 2012, 16, S611–S616. [Google Scholar] [CrossRef]
- Hess, S.Y.; McLain, A.C.; Lescinsky, H.; Brown, K.H.; Afshin, A.; Atkin, R.; Osendarp, S.J. Basis for changes in the disease burden estimates related to vitamin A and zinc deficiencies in the 2017 and 2019 Global Burden of Disease Studies. Public Health Nutr. 2021, 25, 2225–2231. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Veronese, N.; Barbagallo, M. Magnesium and Micro-Elements in Older Persons. Nutrients 2021, 13, 847. [Google Scholar] [CrossRef]
- Stefanache, A.; Lungu, I.-I.; Butnariu, I.-A.; Calin, G.; Gutu, C.; Marcu, C.; Grierosu, C.; Goroftei, E.R.B.; Duceac, L.-D.; Dabija, M.G.; et al. Understanding How Minerals Contribute to Optimal Immune Function. J. Immunol. Res. 2023, 2023, 3355733. [Google Scholar] [CrossRef]
- Gholami, H.; Chmiel, J.A.; Burton, J.P.; Maleki Vareki, S. The Role of Microbiota-Derived Vitamins in Immune Homeostasis and Enhancing Cancer Immunotherapy. Cancers 2023, 15, 1300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; R D’Arcy, B.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol derived phenolic acid mediated attenuation of Alzheimer’s disease b-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Haces, M.L.; Hernández-Fonseca, K.; Medina-Campos, O.N.; Montiel, T.; Pedraza-Chaverri, J.; Massieu, L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008, 211, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, S.R.; Lee, J.E.; Lee, S.; Son, H.J.; Choe, W.; Yoon, K.-S.; Kim, S.S.; Yeo, E.-J.; Kang, I. Molecular Mechanisms of Neuroprotection by Ketone Bodies and Ketogenic Diet in Cerebral Ischemia and Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 124. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, M.; Elfving, Å.; Ungerstedt, U.; Åmark, P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005, 64, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Szili-Torok, T.; de Borst, M.H.; Garcia, E.; Gansevoort, R.T.; Dullaart, R.P.F.; Connelly, M.A.; Bakker, S.J.L.; Tietge, U.J.F. Fasting Ketone Bodies and Incident Type 2 Diabetes in the General Population. Diabetes 2023, 72, 1187–1192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, S.B.; Yoon, B.W. Mechanisms of functional recovery after stroke. Front. Neurol. Neurosci. 2013, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Germanotta, M.; Santoro, M.; Canali, R.; Pascali, S.; Insalaco, S.; Cipollini, V.; Papadopoulou, D.; Antonacci, E.; Aprile, I. Oxidative Stress Status in Post Stroke Patients: Sex Differences. Healthcare 2022, 10, 869. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef]
- Ciancarelli, I.; Di Massimo, C.; De Amicis, D.; Carolei, A.; Ciancarelli, M.G.T. Evidence of redox unbalance in post-acute ischemic stroke patients. Curr. Neurovasc. Res. 2012, 9, 85–90. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, P.; Rao, Y.; Chen, L. Resveratrol Reverses the Synaptic Plasticity Deficits in a Chronic Cerebral Hypoperfusion Rat Model. J. Stroke Cerebrovasc. Dis. 2016, 25, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, Y.; Chen, M.; Liu, G. Application of Nanomicelles in Enhancing Bioavailability and Biological Efficacy of Bioactive Nutrients. Polymers 2022, 14, 3278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermann, D.M.; Zechariah, A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J. Cereb. Blood Flow Metab. 2009, 29, 1620–1643. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, H.; Lu, J.; Qu, Y.; Yu, D.; Tong, Y. Vascular endothelial growth factor: An attractive target in the treatment of hypoxic/ischemic brain injury. Neural Regen. Res. 2016, 11, 174–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, S.; Chang, M.-S.; Koh, S.-H.; Choi, Y.K. Repair Mechanisms of the Neurovascular Unit after Ischemic Stroke with a Focus on VEGF. Int. J. Mol. Sci. 2021, 22, 8543. [Google Scholar] [CrossRef]
- Hermann, D.M.; Chopp, M. Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012, 11, 369–380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dodich, A.; Carli, G.; Cerami, C.; Iannaccone, S.; Magnani, G.; Perani, D. Social and cognitive control skills in long-life occupation activities modulate the brain reserve in the behavioural variant of frontotemporal dementia. Cortex 2018, 99, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Brain plasticity and rehabilitation in stroke patients. J. Nippon. Med. Sch. 2015, 82, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, X.; Qiu, H. Rehabilomics: A state-of-the-art review of framework; application; and future considerations. Front. Neurol. 2023, 14, 1103349. [Google Scholar] [CrossRef]
- Hazelton, C.; McGill, K.; Campbell, P.; Todhunter-Brown, A.; Thomson, K.; Nicolson, D.J.; Cheyne, J.D.; Chung, C.; Dorris, L.; Gillespie, D.C.; et al. Perceptual Disorders After Stroke: A Scoping Review of Interventions. Stroke 2022, 53, 1772–1787. [Google Scholar] [CrossRef]
- Dutta, T.M.; Josiah, A.F.; Cronin, C.A.; Wittenberg, G.F.; Cole, J.W. Altered taste and stroke: A case report and literature review. Top. Stroke Rehabil. 2013, 20, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Melzer, T.M.; Manosso, L.M.; Yau, S.Y.; Gil-Mohapel, J.; Brocardo, P.S. In Pursuit of Healthy Aging: Effects of Nutrition on Brain Function. Int. J. Mol. Sci. 2021, 22, 5026. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Narayanan, R. A Review on Neuronutrition. Asian J. Dairy Food Res. 2019, 38, 128–133. [Google Scholar] [CrossRef]
- Holton, K. F Micronutrients May Be a Unique Weapon Against the Neurotoxic Triad of Excitotoxicity, Oxidative Stress and Neuroinflammation: A Perspective. Front. Neurosci. 2021, 15, 726457. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Rathipriya, A.G.; Mahalakshmi, A.M.; Sharma, S.; Hediyal, T.A.; Ray, B.; Sunanda, T.; Rungratanawanich, W.; Kashyap, R.S.; Qoronfleh, M.W.; et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11, 1239. [Google Scholar] [CrossRef]
- Poblete, R.A.; Yaceczko, S.; Aliakbar, R.; Saini, P.; Hazany, S.; Breit, H.; Louie, S.G.; Lyden, P.D.; Partikian, A. Optimization of Nutrition after Brain Injury: Mechanistic and Therapeutic Considerations. Biomedicines 2023, 11, 2551. [Google Scholar] [CrossRef]
- Garbagnati, F.; Cairella, G.; De Martino, A.; Multari, M.; Scognamiglio, U.; Venturiero, V.; Paolucci, S. Is Antioxidant and n–3 Supplementation Able to Improve Functional Status in Poststroke Patients? Results from the Nutristroke Trial. Cerebrovasc. Dis. 2009, 27, 375–383. [Google Scholar] [CrossRef]
- Cha, K.H.; Kang, N.Y.; Huh, S.; Ko, S.H.; Shin, Y.I.; Min, J.H. The Effects of Autonomic Dysfunction on Functional Outcomes in Patients with Acute Stroke. Brain Sci. 2023, 13, 1694. [Google Scholar] [CrossRef]
- Tavarez, T.; Roehl, K.; Koffman, L. Nutrition in the Neurocritical Care Unit: A New Frontier. Curr. Treat. Options Neurol. 2021, 23, 16–18. [Google Scholar] [CrossRef]
- Sakai, K.; Kinoshita, S.; Tsuboi, M.; Fukui, R.; Momosaki, R.; Wakabayashi, H. Effects of Nutrition Therapy in Older Stroke Patients Undergoing Rehabilitation: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chang, E.; Jiang, Y. Effects of vitamin C stimulation on rehabilitation of dysphagia after stroke: A randomized trial. Eur. J. Phys. Rehabil. Med. 2022, 58, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in cardiovascular therapy: Panacea or false hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum Albumin in Health and Disease: Esterase, Antioxidant, Transporting and Signaling Properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef]
- Shah, Z.; Li, R.-C.; Thimmulappa, R.; Kensler, T.; Yamamoto, M.; Biswal, S.; Doré, S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis After Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transpl. 2018, 27, 1789–1797. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.Y.; Zhou, M.W.; Liu, N.; Xing, H.Y.; Liu, X.X.; Li, F. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci. Lett. 2018, 683, 13–18. [Google Scholar] [CrossRef]
- Milder, J.B.; Liang, L.P.; Patel, M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 2010, 40, 238–244. [Google Scholar] [CrossRef]
- Arya, A.K.; Hu, B. Brain–gut axis after stroke. Brain Circ. 2018, 4, 165–173. [Google Scholar]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693 Pt B, 128–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Chu, Z.; Zhu, L.; Zhou, Y.; Yang, F.; Hu, Z.; Luo, Y.; Li, W.; Luo, F. Targeting Nrf2 by bioactive peptides alleviate inflammation: Expanding the role of gut microbiota and metabolites. Crit. Rev. Food Sci. Nutr. 2024, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Olejar, K.J.; On, S.L.W.; Chelikani, V. The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants 2020, 9, 610. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.V.; Hamr, S.C.; Duca, F.A. Regulation of energy balance by a gut–brain axis and involvement of the gut microbiota. Cell. Mol. Life Sci. 2016, 73, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Liébana-García, R.; Olivares, M.; Sanz, Y. The Microbiota and the Gut–Brain Axis in Controlling Food Intake and Energy Homeostasis. Int. J. Mol. Sci. 2021, 22, 5830. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulba, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxidative Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Houlden, A.; Goldrick, M.; Brough, D.; Vizi, E.S.; Lénárt, N.; Martinecz, B.; Roberts, I.S.; Denes, A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 2016, 57, 10–20. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The Role of Gut Microbiota in an Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef]
- Dang, Y.; Zhang, X.; Zheng, Y.; Yu, B.; Pan, D.; Jiang, X.; Yan, C.; Yu, Q.; Lu, X. Distinctive Gut Microbiota Alteration Is Associated with Poststroke Functional Recovery: Results from a Prospective Cohort Study. Neural Plast. 2021, 2021, 1469339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, X.; Gao, X.; Peng, Y.; Wu, Q.; Zhu, J.; Tan, C.; Xia, G.; You, C.; Xu, R.; Pan, S.; et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell. Infect. Microbiol. 2019, 9, 4. [Google Scholar] [CrossRef]
- Chang, Y.; Woo, H.G.; Jeong, J.H.; Kim, G.H.; Park, K.D.; Song, T.J. Microbiota dysbiosis and functional outcome in acute ischemic stroke patients. Sci. Rep. 2021, 11, 10977. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Sun, Y.; Wang, J.; Wei, S.; Mao, L.; Zheng, J.; Liu, P.; Yang, X.; Chen, Z. Relationship Between the Gut Microbiota and Neurological Deficits in Patients with Cerebral Ischemic Stroke. Neurorehabil. Neural Repair. 2024, 38, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Brea, J.; Garcia-Montes, M.; Geuna, S.; Herrera-Rincon, C. Gut Microbiota and Neuroplasticity. Cells 2021, 10, 2084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.C.; Powell, E.; Green, S.J.; Chlipala, G.; Frank, J.; Yackzan, A.T.; Yanckello, L.M.; Chang, Y.-H.; Xing, X.; Heil, S.; et al. Functional recovery outcomes following acute stroke is associated with abundance of gut microbiota related to inflammation, butyrate and secondary bile acid. Front. Rehabilit. Sci. 2022, 3, 1017180. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Wa’skow, M.; Steliga, A.; Mory´s, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Katche, C.; Slipczuk, L.; Rossato, J.I.; Goldin, A.; Izquierdo, I.; Medina, J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA 2008, 105, 2711–2716. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2021, 38, 223–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.; Xu, N.; Matei, N.; McBride, D.W.; Ding, Y.; Liang, H.; Tang, J.; Zhang, J.H. Sodium Butyrate Attenuated Neuronal Apoptosis via GPR41/Gbetagamma/PI3K/Akt Pathway After MCAO in Rats. J. Cereb. Blood Flow. Metab. 2021, 41, 267–281. [Google Scholar] [CrossRef]
- Mang, C.S.; Campbell, K.L.; Ross, C.J.; Boyd, L.A. Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys. Ther. 2013, 93, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alcantara, C.C.; García-Salazar, L.F.; Silva-Couto, M.A.; Santos, G.L.; Reisman, D.S.; Russo, T.L. Post-stroke BDNF Concentration Changes Following Physical Exercise: A Systematic Review. Front. Neurol. 2018, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Corona, G.; Mills, H.; Chen, L.; Spencer, J.P.E.; Tzortzis, G.; Burnet, P.W.J. Prebiotic feeding elevates central brain derived neurotrophic factor, N-Methyl-d-Aspartate receptor subunits and d-Serine. Neurochem. Int. 2013, 63, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.; Tsiountsioura, M.; Meixner-Goetz, L.; Cvirn, G.; Lamprecht, M. Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials. Nutrients 2023, 15, 3770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Food Sources | Bioactive Substances | Specific Effects | References |
|---|---|---|---|
| Legumes, fruits, vegetables, fish, extra virgin olive oil, fish and nut oils, and leafy vegetables | Omega-3 fatty acids: EPA, DHA, and DPA | Improved neurotransmission, neuronal membrane fluidity, cell signalling, neuronal plasticity, BDNF, and microbiota composition (taxonomic groups producing SCFA); improved rehabilitation outcomes | [5,28,29,31,35,37,38,39,40,41] |
| Vegetables, green tea, coffee, wine, extra virgin olive oil, and well-balanced diet (the Mediterranean diet, MD) | Polyphenols | Antioxidant and anti-inflammatory properties; neuroprotection; scavenging free radicals, chelating metals, dampening pro-oxidative enzymes | [27,32,35,37,38,39,42,43] |
| Fruits, legumes, animal red meats, poultry, beef or sheep liver, seafood, eggs, herbs, spices, high dietary fiber intake, nuts, seeds, and whole grain products | Minerals (Zn, Mg, Se, and Cu) | Co-factors of antioxidant enzyme, long-term regulation of systemic inflammation, and metabolic homeostasis | [24,25,26,27,43,44,45,46,47,48,49] |
| Well-balanced diet, vegetables, fruits, dairy products, egg yolk, and offal and liver of pigs, sheep, and cattle. | Vitamins (A, C, E, D, B group, and K2) | Antioxidants and anti-inflammatory; anti-apoptosis; and neurorestorative (post-stroke recovery, rehabilitation effectiveness) | [27,44,47,50,51] |
| Diet with high fat, moderate protein content, and low carbohydrates (ketogenic diet) | Ketone bodies (acetone, and β hydroxybutyrate acetoacetate) | Prevention of mitochondrial dysfunction, decreased oxidative and inflammatory damage, and GABA release | [52,53,54,55] |
| Fruits, legumes, and vegetables | dietary fiber (carbohydrate polymers, and non-digestible carbohydrates) | Gut microbiome composition, intestinal peristalsis, acid–base balance, and decreased proinflammatory cytokines | [5,27,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciancarelli, I.; Morone, G.; Iosa, M.; Cerasa, A.; Calabrò, R.S.; Tozzi Ciancarelli, M.G. Neuronutrition and Its Impact on Post-Stroke Neurorehabilitation: Modulating Plasticity Through Diet. Nutrients 2024, 16, 3705. https://doi.org/10.3390/nu16213705
Ciancarelli I, Morone G, Iosa M, Cerasa A, Calabrò RS, Tozzi Ciancarelli MG. Neuronutrition and Its Impact on Post-Stroke Neurorehabilitation: Modulating Plasticity Through Diet. Nutrients. 2024; 16(21):3705. https://doi.org/10.3390/nu16213705
Chicago/Turabian StyleCiancarelli, Irene, Giovanni Morone, Marco Iosa, Antonio Cerasa, Rocco Salvatore Calabrò, and Maria Giuliana Tozzi Ciancarelli. 2024. "Neuronutrition and Its Impact on Post-Stroke Neurorehabilitation: Modulating Plasticity Through Diet" Nutrients 16, no. 21: 3705. https://doi.org/10.3390/nu16213705
APA StyleCiancarelli, I., Morone, G., Iosa, M., Cerasa, A., Calabrò, R. S., & Tozzi Ciancarelli, M. G. (2024). Neuronutrition and Its Impact on Post-Stroke Neurorehabilitation: Modulating Plasticity Through Diet. Nutrients, 16(21), 3705. https://doi.org/10.3390/nu16213705









