Association of Polyamine Intake, Other Dietary Components, and Fecal Content of N-acetyl Putrescine and Cadaverine with Patients’ Colorectal Lesions
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants, Study Design, and Variables
2.2. Daily Intake of Food and Polyamines
2.3. Determination of Polyamines in Feces via High-Performance Liquid Chromatography (HPLC)
2.4. Statistical Analyses
3. Results
3.1. Study Participants and Types of Colorectal Lesion
3.2. Association of Nutritional Survey Data and the Presence of Colorectal Lesions
3.3. Association of Dietary Intake of Polyamines with Colorectal Lesions in Patients
3.4. Association of Biogenic Amines in Feces with Colorectal Lesions in Patients
3.5. Correlation between Dietary Intake of Polyamines and Their Fecal Content in Relation to Colorectal Lesion Types
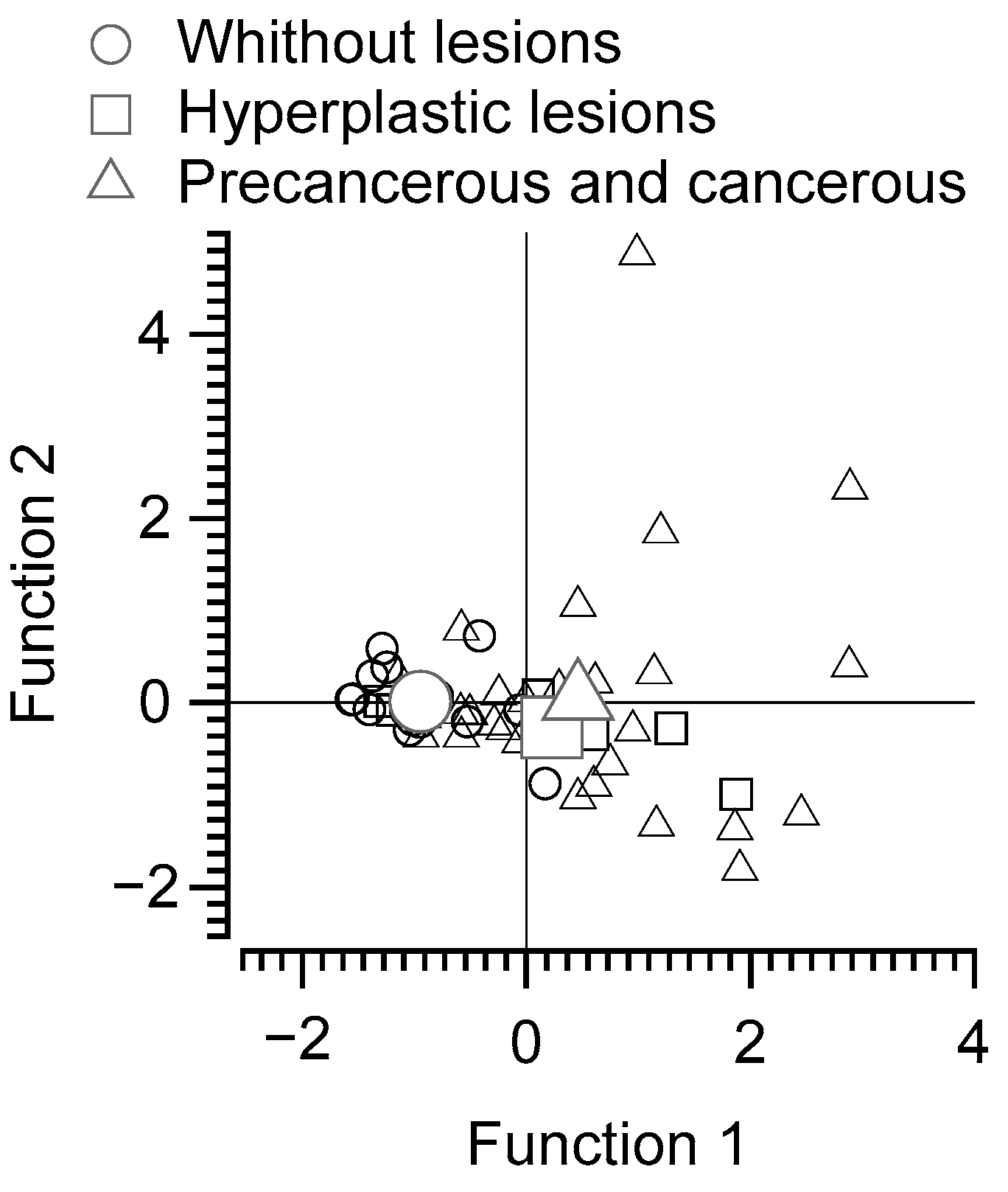
3.6. Discriminant Function Analysis of Subjects without Lesions, with Benign Lesions, and with Precancerous or Malignant Lesions Based on Polyamine Intake and Fecal Content of N-acetyl Putrescine and Cadaverine
3.7. Multiple Regression Analysis on Fecal Polyamines as Dependent Variables
3.8. Logistic Regression Analysis to Predict the Presence of Colorectal Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.; Mininni, M.; Deiana, G.; Marino, G.; Divella, R.; Bochicchio, I.; Giuliano, A.; Lapadula, S.; Lettini, A.R.; Sanseverino, F. Healthy Lifestyle and Cancer Risk: Modifiable Risk Factors to Prevent Cancer. Nutrients 2024, 16, 800. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Beaumont, M.; Andriamihaja, M.; Davila, A.M.; Lan, A.; Grauso, M.; Armand, L.; Benamouzig, R.; Tomé, D. Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences. Am. J. Pathol. 2017, 187, 476–486. [Google Scholar] [CrossRef]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Cantabrana, B.; Peña-Iglesias, P.; Castro-Estrada, P.; Suarez, L.; Bordallo, J.; Barreiro-Alonso, E.; Sánchez, M. Dietary intake of polyamines in the Spanish adult population showed a direct correlation with the healthy dietary index score and inverse with the dietary inflammatory index score. Nutrition 2024. submitted. [Google Scholar]
- Liu, B.; Jiang, X.; Cai, L.; Zhao, X.; Dai, Z.; Wu, G.; Li, X. Putrescine mitigates intestinal atrophy through suppressing inflammatory response in weanling piglets. J. Anim. Sci. Biotechnol. 2019, 10, 69. [Google Scholar] [CrossRef]
- Sánchez, M.; Suárez, L.; Andrés, M.T.; Flórez, B.H.; Bordallo, J.; Riestra, S.; Cantabrana, B. Modulatory effect of intestinal polyamines and trace amines on the spontaneous phasic contractions of the isolated ileum and colon rings of mice. Food Nutr. Res. 2017, 61, 1321948. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Hu, S.; Wang, X. Eflornithine for chemoprevention in the high-risk population of colorectal cancer: A systematic review and meta-analysis with trial sequential analysis. Front. Oncol. 2023, 13, 1281844. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Das, C.; Mande, S.S. In Silico Analysis of Putrefaction Pathways in Bacteria and Its Implication in Colorectal Cancer. Front. Microbiol. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Sittipo, P.; Shim, J.W.; Lee, Y.K. Microbial Metabolites Determine Host Health and the Status of Some Diseases. Int. J. Mol. Sci. 2019, 20, 5296. [Google Scholar] [CrossRef]
- Gong, D.; Adomako-Bonsu, A.G.; Wang, M.; Li, J. Three specific gut bacteria in the occurrence and development of colorectal cancer: A concerted effort. PeerJ 2023, 11, e15777. [Google Scholar] [CrossRef]
- Abdulla, M.H.; Agarwal, D.; Singh, J.K.; Traiki, T.B.; Pandey, M.K.; Ahmad, R.; Srivastava, S.K. Association of the microbiome with colorectal cancer development (Review). Int. J. Oncol. 2021, 58, 17. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstern, C.R.; Lamichhane-Khadka, R. A tale of two bacteria—Bacteroides fragilis, Escherichia coli, and colorectal cancer. Front. Bacteriol. 2023, 2, 1229077. [Google Scholar] [CrossRef]
- Hanus, M.; Parada-Venegas, D.; Landskron, G.; Wielandt, A.M.; Hurtado, C.; Alvarez, K.; Hermoso, M.A.; López-Köstner, F.; De la Fuente, M. Immune System, Microbiota, and Microbial Metabolites: The Unresolved Triad in Colorectal Cancer Microenvironment. Front. Immunol. 2021, 12, 612826. [Google Scholar] [CrossRef]
- Ye, C.; Liu, X.; Liu, Z.; Pan, C.; Zhang, X.; Zhao, Z.; Sun, H. Fusobacterium nucleatum in tumors: From tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 2024, 25, 2306676. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.J.; Hendrix, J.M.; Garmon, E.H. American Society of Anesthesiologists Classification. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kristal, A.R.; Kolar, A.S.; Fisher, J.L.; Plascak, J.J.; Stumbo, P.J.; Weiss, R.; Paskett, E.D. Evaluation of web-based, self-administered, graphical food frequency questionnaire. J. Acad. Nutr. Diet. 2014, 114, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Basiotis, P.; Carlson, A.; Gerrior, S.; Juan, W.; Lino, M. The Healthy Eating Index: 1999–2000; USDA, Center for Nutrition Policy and Promotion, CNPP-12: Washington, DC, USA, 2002. [Google Scholar]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Escribano, M.I.; Legaz, M.E. High performance liquid chromatography of the dansyl derivatives of putrescine, spermidine, and spermine. Plant Physiol. 1988, 87, 519–522. [Google Scholar] [CrossRef]
- Castelló, A.; Amiano, P.; Fernández de Larrea, N.; Martín, V.; Alonso, M.H.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Olmedo-Requena, R.; Guevara, M.; Fernandez-Tardon, G.; et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur. J. Nutr. 2019, 58, 1495–1505. [Google Scholar] [CrossRef]
- Arthur, R.S.; Kirsh, V.A.; Rohan, T.E. The association of the healthy eating index with risk of colorectal cancers (overall and by subsite) among Canadians. Cancer Epidemiol. 2023, 87, 102454. [Google Scholar] [CrossRef]
- Nardone, O.M.; Zammarchi, I.; Santacroce, G.; Ghosh, S.; Iacucci, M. Inflammation-Driven Colorectal Cancer Associated with Colitis: From Pathogenesis to Changing Therapy. Cancers 2023, 15, 2389. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Rodríguez-Barranco, M.; Fernández de Larrea, N.; Jakszyn, P.; Dorronsoro, A.; Amiano, P.; Chirlaque, M.D.; Colorado-Yohar, S.; Guevara, M.; Moreno-Iribas, C.; et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients 2022, 14, 3085. [Google Scholar] [CrossRef] [PubMed]
- ter Steege, J.C.; Buurman, W.A.; Forget, P.P. Spermine induces maturation of the immature intestinal immune system in neonatal mice. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 332–340. [Google Scholar] [CrossRef] [PubMed]
- van Wettere, W.H.; Willson, N.L.; Pain, S.J.; Forder, R.E. Effect of oral polyamine supplementation pre-weaning on piglet growth and intestinal characteristics. Animal Int. J. Anim. Biosci. 2016, 10, 1655–1659. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-Promoting Effects of Dietary Polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Madeo, F.; Hofer, S.J.; Pendl, T.; Bauer, M.A.; Eisenberg, T.; Carmona-Gutierrez, D.; Kroemer, G. Nutritional Aspects of Spermidine. Annu. Rev. Nutr. 2020, 40, 135–159. [Google Scholar] [CrossRef]
- Vargas, A.J.; Ashbeck, E.L.; Wertheim, B.C.; Wallace, R.B.; Neuhouser, M.L.; Thomson, C.A.; Thompson, P.A. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am. J. Clin. Nutr. 2015, 102, 411–419. [Google Scholar] [CrossRef]
- Vargas, A.J.; Wertheim, B.C.; Gerner, E.W.; Thomson, C.A.; Rock, C.L.; Thompson, P.A. Dietary polyamine intake and risk of colorectal adenomatous polyps. Am. J. Clin. Nutr. 2012, 96, 133–141. [Google Scholar] [CrossRef]
- Huang, C.Y.; Fang, Y.J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.Y.; Zhang, X.; Feng, X.L.; Chen, Y.M.; Zhang, C.X. Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrients 2020, 12, 3575. [Google Scholar] [CrossRef]
- Gobert, A.P.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Finley, J.L.; Smith, T.M.; McNamara, K.M.; Singh, K.; Sierra, J.C.; et al. Protective Role of Spermidine in Colitis and Colon Carcinogenesis. Gastroenterology 2022, 162, 813–827.e818. [Google Scholar] [CrossRef]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; Boer, V.C.J. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Milovic, V. Polyamines in the gut lumen: Bioavailability and biodistribution. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1021–1025. [Google Scholar] [CrossRef]
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P.; Akdis, C.A.; O’Mahony, L. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis. 2017, 28, 1353881. [Google Scholar] [CrossRef]
- Yang, Y.; Misra, B.B.; Liang, L.; Bi, D.; Weng, W.; Wu, W.; Cai, S.; Qin, H.; Goel, A.; Li, X.; et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019, 9, 4101–4114. [Google Scholar] [CrossRef] [PubMed]
- Coradduzza, D.; Arru, C.; Culeddu, N.; Congiargiu, A.; Azara, E.G.; Scanu, A.M.; Zinellu, A.; Muroni, M.R.; Rallo, V.; Medici, S.; et al. Quantitative Metabolomics to Explore the Role of Plasma Polyamines in Colorectal Cancer. Int. J. Mol. Sci. 2022, 24, 101. [Google Scholar] [CrossRef]
- Venäläinen, M.K.; Roine, A.N.; Häkkinen, M.R.; Vepsäläinen, J.J.; Kumpulainen, P.S.; Kiviniemi, M.S.; Lehtimäki, T.; Oksala, N.K.; Rantanen, T.K. Altered Polyamine Profiles in Colorectal Cancer. Anticancer. Res. 2018, 38, 3601–3607. [Google Scholar] [CrossRef]
- Kuwabara, H.; Katsumata, K.; Iwabuchi, A.; Udo, R.; Tago, T.; Kasahara, K.; Mazaki, J.; Enomoto, M.; Ishizaki, T.; Soya, R.; et al. Salivary metabolomics with machine learning for colorectal cancer detection. Cancer Sci. 2022, 113, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
| Number of Colorectal Lesions | Number of Patients | Percentage |
| 1 | 14 | 34.15 |
| 2 | 11 | 26.83 |
| 3–5 | 11 | 26.83 |
| 6–10 | 5 | 12.20 |
| Location of Colorectal Lesions | Number of Lesions | Percentage |
| Ascending colon | 20 | 18.87 |
| Transverse colon | 11 | 10.38 |
| Descending colon | 9 | 8.49 |
| Sigmoid colon | 47 | 44.34 |
| Rectum | 19 | 17.92 |
| Anatomical Pathology Classification | Number of Lesions | Percentage |
| Hyperplastic lesions | 23 | 21.70 |
| Tubular adenomas | 69 | 65.09 |
| Tubulovillous adenomas | 11 | 10.38 |
| Adenocarcinomas | 3 | 2.83 |
| Variable | Without Colorectal Lesions | With Colorectal Lesions | Hyperplastic Lesions | Precancerous and Adenocarcinomas |
|---|---|---|---|---|
| Characteristics of participants and of diets | ||||
| Age (years) | 58.42 (52.23–65.41) | 61.25 (56.24–64.93) | 58.73 (54.86–61.16) | 62.17 (56.44–65.22) |
| Calories (kcal/day) | 1973.83 (1642.46–2162.3) | 2120.13 (1909.81–2618.88) | 2918.25 (1923.8–3070.51) | 2108.4 (1907.34–2470.14) |
| BMI (kg/m2) | 26.45 (22.78–34.23) | 27 (25–29.7) | 24.85 (24.05–34.05) | 27.1 (25.4–29.4) |
| HEI Score | 75.8 (67.05–80.23) | 68.4 (61.85–79.65) | 65.7 (56.25–79.4) | 72.1 (61.9–80) |
| DII Score | −2.09 (−3.4–−0.44) | −1.32 (−3.01–0.59) | −1.1 (−3.48–1.15) | −1.32 (−2.9–−0.19) |
| Components of daily diet | ||||
| Alcohol calories (kcal) | 26.82 (13.84–39.71) | 105.92 (20.13–260.43) * | 228.7 (9.62–362.92) | 105.02 (20.76–253.83) |
| Alcohol servings | 0.2 (0.11–0.28) | 0.85 (0.17–2.19) ** | 1.93 (0.1–2.84) | 0.73 (0.18–2.16) ɸ |
| Cholesterol (mg) | 211.22 (150.75–303.79) | 296.86 (183.75–341.12) | 365.47 (288.64–453.69) ɸ | 265.89 (167.32–337.76) |
| Number of citrus, melon, berry cup equivalents (cups) | 0.56 (0.18–0.64) | 0.77 (0.3–1.36) * | 0.93 (0.3–2.69) | 0.77 (0.27–1.3) |
| Fish servings | 1.92 (0.87–2.94) | 2.21 (1.31–3.43) | 4.64 (2.19–7.79) | 1.7 (1.16–3.08) |
| Fructose (g) | 18.22 (14.97–22.41) | 22.36 (17.74–28.15) | 27.94 (23.39–34.54) ɸ | 20.68 (17.09–25.75) |
| Galactose (g) | 0.26 (0.13–0.47) | 0.48 (0.21–1.96) * | 1.22 (0.23–2.12) | 0.42 (0.21–1.95) |
| Glucose (g) | 16.71 (15.12–18.49) | 21.17 (16.09–27.94) * | 28.64 (23.74–32.92) ɸɸ | 20.44 (15.26–24.7) |
| Inositol (g) | 0.42 (0.33–0.52) | 0.56 (0.4–0.85) * | 0.65 (0.39–1.33) | 0.56 (0.4–0.83) |
| Lignan secoisolariciresinol | 65.21 (54.38–102.47) | 103.97 (65.44–139.35) * | 132.23 (90.22–151.01) | 91.99 (59.79–139.95) |
| Low-fat dairy servings | 1.62 (0.49–2.41) | 0.53 (0.06–1.5) * | 0.64 (0.2–0.98) | 0.45 (0.05–1.5) |
| Eggs (equivalent to 28.3 g of lean meat) | 0.38 (0.29–0.63) | 0.63 (0.41–1.04) * | 1.05 (0.52–1.11) | 0.6 (0.41–0.86) |
| Cooked lean meat from fish, other seafood low in omega-3 (28.3 g) | 0.78 (0.31–1.06) | 0.84 (0.42–1.21) | 1.6 (0.94–2.62) ¥ | 0.63 (0.35–1.08) |
| Cooked lean meat from meat, poultry, fish (28.3 g) | 3.36 (2.15–5.47) | 3.38 (2.47–4.88) | 5.57 (4.27–6.98) ¥ | 3 (2.38–4.3) |
| MUFA 16:1 (palmitoleic acid) (g) | 0.86 (0.54–1.07) | 0.98 (0.75–1.23) | 1.36 (0.97–1.57) ɸ | 0.94 (0.72–1.2) |
| MUFA 20:1 (gadoleic acid) (g) | 0.2 (0.16–0.28) | 0.22 (0.18–0.32) | 0.35 (0.25–0.49) ɸ | 0.21 (0.17–0.27) |
| Niacin equivalents (mg) | 39.02 (29.09–51.83) | 39.14 (33.86–47.22) | 51.12 (43.27–57.46) ¥ | 38.08 (33.29–45.98) |
| Non-fried fish servings | 1.31 (0.59–2.58) | 2.08 (0.99–3.32) | 4.35 (2–6.46) ɸ | 1.7 (0.87–2.81) |
| PUFA 18:3 n-6 (g) | 0.02 (0.01–0.02) | 0.02 (0.01–0.03) | 0.03 (0.02–0.04) ¥ | 0.01 (0.01–0.02) |
| PUFA 20:4 (arachidonic acid) (g) | 0.11 (0.06–0.14) | 0.12 (0.09–0.16) | 0.17 (0.14–0.21) ɸ | 0.11 (0.08–0.16) |
| SFA 17:0 (margaric acid) (g) | 0.08 (0.06–0.12) | 0.1 (0.07–0.14) | 0.16 (0.1–0.21) ɸ | 0.1 (0.06–0.12) |
| SFA 4:0 (butyric acid) (g) | 0.28 (0.12–0.43) | 0.4 (0.23–0.74) | 0.66 (0.33–0.99) ɸ | 0.37 (0.19–0.69) |
| Variable | Without Colorectal Lesions | With Colorectal Lesions | Hyperplastic Lesions | Precancerous and Adenocarcinomas |
|---|---|---|---|---|
| Dietary intake of polyamines in mg per person and day | ||||
| Putrescine | 15.58 (11.11–20.66) | 23.81 (12.6–33.88) * | 27.51 (14.52–35.63) | 22.17 (11.88–34.09) |
| Spermidine | 10.93 (8.1–14.4) | 11.22 (8.47–12.5) | 11.24 (10.16–14.24) | 11.19 (8.05–12.5) |
| Spermine | 5.98 (4.2–11.45) | 7.35 (5.47–9.01) | 8.89 (7.96–12.91) | 7.09 (4.98–8.5) |
| Total polyamines | 33.37 (24.71–46.66) | 42.91 (28.09–50.61) | 46.88 (35.56–58.25) | 42.19 (27.36–50.61) |
| Ratios of dietary intake of polyamines | ||||
| Putrescine–spermidine ratio | 1.46 (0.97–1.85) | 2.12 (1.46–3.06) ** | 2.47 (1.18–3.04) | 2.01 (1.47–3.12) ɸ |
| Putrescine–spermine ratio | 1.91 (1.41–3.14) | 3.17 (2.13–4.28) * | 2.71 (1.66–3.47) | 3.4 (2.15–4.33) |
| Spermidine–spermine ratio | 1.56 (1.24–1.86) | 1.44 (1.31–1.81) | 1.3 (1.01–1.47) | 1.48 (1.35–1.86) |
| Dietary intake of polyamines in mg per kcal per person and day | ||||
| Putrescine | 8.25 (5.34–10.48) | 10.46 (5.96–14.95) | 9.43 (5.33–15.99) | 10.49 (6.13–14.12) |
| Spermidine | 5.76 (4.38–7.19) | 5.01 (3.87–6.01) | 4.96 (3.55–5.95) | 5.01 (3.9–6.06) |
| Spermine | 3.83 (2.22–5.25) | 3.29 (2.81–4.19) | 3.88 (2.86–4.84) | 3.21 (2.73–4.18) |
| Total polyamines | 18.73 (12.18–22.65) | 18.27 (14.09–25.87) | 16.16 (14.77–26.6) | 19.06 (13.74–25.35) |
| Feces polyamines in nmol per mg of sample | ||||
| Putrescine | 0.55 (0.21–1.02) | 0.64 (0.38–1.95) | 0.91 (0.33–2.62) | 0.58 (0.35–1.9) |
| Spermidine | 0.61 (0.43–0.94) | 0.99 (0.55–1.43) | 1.15 (0.56–1.51) | 0.87 (0.53–1.4) |
| Spermine | 0.03 (0.01–0.04) | 0.03 (0.02–0.05) | 0.04 (0.03–0.07) | 0.03 (0.02–0.05) |
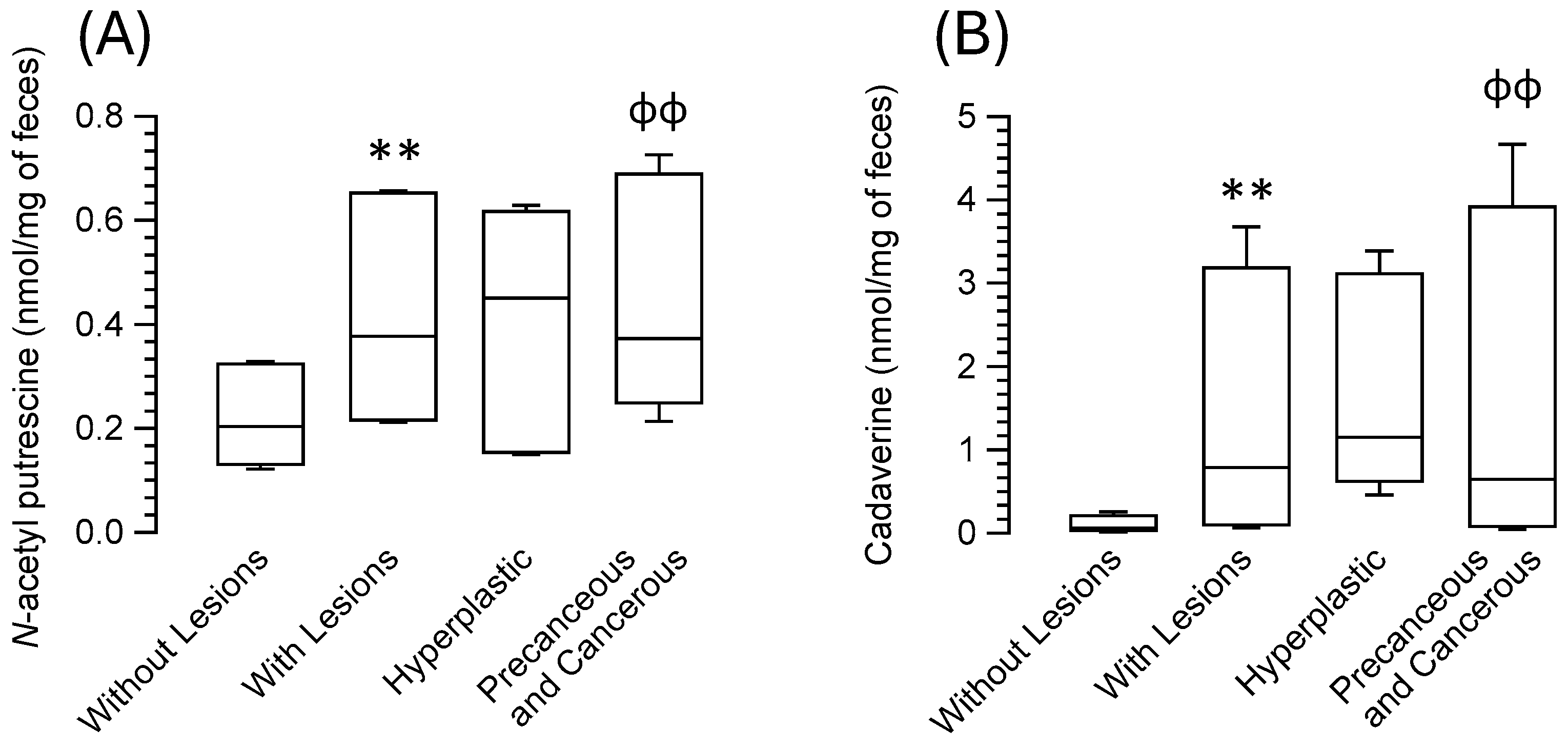
| N-acetyl putrescine | 0.89 (0.56–1.39) | 1.58 (0.92–2.7) *** | 1.88 (0.67–2.59) | 1.56 (0.93–2.98) ɸɸ |
| N-acetyl spermidine | 0.17 (0.11–0.22) | 0.29 (0.16–0.43) | 0.27 (0.14–0.54) | 0.29 (0.16–0.43) |
| Cadaverine | 0.24 (0.06–1.02) | 3.14 (0.25–14.71) *** | 4.6 (1.83–13.55) | 2.58 (0.18–18.66) ɸɸ |
| Tyramine | 0.08 (0.06–0.13) | 0.09 (0.06–0.16) | 0.1 (0.07–0.13) | 0.08 (0.06–0.21) |
| Isoamylamine | 2.04 (1.37–2.72) | 2.1 (0.38–2.74) | 1.51 (0.18–2.76) | 2.1 (0.77–2.81) |
| Ratios of feces content of polyamines | ||||
| Putrescine–spermidine ratio | 0.8 (0.36–1.41) | 0.93 (0.32–2.67) | 0.8 (0.27–2.65) | 1.08 (0.35–2.87) |
| Putrescine–spermine ratio | 19.56 (10.47–32.67) | 22.45 (7.89–50.18) | 23.35 (5.14–67.38) | 22.45 (7.92–55.05) |
| Spermidine–spermine ratio | 25.53 (17.99–35.76) | 22.85 (16.46–35.87) | 23.34 (18.64–31.82) | 22.85 (16.11–38.78) |
| Putrescine–cadaverine ratio | 1.77 (0.45–9.22) | 0.52 (0.1–1.47) * | 0.19 (0.07–2.76) | 0.67 (0.13–1.55) |
| Putrescine–N-acetyl putrescine ratio | 0.55 (0.28–0.85) | 0.6 (0.23–1.22) | 0.78 (0.14–1.34) | 0.53 (0.24–1.23) |
| N-acetyl putrescine–cadaverine ratio | 2.88 (0.79–14.67) | 0.7 (0.21–5.19) ** | 0.33 (0.15–2.59) | 0.79 (0.21–5.2) ¥ |
| Cadaverine–tyramine ratio | 2.31 (0.92–11.23) | 20.04 (3.67–112.14) *** | 48.8 (27.41–153.41) ɸ | 14.26 (3.55–111.52) ɸɸ |
| Regression Model Variables for Each Amine | B | SE B | β | p |
|---|---|---|---|---|
| N-acetyl putrescine in feces (R2 = 0.41, ANOVA p < 0.001) | ||||
| Constant | 0.579 | 0.101 | <0.001 | |
| Wholegrain servings | 0.177 | 0.030 | 0.709 | <0.001 |
| Lignane secoisolariciresinol (mg) | −0.001 | 0.000 | −0.357 | 0.005 |
| Cadaverine in feces (R2 = 0.1, ANOVA p = 0.021) | ||||
| Constant | 10.148 | 0.403 | 0.06 | |
| Wholegrain servings | 0.476 | 0.200 | 0.316 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Alonso, E.; Castro-Estrada, P.; Sánchez, M.; Peña-Iglesias, P.; Suárez, L.; Cantabrana, B. Association of Polyamine Intake, Other Dietary Components, and Fecal Content of N-acetyl Putrescine and Cadaverine with Patients’ Colorectal Lesions. Nutrients 2024, 16, 2894. https://doi.org/10.3390/nu16172894
Barreiro-Alonso E, Castro-Estrada P, Sánchez M, Peña-Iglesias P, Suárez L, Cantabrana B. Association of Polyamine Intake, Other Dietary Components, and Fecal Content of N-acetyl Putrescine and Cadaverine with Patients’ Colorectal Lesions. Nutrients. 2024; 16(17):2894. https://doi.org/10.3390/nu16172894
Chicago/Turabian StyleBarreiro-Alonso, Eva, Paula Castro-Estrada, Manuel Sánchez, Pablo Peña-Iglesias, Lorena Suárez, and Begoña Cantabrana. 2024. "Association of Polyamine Intake, Other Dietary Components, and Fecal Content of N-acetyl Putrescine and Cadaverine with Patients’ Colorectal Lesions" Nutrients 16, no. 17: 2894. https://doi.org/10.3390/nu16172894
APA StyleBarreiro-Alonso, E., Castro-Estrada, P., Sánchez, M., Peña-Iglesias, P., Suárez, L., & Cantabrana, B. (2024). Association of Polyamine Intake, Other Dietary Components, and Fecal Content of N-acetyl Putrescine and Cadaverine with Patients’ Colorectal Lesions. Nutrients, 16(17), 2894. https://doi.org/10.3390/nu16172894






