Modifiable and Non-Modifiable Factors That Affect Human Milk Oligosaccharides Composition
Abstract
1. Introduction
2. Non-Modifiable Factors
2.1. Genetic Background
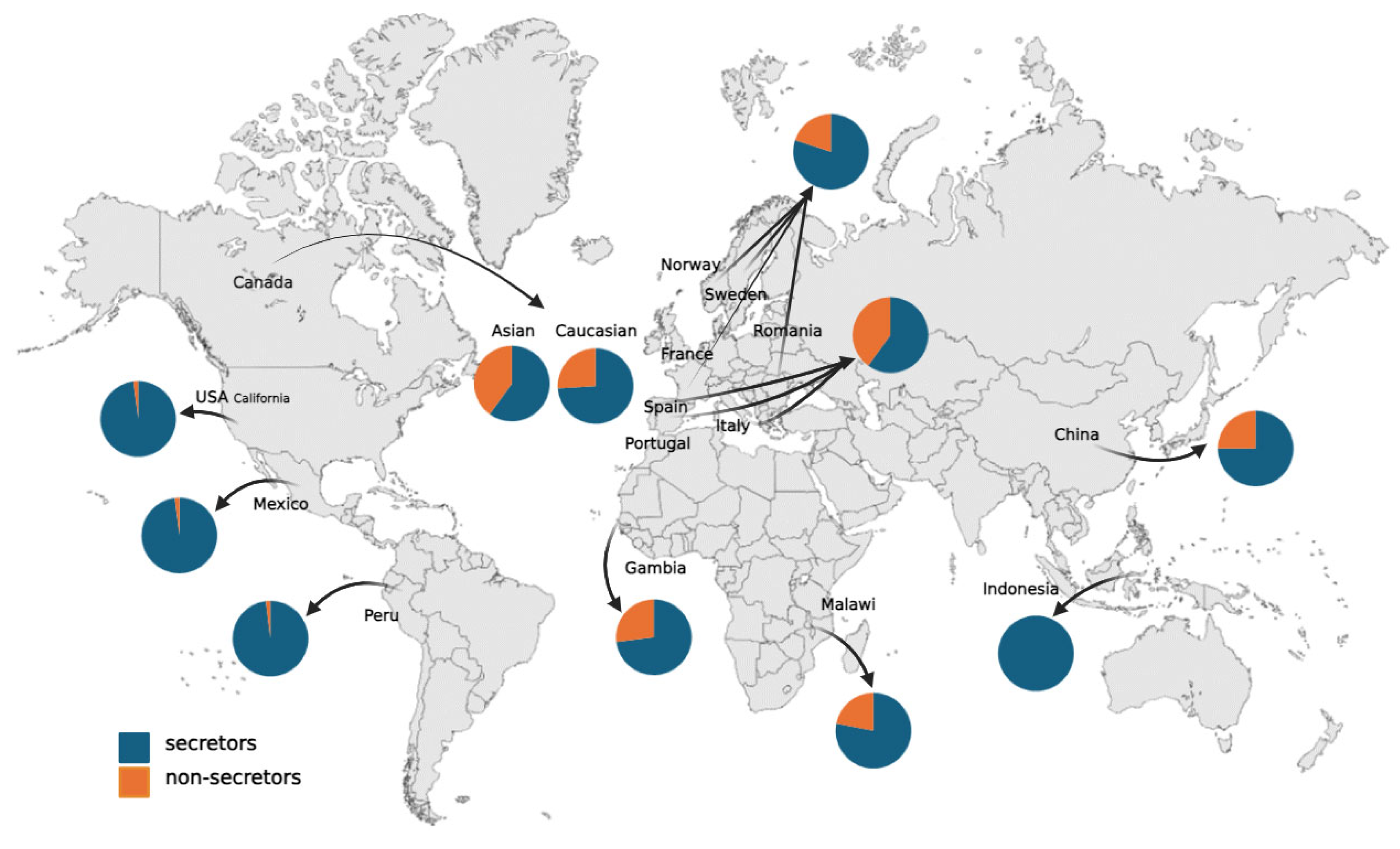
2.2. Race/Ethnicity
2.3. Lactational Stage
2.4. Mother’s Age
2.5. Parity
3. Modifiable Factors
3.1. Mode of Delivery
3.2. Gestational Age
3.3. Breastfeeding Frequency and Duration
3.4. Maternal Diet
3.5. Maternal ppBMI
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Friedmann, H.C. Escherich and Escherichia. EcoSal Plus 2014, 6, 10–128. [Google Scholar] [CrossRef]
- Torrey, J.C. New differential plating methods for B. Bifidus (tissier) and B. Acidophilus (moro). J. Bacteriol. 1917, 2, 435–439. [Google Scholar] [CrossRef]
- Montreuil, J.; Renner, B.; Sawatzki, G. The Saga of Human Milk Gynolactose. In New Perspectives in Infant Nutrition; Georg Thieme Verlag: New York, NY, USA, 1992; pp. 3–11. [Google Scholar]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- György, P.; Norris, R.F.; Rose, C.S. Bifidus Factor. I. A Variant of Lactobacillus Bifidus Requiring a Special Growth Factor. Arch. Biochem. Biophys. 1954, 48, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gauhe, A.; György, P.; Hoover, J.R.E.; Kuhn, R.; Rose, C.S.; Ruelius, H.W.; Zilliken, F. Bifidus Factor. IV. Preparations Obtained from Human Milk. Arch. Biochem. Biophys. 1954, 48, 214–224. [Google Scholar] [CrossRef]
- György, P.; Hoover, J.R.E.; Kuhn, R.; Rose, C.S. Bifidus Factor. Bifidus Factor. III. The Rate of Dialysis. Arch. Biochem. Biophys. 1954, 48, 209–213. [Google Scholar] [CrossRef]
- Watkins, W.M. Structure, genetics and biosynthesis of blood-group-specific glycoproteins. Their composition, structure and function. Biochem. J. 1972, 128, 114P. [Google Scholar] [CrossRef]
- Kobata, A. Structures and Application of Oligosaccharides in Human Milk. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 731–747. [Google Scholar] [CrossRef]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A Strategy for Annotating the Human Milk Glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar] [CrossRef]
- Pfenninger, A.; Karas, M.; Finke, B.; Stahl, B. Structural Analysis of Underivatized Neutral Human Milk Oligosaccharides in the Negative Ion Mode by Nano-Electrospray MSn (Part 1: Methodology). J. Am. Soc. Mass. Spectrom. 2002, 13, 1331–1340. [Google Scholar] [CrossRef]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of Each Neutral Oligosaccharide in the Milk of Japanese Women during the Course of Lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Kunz, C. Oligosaccharides from Human Milk Influence Growth-Related Characteristics of Intestinally Transformed and Non-Transformed Intestinal Cells. Br. J. Nutr. 2008, 99, 462–471. [Google Scholar] [CrossRef]
- Kuntz, S.; Kunz, C.; Rudloff, S. Oligosaccharides from Human Milk Induce Growth Arrest via G2/M by Influencing Growth-Related Cell Cycle Genes in Intestinal Epithelial Cells. Br. J. Nutr. 2009, 101, 1306–1315. [Google Scholar] [CrossRef]
- Garrido, D.; Kim, J.H.; German, J.B.; Raybould, H.E.; Mills, D.A. Oligosaccharide Binding Proteins from Bifidobacterium Longum Subsp. Infantis Reveal a Preference for Host Glycans. PLoS ONE 2011, 6, e17315. [Google Scholar] [CrossRef]
- Sela, D.A.; Garrido, D.; Lerno, L.; Wu, S.; Tan, K.; Eom, H.J.; Joachimiak, A.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium Longum Subsp. Infantis ATCC 15697 α-Fucosidases Are Active on Fucosylated Human Milk Oligosaccharides. Appl. Environ. Microbiol. 2012, 78, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human Milk Oligosaccharide Blood Group Epitopes and Innate Immune Protection against Campylobacter and Calicivirus Diarrhea in Breastfed Infants. In Protecting Infants through Human Milk, Proceedings of the 11th International Conference of the International Society for Research in Human Milk and Lactat ion (ISRHML), Mexico City, Mexico, 4–8 October 2002; Pickering, L.K., Morrow, A.L., Ruiz-Palacios, G.M., Schandler, R.J., Eds.; Springer: New York, NY, USA, 2004; pp. 443–446. [Google Scholar]
- Facinelli, B.; Marini, E.; Magi, G.; Zampini, L.; Santoro, L.; Catassi, C.; Monachesi, C.; Gabrielli, O.; Coppa, G.V. Breast Milk Oligosaccharides: Effects of 2′-Fucosyllactose and 6′-Sialyllactose on the Adhesion of Escherichia Coli and Salmonella Fyris to Caco-2 Cells. J. Matern. Fetal. Neonatal. Med. 2019, 32, 2950–2952. [Google Scholar] [CrossRef] [PubMed]
- Verkhnyatskaya, S.A.; Kong, C.; Klostermann, C.E.; Schols, H.A.; de Vos, P.; Walvoort, M.T.C. Digestion, Fermentation, and Pathogen Anti-Adhesive Properties of the HMO-Mimic Di-Fucosyl-β-Cyclodextrin. Food Funct. 2021, 12, 5018–5026. [Google Scholar] [CrossRef]
- Comstock, S.S.; Li, M.; Wang, M.; Monaco, M.H.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Dietary Human Milk Oligosaccharides but Not Prebiotic Oligosaccharides Increase Circulating Natural Killer Cell and Mesenteric Lymph Node Memory T Cell Populations in Noninfected and Rotavirus-Infected Neonatal Piglets. J. Nutr. 2017, 147, 1041–1047. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann. Nutr. Metab. 2017, 69, 41–51. [Google Scholar] [CrossRef]
- de Kivit, S.; Kraneveld, A.D.; Garssen, J.; Willemsen, L.E.M. Glycan Recognition at the Interface of the Intestinal Immune System: Target for Immune Modulation via Dietary Components. Eur. J. Pharmacol. 2011, 668, S124–S132. [Google Scholar] [CrossRef]
- Vázquez, E.; Barranco, A.; Ramírez, M.; Gruart, A.; Delgado-García, J.M.; Martínez-Lara, E.; Blanco, S.; Martín, M.J.; Castanys, E.; Buck, R.; et al. Effects of a Human Milk Oligosaccharide, 2′-Fucosyllactose, on Hippocampal Long-Term Potentiation and Learning Capabilities in Rodents. J. Nutr. Biochem. 2015, 26, 455–465. [Google Scholar] [CrossRef]
- Matthies, H.; Staak, S.; Krug, M. Fucose and Fucosyllactose Enhance In-Vitro Hippocampal Long-Term Potentiation. Brain Res. 1996, 725, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal Breastfeeding Practices and Infant and Child Mortality: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm Milk Oligosaccharides during the First Month of Lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Stroble, C.; Underwood, M.A.; Lebrilla, C.B. Detection of Milk Oligosaccharides in Plasma of Infants. Anal. Bioanal. Chem. 2014, 406, 5775–5784. [Google Scholar] [CrossRef]
- Wang, A.; Koleva, P.; du Toit, E.; Geddes, D.T.; Munblit, D.; Prescott, S.L.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; et al. The Milk Metabolome of Non-Secretor and Lewis Negative Mothers. Front. Nutr. 2021, 7, 576966. [Google Scholar] [CrossRef]
- Blank, D.; Dotz, V.; Geyer, R.; Kunz, C. Human Milk Oligosaccharides and Lewis Blood Group: Individual High-Throughput Sample Profiling to Enhance Conclusions From Functional Studies. Adv. Nutr. 2012, 3, 440S–449S. [Google Scholar] [CrossRef]
- Sprenger, G.A.; Baumgärtner, F.; Albermann, C. Production of Human Milk Oligosaccharides by Enzymatic and Whole-Cell Microbial Biotransformations. J. Biotechnol. 2017, 258, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of Four Human Milk Groups with Respect to Lewis Blood Group Dependent Oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef]
- Lefebvre, G.; Shevlyakova, M.; Charpagne, A.; Marquis, J.; Vogel, M.; Kirsten, T.; Kiess, W.; Austin, S.; Sprenger, N.; Binia, A. Time of Lactation and Maternal Fucosyltransferase Genetic Polymorphisms Determine the Variability in Human Milk Oligosaccharides. Front. Nutr. 2020, 7, 574459. [Google Scholar] [CrossRef]
- Williams, J.E.; McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Brooker, S.L.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; Prentice, A.M.; et al. Key Genetic Variants Associated with Variation of Milk Oligosaccharides from Diverse Human Populations. Genomics 2021, 113, 1867–1875. [Google Scholar] [CrossRef]
- Castanys-Muñoz, E.; Martin, M.J.; Prieto, P.A. 2’-Fucosyllactose: An Abundant, Genetically Determined Soluble Glycan Present in Human Milk. Nutr. Rev. 2013, 71, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Le Pendu, J. Histo-Blood Group Antigen and Human Milk Oligosaccharides: Genetic Polymorphism and Risk of Infectious Diseases. In Protecting Infants through Human Milk, Proceedings of the 11th International Conference of the International Society for Research in Human Milk and Lactat ion (ISRHML), Mexico City, Mexico, 4–8 October 2002; Springer: New York, NY, USA, 2004; Volume 554. [Google Scholar]
- Erney, R.M.; Malone, W.T.; Skelding, M.B.; Marcon, A.A.; Kleman-Leyer, K.M.; O’Ryan, M.L.; Ruiz-Palacios, G.; Hilty, M.D.; Pickering, L.K.; Prieto, P.A. Variability of Human Milk Neutral Oligosaccharides in a Diverse Population. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 181–192. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Mller-Werner, B.; Jelinek, J.; Stahl, B. Variation of Human Milk Oligosaccharides in Relation to Milk Groups and Lactational Periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef]
- Dessì, A.; Briana, D.; Corbu, S.; Gavrili, S.; Marincola, F.C.; Georgantzi, S.; Pintus, R.; Fanos, V.; Malamitsi-Puchner, A. Metabolomics of Breast Milk: The Importance of Phenotypes. Metabolites 2018, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Koda, Y. Genetic Variation of FUT2 in a Peruvian Population: Identification of a Novel LTR-Mediated Deletion and Characterization of 4 Nonsynonymous Single-Nucleotide Polymorphisms. Transfusion 2019, 59, 1528. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Pang, H.; Koda, Y. Genetic Variation of FUT2 in a Ghanaian Population: Identification of Four Novel Mutations and Inference of Balancing Selection. Ann. Hematol. 2007, 86, 199–204. [Google Scholar] [CrossRef]
- Koda, Y.; Soejima, M.; Kimura, H. The Polymorphisms of Fucosyltransferases. Leg. Med. 2001, 3, 2–14. [Google Scholar] [CrossRef]
- Koda, Y.; Soejima, M.; Munkhtulga, L.; Iwamoto, S. Genetic Variation of FUT3 in Ghanaians, Caucasians, and Mongolians. Transfusion 2009, 49, 2069. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s Normal? Oligosaccharide Concentrations and Profiles in Milk Produced by Healthy Women Vary Geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Altaye, M.; Chaturvedi, P.; Meinzen-Derr, J.; de Lourdes Guerrero, M.; Morrow, A.L. Innate Protection Conferred by Fucosylated Oligosaccharides of Human Milk against Diarrhea in Breastfed Infants. Glycobiology 2004, 14, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Sudarma, V.; Sunardi, D.; Marzuki, N.S.; Munasir, Z.; Asmarinah; Hidayat, A.; Hegar, B. Human Milk Oligosaccharide Profiles and the Secretor and Lewis Gene Status of Indonesian Lactating Mothers. Pediatr. Gastroenterol. Hepatol. Nutr. 2023, 26, 266. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of Maternal Characteristics on Human Milk Oligosaccharide Composition over the First 4 Months of Lactation in a Cohort of Healthy European Mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Robertson, B.M.; Bode, L.; Sharma, A.K.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; Azad, M.B. Maternal Factors and Human Milk Oligosaccharide Composition in the CHILD Cohort. FASEB J. 2017, 31, 650.36. [Google Scholar] [CrossRef]
- Ren, X.; Yan, J.; Bi, Y.; Shuttleworth, P.W.; Wang, Y.; Jiang, S.; Wang, J.; Duan, Y.; Lai, J.; Yang, Z. Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population. Nutrients 2023, 15, 1408. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Totten, S.M.; Zivkovic, A.M.; Wu, S.; Ngyuen, U.; Freeman, S.L.; Ruhaak, L.R.; Darboe, M.K.; German, J.B.; Prentice, A.M.; Lebrilla, C.B. Comprehensive Profiles of Human Milk Oligosaccharides Yield Highly Sensitive and Specific Markers for Determining Secretor Status in Lactating Mothers. J. Proteome Res. 2012, 11, 6124–6133. [Google Scholar] [CrossRef]
- Larsson, M.M.; Rydell, G.E.P.; Grahn, A.; Rodríguez-Díaz, J.; Åkerlind, B.; Hutson, A.M.; Estes, M.K.; Larson, G.; Svensson, L. Antibody Prevalence and Titer to Norovirus (Genogroup II) Correlate with Secretor (FUT2) but Not with ABO Phenotype or Lewis (FUT3) Genotype. J. Infect. Dis. 2006, 194, 422–1427. [Google Scholar] [CrossRef]
- Corvelo, T.C.O.; Aguiar, D.C.F.; Sagica, F.E.S. The Expression of ABH and Lewis Antigens in Brazilian Semi-Isolated Black Communities. Genet. Mol. Biol. 2002, 25, 259–263. [Google Scholar] [CrossRef]
- Nordgren, J.; Nitiema, L.W.; Ouermi, D.; Simpore, J.; Svensson, L. Host Genetic Factors Affect Susceptibility to Norovirus Infections in Burkina Faso. PLoS ONE 2013, 8, e69557. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, C.; Morales, J.M.; Monleón, D.; du Toit, E.; Kumar, H.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Isolauri, E.; Salminen, S.; et al. Human Breast Milk NMR Metabolomic Profile across Specific Geographical Locations and Its Association with the Milk Microbiota. Nutrients 2018, 10, 1355. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic Review of the Concentrations of Oligosaccharides in Human Milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Soyyilmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-matwiejuk, A.; Vigsnæs, L.K. The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, B.; Jiang, T.; Yang, C.; Qiao, W.; Hou, J.; Han, Y.; Xiao, H.; Chen, L. Improved Simple Sample Pretreatment Method for Quantitation of Major Human Milk Oligosaccharides Using Ultrahigh Pressure Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2019, 67, 3445. [Google Scholar] [CrossRef]
- Xu, G.; Davis, J.C.C.; Goonatilleke, E.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Absolute Quantitation of Human Milk Oligosaccharides Reveals Phenotypic Variations during Lactation. J. Nutr. 2017, 147, 117–124. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; Van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal Change of Selected Human Milk Oligosaccharides and Association to Infants’ Growth, an Observatory, Single Center, Longitudinal Cohort Study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef]
- Poulsen, K.O.; Meng, F.; Lanfranchi, E.; Young, J.F.; Stanton, C.; Ryan, C.A.; Kelly, A.L.; Sundekilde, U.K. Dynamic Changes in the Human Milk Metabolome Over 25 Weeks of Lactation. Front. Nutr. 2022, 9, 917659. [Google Scholar] [CrossRef]
- Ma, L.; McJarrow, P.; Jan Mohamed, H.J.B.; Liu, X.; Welman, A.; Fong, B.Y. Lactational Changes in the Human Milk Oligosaccharide Concentration in Chinese and Malaysian Mothers’ Milk. Int. Dairy J. 2018, 87, 1–10. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Najera, J.A.; Khwajazada, S.; Bode, L.; Goran, M.I. Longitudinal Changes in Human Milk Oligosaccharides (HMOs) over the Course of 24 Months of Lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Thum, C.; Wall, C.R.; Weiss, G.A.; Wang, W.; Szeto, I.M.Y.; Day, L. Changes in Hmo Concentrations throughout Lactation: Influencing Factors, Health Effects and Opportunities. Nutrients 2021, 13, 2272. [Google Scholar] [CrossRef] [PubMed]
- Asakuma, S.; Urashima, T.; Akahori, M.; Obayashi, H.; Nakamura, T.; Kimura, K.; Watanabe, Y.; Arai, I.; Sanai, Y. Variation of Major Neutral Oligosaccharides Levels in Human Colostrum. Eur. J. Clin. Nutr. 2008, 62, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, S.; Yan, J.; Yan, S.; Chen, J.; Lu, Z.; Zhang, B.; Lane, J. Longitudinal Changes of Human Milk Oligosaccharides, Breastmilk Microbiome and Infant Gut Microbiome Are Associated with Maternal Characteristics. Int. J. Food Sci. Technol. 2022, 57, 2793–2807. [Google Scholar] [CrossRef]
- Liu, S.; Mao, Y.; Wang, J.; Tian, F.; Hill, D.R.; Xiong, X.; Li, X.; Zhao, Y.; Wang, S. Lactational and Geographical Variation in the Concentration of Six Oligosaccharides in Chinese Breast Milk: A Multicenter Study over 13 Months Postpartum. Front. Nutr. 2023, 10, 1267287. [Google Scholar] [CrossRef]
- Asher, A.T.; Mangel, L.; Ari, J.B.; Gover, O.; Ahmad, W.A.; Herzlich, J.; Mandel, D.; Schwartz, B.; Lubetzky, R. Human Milk Oligosaccharide Profile across Lactation Stages in Israeli Women—A Prospective Observational Study. Nutrients 2023, 15, 2548. [Google Scholar] [CrossRef]
- Asakuma, S.; Akahori, M.; Kimura, K.; Watanabe, Y.; Nakamura, T.; Tsunemi, M.; Arai, I.; Sanai, Y.; Urashima, T. Sialyl Oligosaccharides of Human Colostrum: Changes in Concentration during the First Three Days of Lactation. Biosci. Biotechnol. Biochem. 2007, 71, 1447–1451. [Google Scholar] [CrossRef]
- Mank, M.; Hauner, H.; Heck, A.J.R.; Stahl, B. Targeted LC-ESI-MS2 Characterization of Human Milk Oligosaccharide Diversity at 6 to 16 Weeks Post-Partum Reveals Clear Staging Effects and Distinctive Milk Groups. Anal. Bioanal. Chem. 2020, 412, 6887–6907. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhao, A.; Zhang, J.; Wu, W.; Ren, Z.; Wang, P.; Zhang, Y. Neutral Human Milk Oligosaccharides Are Associated with Multiple Fixed and Modifiable Maternal and Infant Characteristics. Nutrients 2020, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Tonon, K.M.; de Morais, M.B.; Abrão, A.C.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef]
- Austin, S.; de Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Thielecke, F.; Lavalle, L.; Chen, C.; Fogel, P.; Giuffrida, F.; Dubascoux, S.; Martínez-Costa, C.; Haaland, K.; Marchini, G.; et al. Mode of Neonatal Delivery Influences the Nutrient Composition of Human Milk: Results From a Multicenter European Cohort of Lactating Women. Front. Nutr. 2022, 9, 834394. [Google Scholar] [CrossRef]
- De Leoz, M.L.A.; Gaerlan, S.C.; Strum, J.S.; Dimapasoc, L.M.; Mirmiran, M.; Tancredi, D.J.; Smilowitz, J.T.; Kalanetra, K.M.; Mills, D.A.; German, J.B.; et al. Lacto-N-Tetraose, Fucosylation, and Secretor Status Are Highly Variable in Human Milk Oligosaccharides from Women Delivering Preterm. J. Proteome. Res. 2012, 11, 4662–4672. [Google Scholar] [CrossRef]
- Nakhla, T.; Fu, D.; Zopf, D.; Brodsky, N.L.; Hurt, H. Neutral Oligosaccharide Content of Preterm Human Milk. Br. J. Nutr. 1999, 82, 361–367. [Google Scholar] [CrossRef]
- Davidson, B.; Meinzen-Derr, J.K.; Wagner, C.L.; Newburg, D.S.; Morrow, A.L. Fucosylated Oligosaccharides in Human Milk in Relation to Gestational Age and Stage of Lactation. In Protecting Infants through Human Milk, Proceedings of the 11th International Conference of the International Society for Research in Human Milk and Lactat ion (ISRHML), Mexico City, Mexico, 4–8 October 2002; Pickering, L.K., Morrow, A.L., Ruiz-Palacios, G.M., Schandler, R.J., Eds.; Springer: New York, NY, USA, 2004; pp. 427–430. [Google Scholar]
- Austin, S.; De Castro, C.A.; Sprenger, N.; Binia, A.; Affolter, M.; Garcia-Rodenas, C.L.; Beauport, L.; Tolsa, J.F.; Fumeaux, C.J.F. Human Milk Oligosaccharides in the Milk of Mothers Delivering Term versus Preterm Infants. Nutrients 2019, 11, 11282. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The Human Milk Oligosaccharide Disialyllacto-N-Tetraose Prevents Necrotising Enterocolitis in Neonatal Rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef]
- Berger, P.K.; Hampson, H.E.; Schmidt, K.A.; Alderete, T.L.; Furst, A.; Yonemitsu, C.; Demerath, E.; Goran, M.I.; Fields, D.A.; Bode, L. Stability of Human-Milk Oligosaccharide Concentrations Over 1 Week of Lactation and Over 6 Hours Following a Standard Meal. J. Nutr. 2022, 152, 214. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal Diet Alters Human Milk Oligosaccharide Composition with Implications for the Milk Metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Meyer, K.M.; Mohammad, M.; Bode, L.; Chu, D.M.; Ma, J.; Haymond, M.; Aagaard, K. 20: Maternal Diet Structures the Breast Milk Microbiome in Association with Human Milk Oligosaccharides and Gut-Associated Bacteria. Am. J. Obstet. Gynecol. 2017, 216, S15. [Google Scholar] [CrossRef][Green Version]
- Selma-Royo, M.; González, S.; Gueimonde, M.; Chang, M.; Fürst, A.; Martínez-Costa, C.; Bode, L.; Collado, M.C. Maternal Diet Is Associated with Human Milk Oligosaccharide Profile. Mol. Nutr. Food Res. 2022, 66, 2200058. [Google Scholar] [CrossRef] [PubMed]
- Seppo, A.E.; Kukkonen, A.K.; Kuitunen, M.; Savilahti, E.; Yonemitsu, C.; Bode, L.; Järvinen, K.M. Association of Maternal Probiotic Supplementation with Human Milk Oligosaccharide Composition. JAMA Pediatr. 2019, 173, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mao, Y.; Liu, S.; Wang, J.; Li, X.; Zhao, Y.; Hill, D.R.; Wang, S. Vitamins, Vegetables and Metal Elements Are Positively Associated with Breast Milk Oligosaccharide Composition among Mothers in Tianjin, China. Nutrients 2022, 14, 4131. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F. Influence of Sulfonated and Diet-Derived Human Milk Oligosaccharides on the Infant Microbiome and Immune Markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, J.; Yang, J.; Gu, G. The Relationship between Dietary Vitamin A Intake and the Levels of Sialic Acid in the Breast Milk of Lactating Women. J. Nutr. Sci. Vitaminol. 2013, 59, 347–351. [Google Scholar] [CrossRef][Green Version]
- Biddulph, C.; Holmes, M.; Tran, T.D.; Kuballa, A.; Davies, P.S.W.; Koorts, P.; Maher, J. Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women. Nutrients 2023, 15, 2093. [Google Scholar] [CrossRef]
- Jorgensen, J.M.; Arnold, C.; Ashorn, P.; Ashorn, U.; Chaima, D.; Cheung, Y.B.; Davis, J.C.C.; Fan, Y.-M.; Goonatilleke, E.; Kortekangas, E.; et al. Lipid-Based Nutrient Supplements During Pregnancy and Lactation Did Not Affect Human Milk Oligosaccharides and Bioactive Proteins in a Randomized Trial12. J. Nutr. 2017, 147, 1867–1874. [Google Scholar] [CrossRef]
- Neville, J.; Pawlak, R.; Chang, M.; Furst, A.; Bode, L.; Perrin, M.T. A Cross-Sectional Assessment of Human Milk Oligosaccharide Composition of Vegan, Vegetarian, and Nonvegetarian Mothers. Breastfeed. Med. 2022, 17, 210–217. [Google Scholar] [CrossRef]
- Bottin, J.H.; Eussen, S.R.B.M.; Igbinijesu, A.J.; Mank, M.; Koyembi, J.C.J.; Nyasenu, Y.T.; Ngaya, G.; Mad-Bondo, D.; Kongoma, J.B.; Stahl, B.; et al. Food Insecurity and Maternal Diet Influence Human Milk Composition between the Infant’s Birth and 6 Months after Birth in Central-Africa. Nutrients 2022, 14, 4015. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Labiner-Wolfe, J.; Geraghty, S.R.; Rasmussen, K.M. Associations between High Prepregnancy Body Mass Index, Breast-Milk Expression, and Breast-Milk Production and Feeding. Am. J. Clin. Nutr. 2011, 93, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Lagström, H.; Rautava, S.; Ollila, H.; Kaljonen, A.; Turta, O.; Mäkelä, J.; Yonemitsu, C.; Gupta, J.; Bode, L. Associations between Human Milk Oligosaccharides and Growth in Infancy and Early Childhood. Am. J. Clin. Nutr. 2020, 111, 769–778. [Google Scholar] [CrossRef]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated With Excessive Weight Gain During Exclusive Breastfeeding—An Explorative Study. Front. Pediatr. 2019, 7, 297. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human Milk Oligosaccharide 2’-Fucosyllactose Links Feedings at 1 Month to Cognitive Development at 24 Months in Infants of Normal and Overweight Mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Puopolo, K.M.; Newburg, D.S.; Lönnerdal, B.; Chen, C.; Allen, M.; Merewood, A.; Worden, S.; Welt, C.K. Effects of Recombinant Human Prolactin on Breast Milk Composition. Pediatrics 2011, 127, e359–e366. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Sobieraj, P.; Szostak-Węgierek, D.; Wesołowska, A. Impact of Infant and Maternal Factors on Energy and Macronutrient Composition of Human Milk. Nutrients 2020, 12, 2591. [Google Scholar] [CrossRef]
| Oligosaccharide * | Factor | Association | References |
|---|---|---|---|
| 2’-FL | genetic | Absent or very low amounts in non-secretors, presence correlated with FUT2 gene | [32,33,34,35] |
| race/ethnicity | ↑ in milk from Peruvian mothers | [43,44] | |
| lactational stage | ↓ over lactation | [36,45,63,65,67,69,70] | |
| mother age | ↑ with age | [43,74] | |
| parity | ↑ in primiparous mothers | [43,46,47] | |
| mode of delivery | ↓ in C-section deliveries | [55,77] | |
| maternal diet | ↑ with vitamin B intake | [89] | |
| 3’-FL | genetic | ↑ with certain FUT3 gene variants, found in Lewis-negative (le-) individuals | [33,36] [36,37] |
| race/ethnicity | ↑ levels in milk from Peruvian mothers, ↑ in Asian mothers | [43,44] [47] | |
| lactational stage | ↑ over lactation | [36,45,63,65,67,69,70] | |
| parity | ↓ in multiparous mothers | [43,46,47] | |
| gestational age | negative association with gestational age | [74] | |
| 3’-SL | race/ethnicity | ↑ levels in milk from Peruvian mothers | [43,44] |
| lactational stage | ↑ after 4 months of lactation, before that time levels debatable | [32,47,65] | |
| mode of delivery | ↓ in C-section deliveries | [55,77] | |
| breastfeeding frequency | ↓ with breastfeeding duration | [47,84,85] | |
| 6’-SL | lactational stage | ↓ over lactation | [32,49,62,64,65,66] |
| 6’-GL | mode of delivery | ↓ in C-section deliveries | [55,77] |
| LNT | lactational stage | ↓ after 4 months of lactation | [62,63,64,65,66] |
| breastfeeding frequency | ↑ with breastfeeding duration | [47,84,85] | |
| LNnT | lactational stage | ↓ after 4 months of lactation | [62,63,64,65,66] |
| mother age | ↓ with age | [43,47,74] | |
| parity | ↓ in multiparous mothers | [43,46,47] | |
| gestational age | more abundant in preterm milk | [78] | |
| LST | mother age | ↓ with age | [43,47,74] |
| LSTb | gestational age | ↑ in preterm milk | [81,82] |
| LSTc | race/ethnicity | ↑ in Asian mothers | [47] |
| lactational stage | ↓ over lactation | [32,49,65,66,69] | |
| LNFP-I | genetic | ↓ in non-secretors | [32,33,34,35] |
| LNFP-II | genetic | absent in some Lewis-positive individuals | [32] |
| gestational age | negative association with gestational age | [74] | |
| LNFP-III | genetic | found in Lewis-negative (Le−) individuals | [36,37] |
| race/ethnicity | ↑ in Swedish and Chinese mothers | [43,55] | |
| parity | ↑ in primiparous mothers | [43,46,47] | |
| mode of delivery | ↑ in natural deliveries | [55] | |
| LNFP-IV | gestational age | negative association with gestational age | [74] |
| DSLNH | mother age | ↓ with age | [43,47,74] |
| maternal diet | ↑ with multivitamin intake | [47] | |
| DSLNT | gestational age | ↑ in preterm milk | [81,82] |
| DFLNH, FLNH | mother age | ↑ with age | [43,74] |
| FDSLNH | breastfeeding frequency | ↑ with breastfeeding duration | [47,84,85] |
| TFLNH-II, IFLNH-I, DFpLNH-I, DFpLNH-II | gestational age | negative association with gestational age | [74] |
| Fucosylated HMOs (as a whole group) | race/ethnicity | ↓ in non-secretor mothers from Malawi and Gambia (region dependent) | [50,51] |
| maternal diet | ↑ with vitamin B intake | [89] | |
| Sialylated HMOs (as a whole group) | race/ethnicity | ↓ in non-secretor mothers from Malawi and Gambia (probably connected with malnutrition) | [50,51] |
| maternal diet | ↓ with malnutrition ↓ with a lipid-rich diet ↑ with vitamin A intake ↑ fruit intake | [50,85,86,87] [90,91,92] [90] | |
| gestational age | ↑ in preterm milk | [81,82] | |
| General HMOs | maternal age | ↑ with meat and poultry consumption | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczna, M.; Koryszewska-Bagińska, A.; Bzikowska-Jura, A.; Chmielewska-Jeznach, M.; Jarzynka, S.; Olędzka, G. Modifiable and Non-Modifiable Factors That Affect Human Milk Oligosaccharides Composition. Nutrients 2024, 16, 2887. https://doi.org/10.3390/nu16172887
Konieczna M, Koryszewska-Bagińska A, Bzikowska-Jura A, Chmielewska-Jeznach M, Jarzynka S, Olędzka G. Modifiable and Non-Modifiable Factors That Affect Human Milk Oligosaccharides Composition. Nutrients. 2024; 16(17):2887. https://doi.org/10.3390/nu16172887
Chicago/Turabian StyleKonieczna, Małgorzata, Anna Koryszewska-Bagińska, Agnieszka Bzikowska-Jura, Magdalena Chmielewska-Jeznach, Sylwia Jarzynka, and Gabriela Olędzka. 2024. "Modifiable and Non-Modifiable Factors That Affect Human Milk Oligosaccharides Composition" Nutrients 16, no. 17: 2887. https://doi.org/10.3390/nu16172887
APA StyleKonieczna, M., Koryszewska-Bagińska, A., Bzikowska-Jura, A., Chmielewska-Jeznach, M., Jarzynka, S., & Olędzka, G. (2024). Modifiable and Non-Modifiable Factors That Affect Human Milk Oligosaccharides Composition. Nutrients, 16(17), 2887. https://doi.org/10.3390/nu16172887






