Vitamin D and Gestational Diabetes Mellitus in the IEU OpenGWAS Project: A Two-Sample Bidirectional Mendelian Randomization Study
Abstract
:1. Introduction
2. Materials and Methods
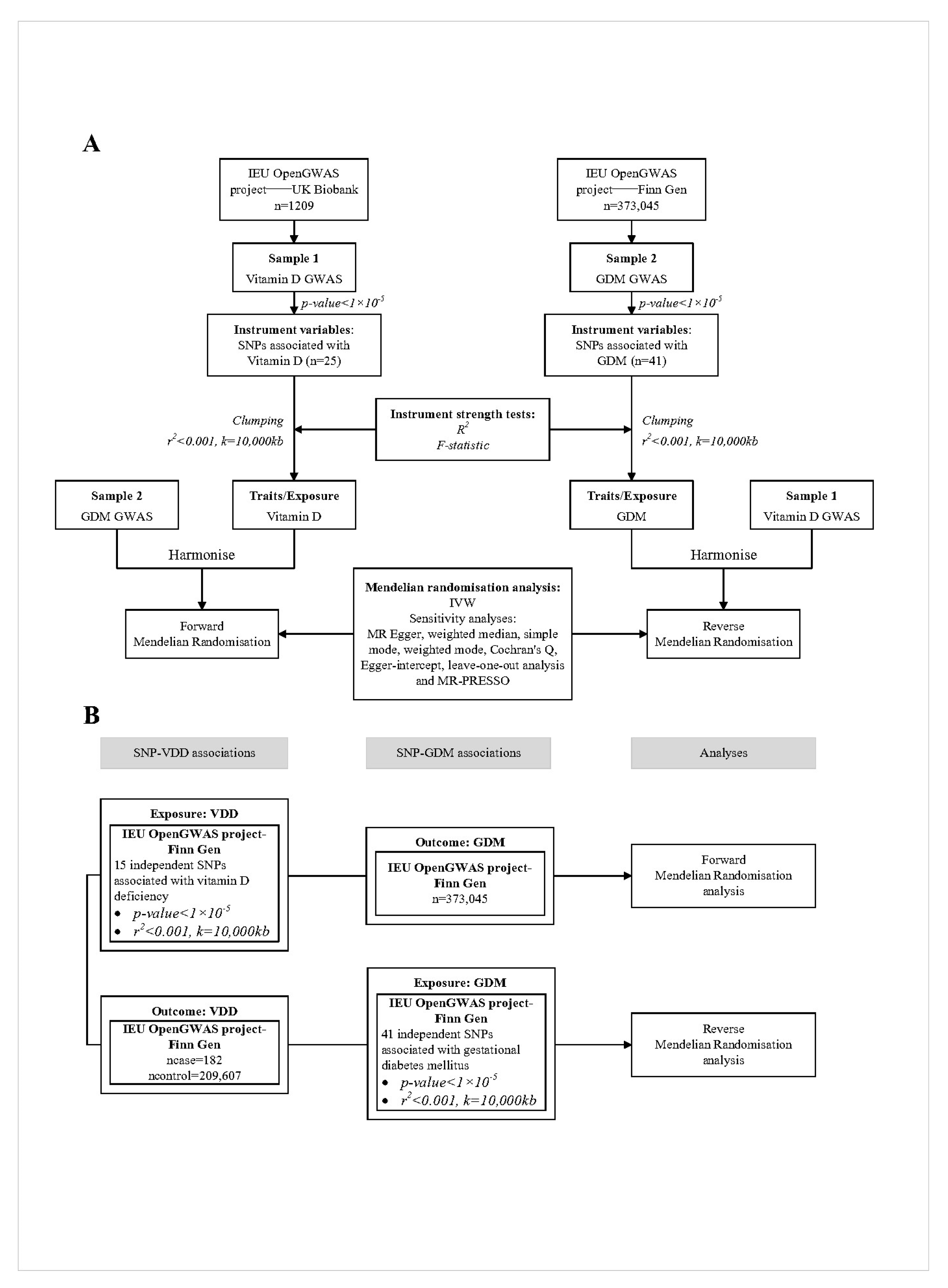
2.1. Study Design and Data Sources
2.1.1. Principles of SNP Selection
2.1.2. Data Sources for Vitamin D Levels and SNP Selection
2.1.3. Data Sources for Gestational Diabetes Mellitus and SNP Selection
2.1.4. Data Sources for Vitamin D Deficiency and SNP Selection
2.2. Statistical Power (R2 and F Value)
2.3. Mendelian Randomization Analysis
2.4. Sensitivity Analysis
3. Results
3.1. Instrumental Variables
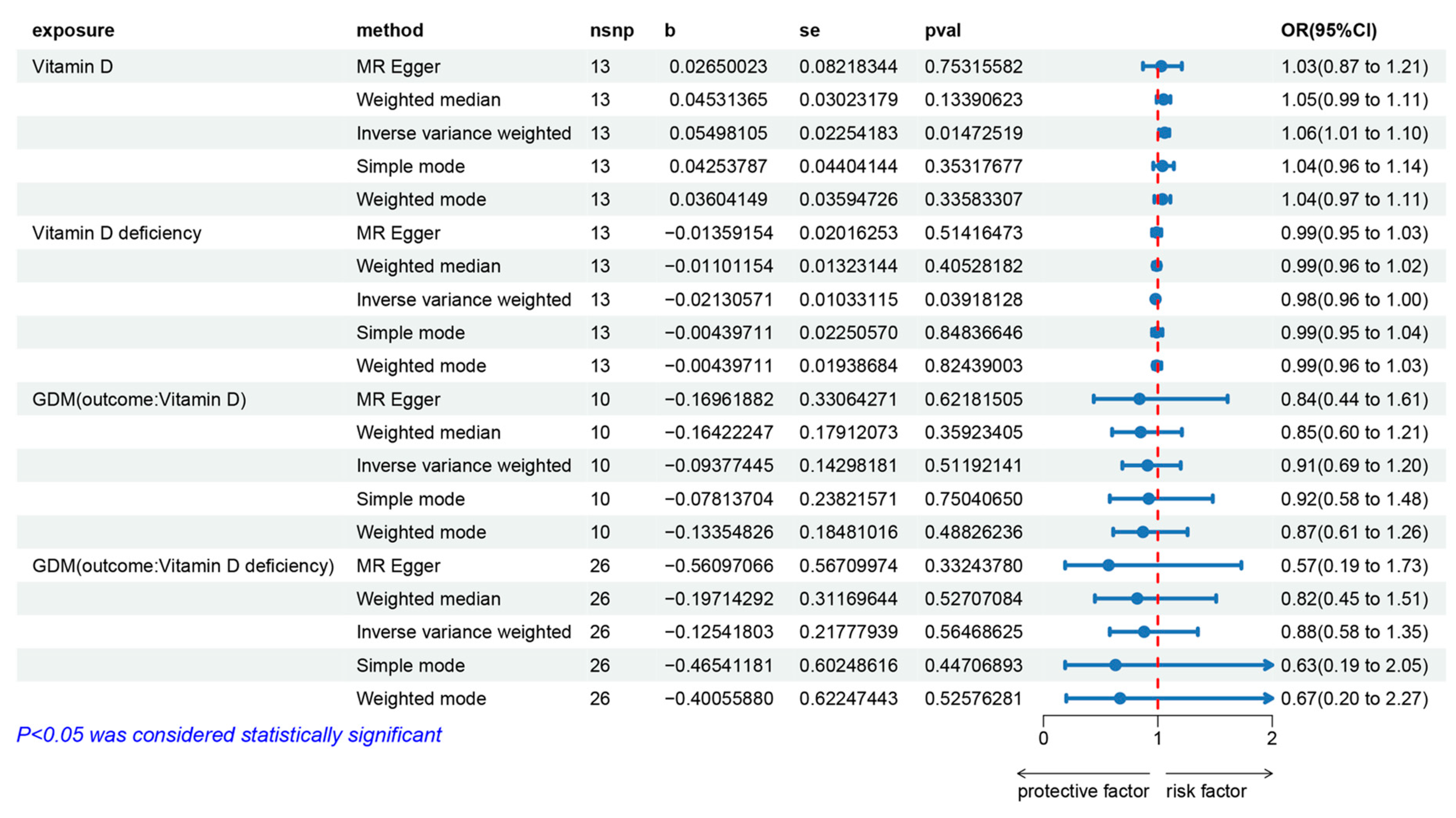
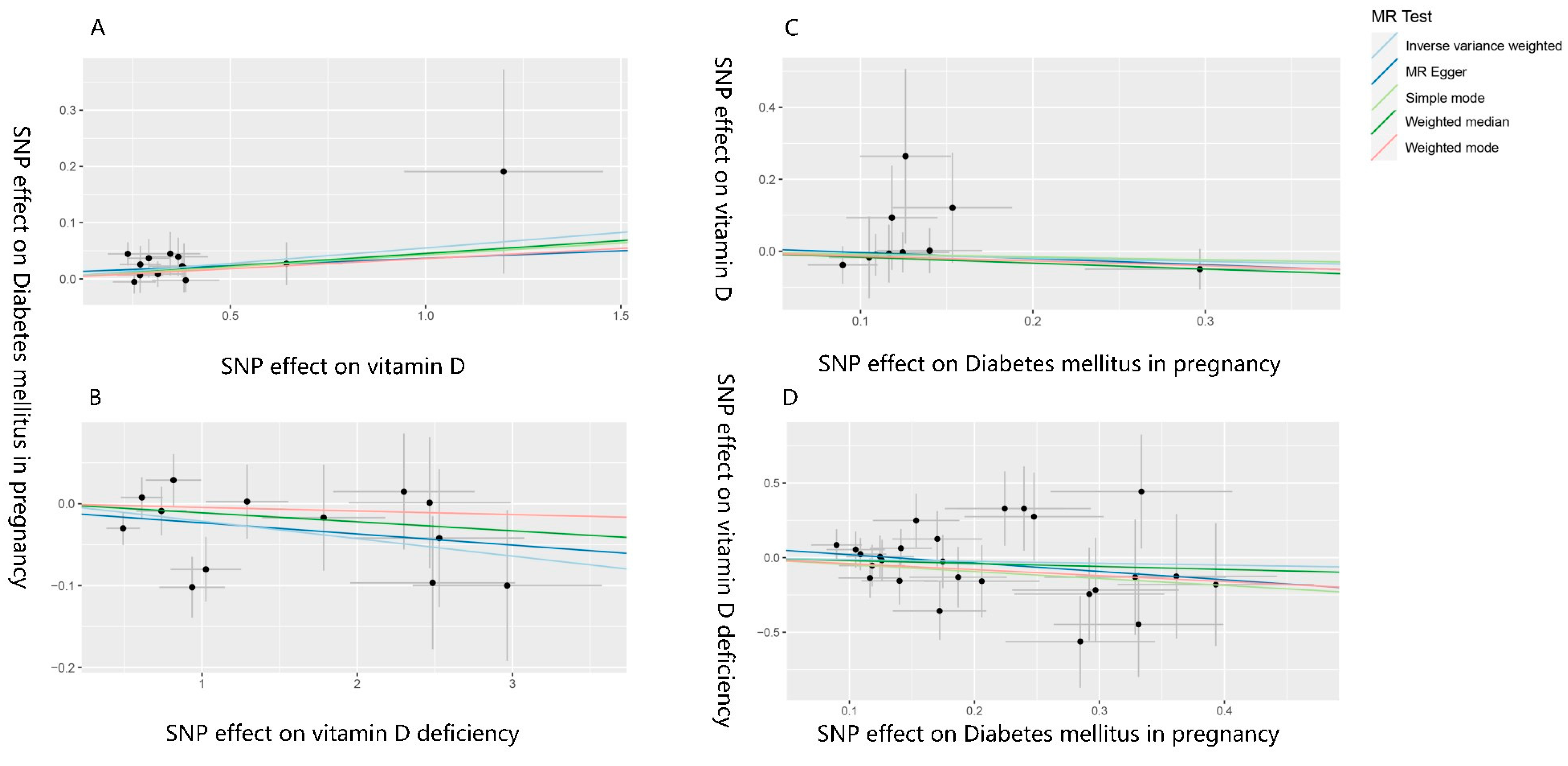
3.2. The Effect of Vitamin D on Gestational Diabetes Mellitus
3.3. The Effect of Gestational Diabetes Mellitus on Vitamin D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haimi, M.; Kremer, R. Vitamin D deficiency/insufficiency from childhood to adulthood: Insights from a sunny country. World J. Clin. Pediatr. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Chau, Y.Y.; Kumar, J. Vitamin D in chronic kidney disease. Indian J. Pediatr. 2012, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2020, 1268, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Brustad, M.; Meyer, H.E. Vitamin D—Hvor mye er nok, og er mer bedre for helsen? [Vitamin D—How much is enough, and is more better for your Health?]. Tidsskr. Nor. Laegeforen. 2014, 134, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Wyse, J.; Mangan, R.; Zgaga, L. Power determination in vitamin D randomised control trials and characterising factors affecting it through a novel simulation-based tool. Sci. Rep. 2021, 11, 10804. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Bandeira, F.; Griz, L.; Dreyer, P.; Eufrazino, C.; Bandeira, C.; Freese, E. Vitamin D deficiency: A global perspective. Arq. Bras. Endocrinol. Metabol. 2006, 50, 640–646. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef]
- Qin, L.L.; Lu, F.G.; Yang, S.H.; Xu, H.L.; Luo, B.A. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients 2016, 8, 301. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. S1), 13–27. [CrossRef]
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef]
- Lowe, W.L., Jr.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Diabetes in Pregnancy Working Group; Maternal Medicine Clinical Study Group; Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: Opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Li, S.; Liu, H.; Liu, L.; Huang, Q.; Hou, Y.; Liang, X.; Cui, B.; Zhang, M.; et al. Joint effect of urinary arsenic species and serum one-carbon metabolism nutrients on gestational diabetes mellitus: A cross-sectional study of Chinese pregnant women. Environ. Int. 2021, 156, 106741. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.C.; Dampil, O.A.; Ong-Lopez, A.M. The Association between Maternal Serum Vitamin D Levels and Gestational Diabetes Mellitus among Filipino Patients: A Cross-Sectional Study. J. ASEAN Fed. Endocr. Soc. 2020, 35, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, R.E.; Gheoca Mutu, D.E.; Sima, R.M.; Toader, O.D.; Stănculescu, R.V. The Impact of Vitamin D Deficiency on Gestational Diabetes Mellitus Risk: A Retrospective Study. Cureus 2024, 16, e65037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Zhang, C.; Song, Y.; Zhang, Z. Serum vitamin D deficiency and risk of gestational diabetes mellitus: A meta-analysis. Arch. Med. Sci. 2020, 16, 742–751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parildar, H.; Dogruk Unal, A.; Aksan Desteli, G.; Cigerli, O.; Guvener Demirag, N. Frequency of Vitamin D deficiency in pregnant diabetics at Baskent University Hospital, Istanbul. Pak. J. Med. Sci. 2013, 29, 15–20. [Google Scholar] [CrossRef]
- Wen, J.; Hong, Q.; Zhu, L.; Xu, P.; Fu, Z.; Cui, X.; You, L.; Wang, X.; Wu, T.; Ding, H.; et al. Association of maternal serum 25-hydroxyvitamin D concentrations in second and third trimester with risk of gestational diabetes and other pregnancy outcomes. Int. J. Obes. 2017, 41, 489–496. [Google Scholar] [CrossRef]
- Pleskačová, A.; Bartáková, V.; Pácal, L.; Kuricová, K.; Bělobrádková, J.; Tomandl, J.; Kaňková, K. Vitamin D Status in Women with Gestational Diabetes Mellitus during Pregnancy and Postpartum. Biomed. Res. Int. 2015, 2015, 260624. [Google Scholar] [CrossRef] [PubMed]
- Makgoba, M.; Nelson, S.M.; Savvidou, M.; Messow, C.M.; Nicolaides, K.; Sattar, N. First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus. Diabetes Care 2011, 34, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Holmes, M.V. Meta-analysis and Mendelian randomization: A review. Res. Synth. Methods 2019, 10, 486–496. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Chen, Z.; Boehnke, M.; Wen, X.; Mukherjee, B. Revisiting the genome-wide significance threshold for common variant GWAS. G3 Genes Genomes Genet. 2021, 11, jkaa056. [Google Scholar] [CrossRef]
- Xiao, G.; He, Q.; Liu, L.; Zhang, T.; Zhou, M.; Li, X.; Chen, Y.; Chen, Y.; Qin, C. Causality of genetically determined metabolites on anxiety disorders: A two-sample Mendelian randomization study. J. Transl. Med. 2022, 20, 475. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Zhang, Z.; Burrows, K.; Fuller, H.; Speliotes, E.K.; Abeysekera, K.W.M.; Thorne, J.L.; Lewis, S.J.; Zulyniak, M.A.; Moore, J.B. Non-Alcoholic Fatty Liver Disease and Vitamin D in the UK Biobank: A Two-Sample Bidirectional Mendelian Randomisation Study. Nutrients 2023, 15, 1442. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ahsan, H.; Vander Weele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Grover, S.; Del Greco, M.F.; Stein, C.M.; Ziegler, A. Mendelian Randomization. Methods Mol. Biol. 2017, 1666, 581–628. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Smith, G.D.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Smith, G.D.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, D.; Harroud, A.; Mitchell, R.E.; Ross, S.; Forgetta, V.; Timpson, N.J.; Smith, G.D.; Polychronakos, C.; Richards, J.B. Correction: Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLoS Med. 2021, 18, e1003624. [Google Scholar] [CrossRef]
- Cohen, J.F.; Chalumeau, M.; Cohen, R.; Korevaar, D.A.; Khoshnood, B.; Bossuyt, P.M. Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J. Clin. Epidemiol. 2015, 68, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Erratum to: Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 391–392. [Google Scholar] [CrossRef]
- Lips, P.; Eekhoff, M.; van Schoor, N.; Oosterwerff, M.; de Jongh, R.; Krul-Poel, Y.; Simsek, S. Vitamin D and type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2017, 173, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Asadi, M.; Rahmani, S.; Akhavan Zanjani, M.; Sadeghi, O.; Hosseini, S.A.; Zare Javid, A. Circulating vitamin D and the risk of gestational diabetes: A systematic review and dose-response meta-analysis. Endocrine 2020, 70, 36–47. [Google Scholar] [CrossRef]
- Gustafsson, M.K.; Romundstad, P.R.; Stafne, S.N.; Helvik, A.-S.; Stunes, A.K.; Mørkved, S.; Salvesen, K.Å.; Thorsby, P.M.; Syversen, U. Alterations in the vitamin D endocrine system during pregnancy: A longitudinal study of 855 healthy Norwegian women. PLoS ONE 2018, 13, e0195041. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, H.K.; Ryu, H.M.; Han, Y.J.; Lee, S.W.; Park, B.K.; Park, S.-Y.; Yim, C.-H.; Kim, S.-H. Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study. J. Nutr. Sci. Vitaminol. 2014, 60, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Boyle, V.T.; Thorstensen, E.B.; Mourath, D.; Jones, M.B.; McCowan, L.M.; Kenny, L.C.; Baker, P.N. The relationship between 25-hydroxyvitamin D concentration in early pregnancy and pregnancy outcomes in a large, prospective cohort. Br. J. Nutr. 2016, 116, 1409–1415. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Chen, X.; Li, S.; Meng, X.; Jia, A.; Yang, X.; Huang, F.; Zhang, X.; Zhang, Q. Association between urinary arsenic species and vitamin D deficiency: A cross-sectional study in Chinese pregnant women. Front. Public Health 2024, 12, 1371920. [Google Scholar] [CrossRef]
- Birney, E. Mendelian Randomization. Cold Spring Harb. Perspect. Med. 2022, 12, a041302. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Gasparri, C.; Perna, S.; Spadaccini, D.; Alalwan, T.; Girometta, C.; Infantino, V.; Rondanelli, M. Is vitamin D-fortified yogurt a value-added strategy for improving human health? A systematic review and meta-analysis of randomized trials. J. Dairy Sci. 2019, 102, 8587–8603. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Wang, X.; Xu, Y.; Jiang, C.; Liu, H.; Xu, Z.; Shen, J.; Zhang, X.; Zhang, Q.; Du, Y. Vitamin D and Gestational Diabetes Mellitus in the IEU OpenGWAS Project: A Two-Sample Bidirectional Mendelian Randomization Study. Nutrients 2024, 16, 2836. https://doi.org/10.3390/nu16172836
Bai Y, Wang X, Xu Y, Jiang C, Liu H, Xu Z, Shen J, Zhang X, Zhang Q, Du Y. Vitamin D and Gestational Diabetes Mellitus in the IEU OpenGWAS Project: A Two-Sample Bidirectional Mendelian Randomization Study. Nutrients. 2024; 16(17):2836. https://doi.org/10.3390/nu16172836
Chicago/Turabian StyleBai, Yuxuan, Xiaoxiao Wang, Yaxuan Xu, Chang Jiang, Haoran Liu, Zixiu Xu, Jinping Shen, Xumei Zhang, Qiang Zhang, and Yue Du. 2024. "Vitamin D and Gestational Diabetes Mellitus in the IEU OpenGWAS Project: A Two-Sample Bidirectional Mendelian Randomization Study" Nutrients 16, no. 17: 2836. https://doi.org/10.3390/nu16172836





