Time-Restricted Eating Benefits on Pulmonary Function and Postural Balance in Overweight or Obese Women
Abstract
:1. Introduction
2. Materials and Methods
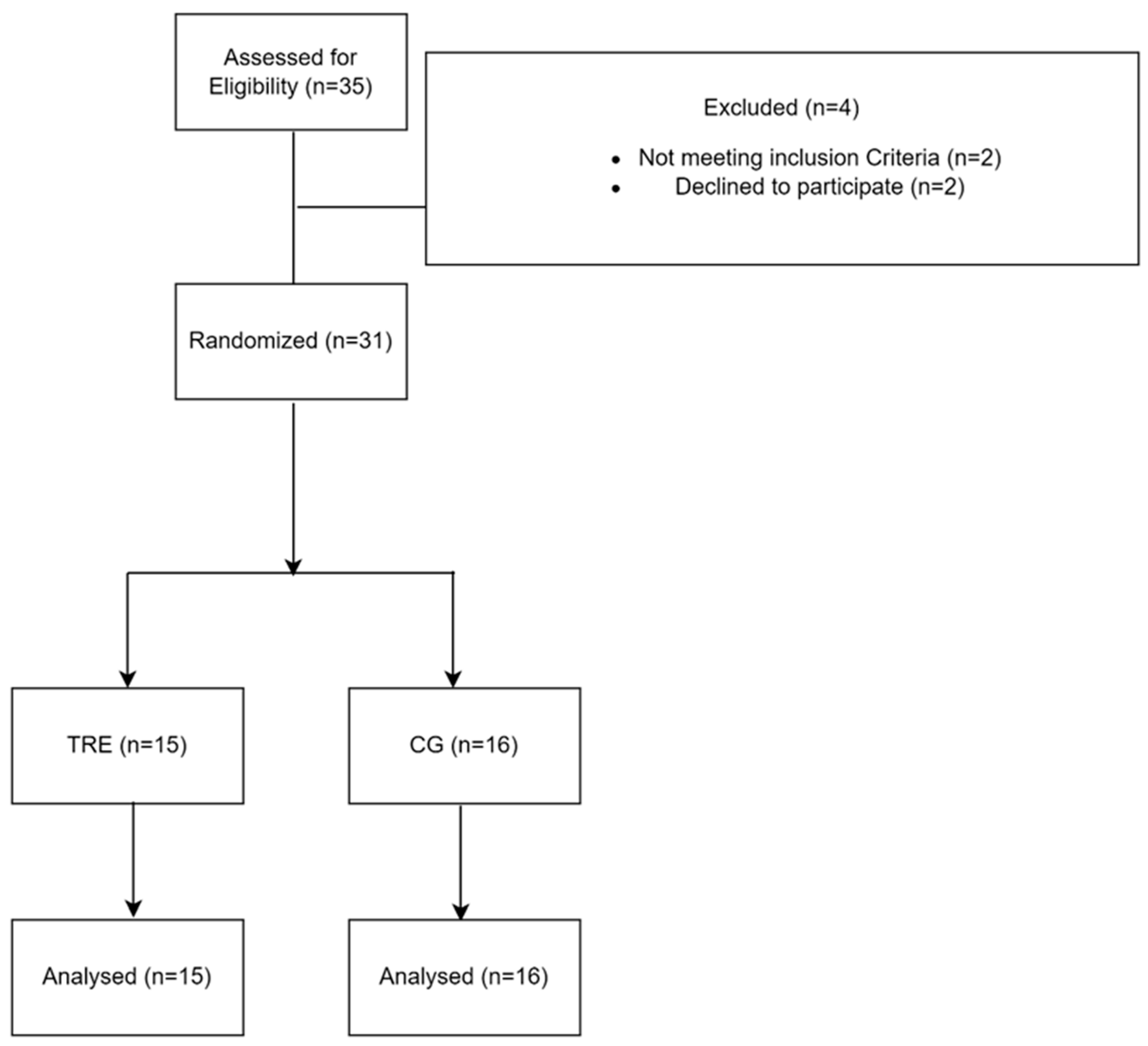
2.1. Participants
2.2. Study Design
2.2.1. Time-Restricted Eating Diet
2.2.2. Control Condition
2.3. HRV Analysis
2.4. Sleep Patterns
2.5. Respiratory Function Measurements
2.6. Y Balance Test (YBT)
2.7. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. HRV
3.2.1. Time Domain
3.2.2. Frequency Domain
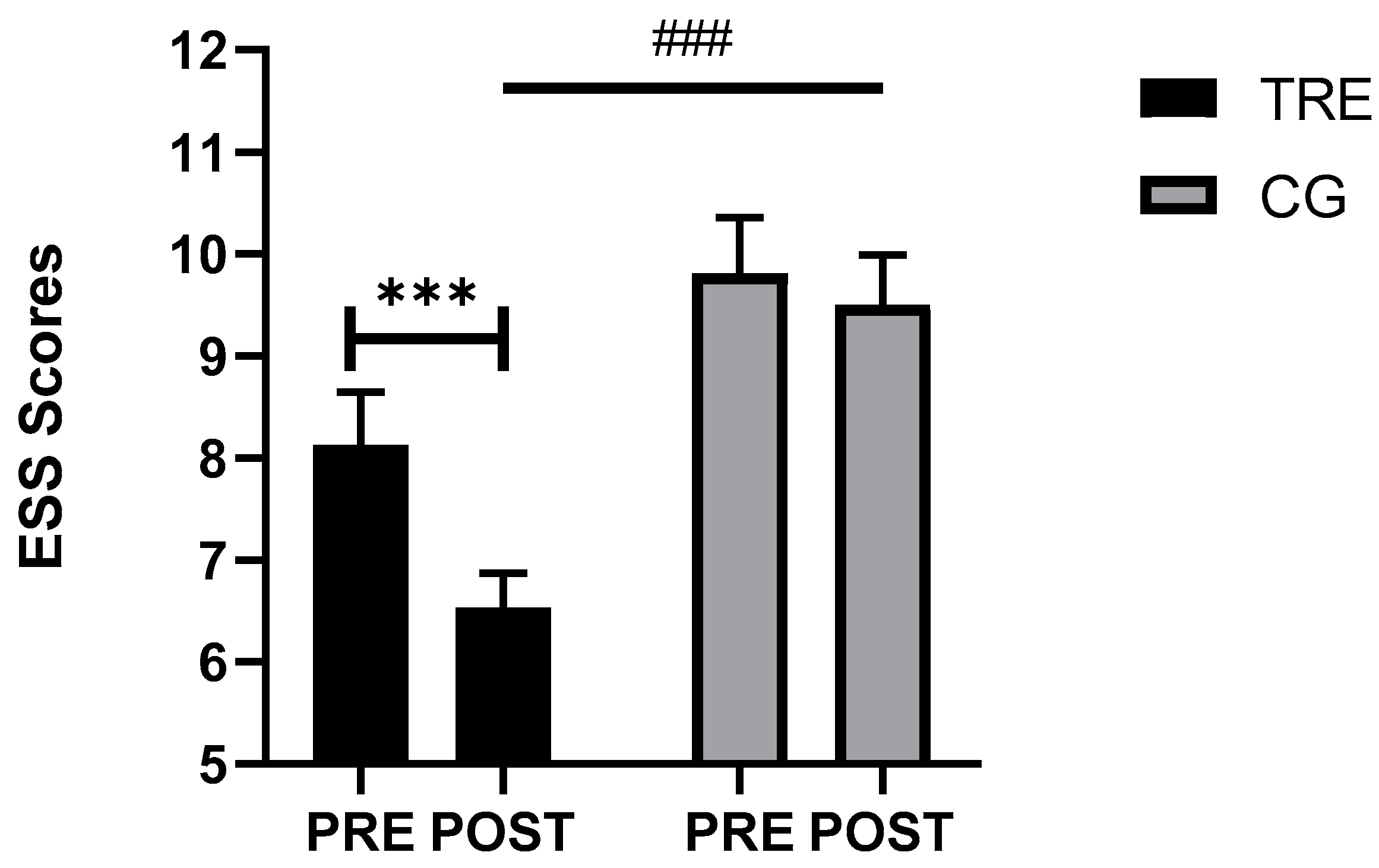
3.3. Sleep Parameters
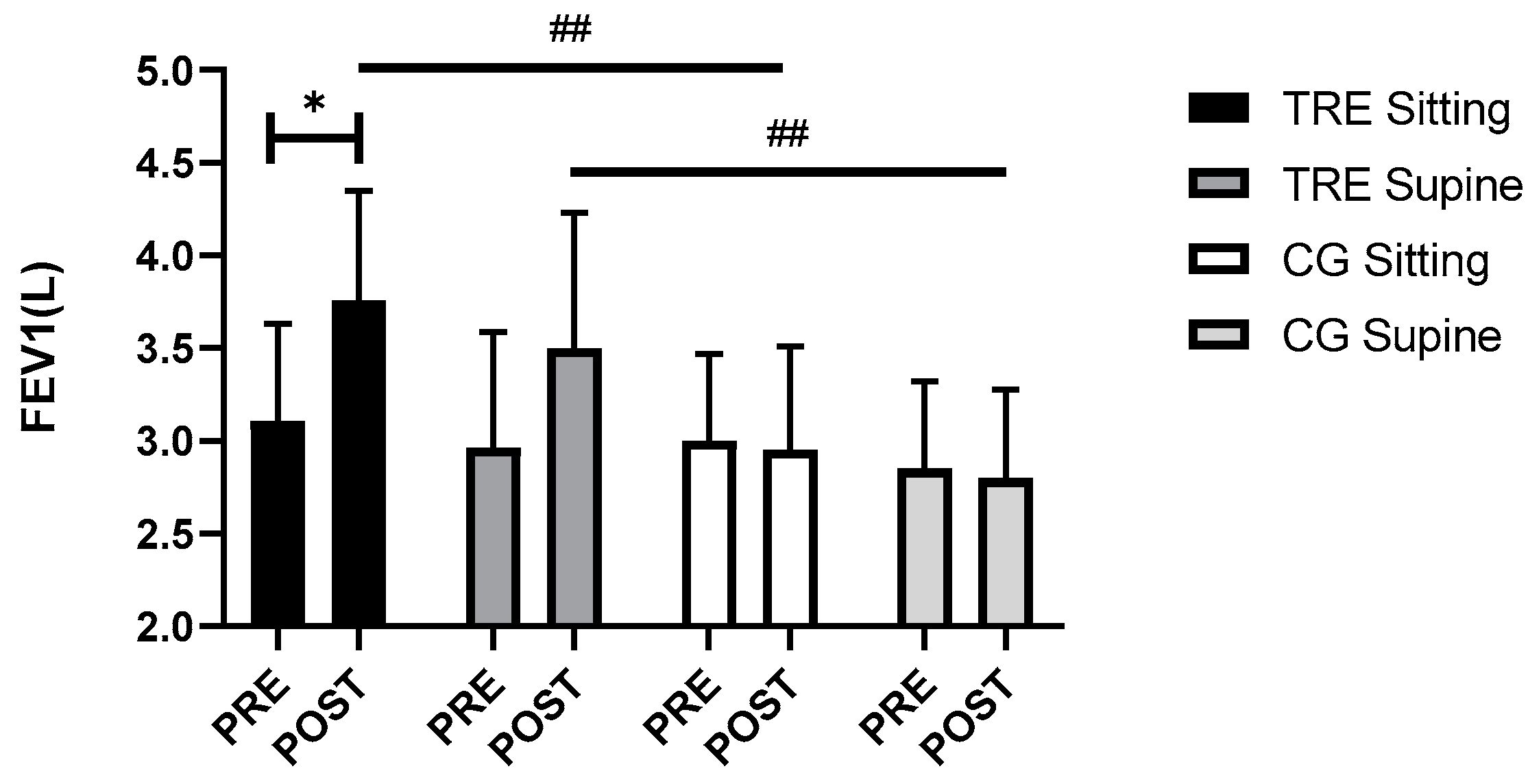
3.4. Respiratory Function
3.5. Postural Balance
3.6. Relationship between Body Composition and Physio-Mechanical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, Y.; Du, T.; Zhuang, X.; Ma, G. Time-restricted eating improves health because of energy deficit and circadian rhythm: A systematic review and meta-analysis. iScience 2024, 27, 109000. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. Heart J. 2022, 29, 2218–2237. [Google Scholar] [CrossRef]
- Patel, M.; Braun, J.; Lambert, G.; Kameneva, T.; Keatch, C.; Lambert, E. Central mechanisms in sympathetic nervous dysregulation in obesity. J. Neurophysiol. 2023, 130, 1414–1424. [Google Scholar] [CrossRef]
- Laederach-Hofmann, K.; Mussgay, L.; Rúddel, H. Autonomic cardiovascular regulation in obesity. J. Endocrinol. 2000, 164, 59–66. [Google Scholar] [CrossRef]
- Espinoza-Salinas, A.; Peiret-Villacura, L.; Molina-Sotomayor, E.; Cigarroa-Cuevas, I.; Arenas-Sánchez, G.; Podestá, I.; González-Jurado, J. Effects of Cardiovagal Training on Autonomic Function, Inflammatory Markers and Insulin Levels in Adults with Obesity. Preprints 2022, 12, 2022030031. [Google Scholar] [CrossRef]
- Shah, N.M.; Kaltsakas, G. Respiratory complications of obesity: From early changes to respiratory failure. Breathe 2023, 19, 220263. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Kacmarek, R.; Berra, L. Ventilatory Mechanics in the Patient with Obesity. Anesthesiology 2020, 132, 1246–1256. [Google Scholar] [CrossRef]
- Lo Mauro, A.; Tringali, G.; Codecasa, F.; Abbruzzese, L.; Sartorio, A.; Aliverti, A. Pulmonary and chest wall function in obese adults. Sci. Rep. 2023, 13, 17753. [Google Scholar] [CrossRef]
- Zhou, N.; Forton, K.; Motoji, Y.; Scoubeau, C.; Klass, M.; Naeije, R.; Faoro, V. Right ventricular-pulmonary arterial coupling impairment and exercise capacity in obese adults. Front. Cardiovasc. Med. 2022, 9, 946155. [Google Scholar] [CrossRef]
- Nanayakkara, B.; McNamara, S. Pathophysiology of Chronic Hypercapnic Respiratory Failure. Sleep. Med. Clin. 2024, 19, 379–389. [Google Scholar] [CrossRef]
- Hagenburg, J.; Bertin, E.; Salmon, J.-H.; Thierry, A.; Perotin, J.-M.; Dormoy, V.; Dury, S.; Gaubil, I.; Bolko, L.; Lebargy, F.; et al. Association between obesity-related dyspnea in daily living, lung function and body composition analyzed by DXA: A prospective study of 130 patients. BMC Pulm. Med. 2022, 22, 103. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.-M.; Ciangura, C.; Bellicha, A. Physical activity and exercise for weight loss and maintenance in people living with obesity. Rev. Endocr. Metab. Disord. 2023, 24, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Rijal, A.; Adhikari, T.B.; Dhakal, S.; Maagaard, M.; Piri, R.; Nielsen, E.E.; Neupane, D.; Jakobsen, J.C.; Olsen, M.H. Effect of exercise on functional capacity and body weight for people with hypertension, type 2 diabetes, or cardiovascular disease: A systematic review with meta-analysis and trial sequential analysis. BMC Sports Sci. Med. Rehabil. 2024, 16, 38. [Google Scholar] [CrossRef]
- Verdú, E.; Homs, J.; Boadas-Vaello, P. Physiological Changes and Pathological Pain Associated with Sedentary Lifestyle-Induced Body Systems Fat Accumulation and Their Modulation by Physical Exercise. Int. J. Environ. Res. Public Health 2021, 18, 13333. [Google Scholar] [CrossRef] [PubMed]
- Tutor, A.W.; Lavie, C.J.; Kachur, S.; Milani, R.V.; Ventura, H.O. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2023, 78, 2–10. [Google Scholar] [CrossRef]
- Tashiro, H.; Kurihara, Y.; Kuwahara, Y.; Takahashi, K. Impact of obesity in asthma: Possible future therapies. Allergol. Int. 2024, 73, 48–57. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef]
- Al Lawati, R.; Al Abri, M.A.; Kuppuswamy, B.; Al-Kharousi, A.; Al-Atbi, A.Y.; Rizvi, S.; Dikshit, M. The Effect of Change in Posture on Spirometry in Patients with Obstructive Sleep Apnoea Syndrome. Sultan Qaboos Univ. Med. J. 2019, 19, e310–e315. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2022, 252, 125–141. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, X.; Liu, Z.; Sun, D.; Liang, Y.; Shen, H.; Li, X.; Mu, J.; Liu, J.; Cao, G.; et al. 6-week time-restricted eating improves body composition, maintains exercise performance, without exacerbating eating disorder in female DanceSport dancers. J. Int. Soc. Sports Nutr. 2024, 21, 2369613. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-restricted Eating for the Prevention and Management of Metabolic Diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef]
- Crose, A.; Alvear, A.; Singroy, S.; Wang, Q.; Manoogian, E.; Panda, S.; Mashek, D.G.; Chow, L.S. Time-Restricted Eating Improves Quality of Life Measures in Overweight Humans. Nutr. J. 2022, 13, 1430. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutr. J. 2019, 11, 1234. [Google Scholar] [CrossRef]
- Wang, R.; Liao, Y.; Deng, Y.; Shuang, R. Unraveling the Health Benefits and Mechanisms of Time-Restricted Feeding: Beyond Caloric Restriction. Nutr. Rev. 2024, nuae074. [Google Scholar] [CrossRef]
- Mackieh, R.; Al-Bakkar, N.; Kfoury, M.; Okdeh, N.; Pietra, H.; Roufayel, R.; Legros, C.; Fajloun, Z.; Sabatier, J.-M. Unlocking the Benefits of Fasting: A Review of Its Impact on Various Biological Systems and Human Health. Curr. Med. Chem. 2023, 31, 1781–1803. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Wang, Y.-T.; Chan, L.-C.; Chu, N.-F. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition 2022, 93, 111504. [Google Scholar] [CrossRef]
- Zimmermann, P.; Herz, D.; Karl, S.; Weiß, J.W.; Lackner, H.K.; Erlmann, M.P.; Sourij, H.; Schierbauer, J.; Haupt, S.; Aberer, F.; et al. Effects of Different Fasting Interventions on Cardiac Autonomic Modulation in Healthy Individuals: A Secondary Outcome Analysis of the EDIF Trial. Biology 2023, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, N.; Hidouri, S.; Ghram, A.; Ammar, A.; Masmoudi, L.; Driss, T.; Knechtle, B.; Weiss, K.; Hammouda, O.; Chlif, M. Effects of Walking Football during Ramadan Fasting on Heart Rate Variability and Physical Fitness in Healthy Middle-Aged Males. Am. J. Mens. Health 2022, 16, 15579883221103418. [Google Scholar] [CrossRef] [PubMed]
- Cansel, M.; Taşolar, H.; Yağmur, J.; Ermiş, N.; Açıkgöz, N.; Eyyüpkoca, F.; Pekdemir, H.; Ozdemir, R. The effects of Ramadan fasting on heart rate variability in healthy individuals: A prospective study. Anadolu Kardiyol. Derg. 2014, 14, 413–416. [Google Scholar] [CrossRef]
- Sibley, K.M.; Mochizuki, G.; Lakhani, B.; McIlroy, W.E. Autonomic contributions in postural control: A review of the evidence. Rev. Neurosci. 2014, 25, 687–697. [Google Scholar] [CrossRef]
- Hall, W.L. The emerging importance of tackling sleep–diet interactions in lifestyle interventions for weight management. Br. J. Nutr. 2022, 128, 561–568. [Google Scholar] [CrossRef]
- Cay, M.; Ucar, C.; Senol, D.; Cevirgen, F.; Ozbag, D.; Altay, Z.; Yildiz, S. Effect of increase in cortisol level due to stress in healthy young individuals on dynamic and static balance scores. North. Clin. Istanb. 2018, 5, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Zouita, A.; Chtourou, H.; Ferchichi, H.; Dziri, C.; Abedelmalek, S.; Souissi, N. The effect of Ramadan intermittent fasting on dynamic postural control in judo athletes. Biol. Rhythm. Res. 2014, 45, 27–36. [Google Scholar] [CrossRef]
- Laatar, R.; Borji, R.; Baccouch, R.; Zahaf, F.; Rebai, H.; Sahli, S. Effects of Ramadan Fasting on Postural Balance and Attentional Capacities in Elderly People. J. Nutr. Health Aging 2016, 20, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Thomas, E.; Thomas, A.C.; Bellafiore, M. The effect of time-of-day and sleep deprivation on postural control: A systematic review. Gait Posture 2022, 97, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Paillard, T. Detrimental effects of sleep deprivation on the regulatory mechanisms of postural balance: A comprehensive review. Front. Hum. Neurosci. 2023, 14, 1146550. [Google Scholar] [CrossRef]
- Kamoun, A.; Hammouda, O.; Yahia, A.; Dhari, O.; Ksentini, H.; Driss, T.; Souissi, N.; Elleuch, M.H. Effects of Melatonin Ingestion Before Nocturnal Sleep on Postural Balance and Subjective Sleep Quality in Older Adults. J. Aging Phys. Act. 2019, 27, 316–324. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef]
- Alamer, R.; Khalifa, K.; Alajlan, S.; Al Ansari, A. Analyzing the Psychometric Properties of the Short Form-36 Quality of Life Questionnaire in Patients with Obesity. Obes. Surg. 2018, 28, 2521–2527. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, M.; Luo, H. Regulation of metabolism by circadian rhythms: Support from time-restricted eating, intestinal microbiota & omics analysis. Life Sci. 2024, 351, 122814. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Martin, J.L.; Hakim, A.D. Wrist actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Pedersen, O.F.; Dirksen, A. A new staging strategy for chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon Dis. 2007, 2, 657–663. [Google Scholar] [PubMed] [PubMed Central]
- Suvarna, T.; Oliver Raj, J.; Prakash, N. Correlation between Balance and BMI in Collegiate students: A cross sectional study. Int. J. Physiother. Res. 2021, 9, 3759–3764. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Hammoud, S.; Mourad, R.; Karam, R.; Saad, I.; van den Bemt, B.J.F.; Kurdi, M. Effect of Ramadan fasting on heart rate variability as a measure of cardiac stress in a Lebanese cohort. Eur. J. Clin. Nutr. 2020, 74, 8. [Google Scholar] [CrossRef]
- Mzoughi, K.; Zairi, I.; Jabeur, M.; Kraiem, S. The effects of fasting on heart rate variability in hypertensive patients. Clin. Exp. Hypertens. 2018, 40, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.A.L.; Yamane, A.C.; Gonçalves, T.C.P.; Kalva-Filho, C.; Papoti, M.; Júnior, C.R.B. Fed and fasted states on heart rate variability, hemodynamic heart rate and blood pressure in adults submited to moderate aerobic exercise. Int. J. Cardiol. Heart Vasc. 2019, 23, 100378. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Varady, K.A. Safety of 8-h time restricted feeding in adults with obesity. Appl. Physiol. Nutr. Metab. 2019, 44, 107–109. [Google Scholar] [CrossRef]
- Metse, A.P.; Bowman, J. A Prevalence of self-reported suboptimal sleep in Australia and receipt of sleep care. Sleep. Health 2020, 6, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Steger, F.L.; Jamshed, H.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-J.; Peterson, C.M. Early time-restricted eating affects weight, metabolic health, mood, and sleep in adherent completers: A secondary analysis. Obesity 2023, 31, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Latiri, I.; Sandid, S.; Fennani, M.A.; Hadrich, M.; Masmoudi, T.; Maatoug, C.; Zammit-Chatti, M.; Chamari, K.; Ben Saad, H. The Effects of Ramadan Fasting on the Spirometric Data of Healthy Adult Males. Am. J. Mens. Health 2017, 11, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Hakala, K.; Stenius-Aarniala, B.; Sovijärvi, A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000, 118, 1315–1321. [Google Scholar] [CrossRef]
- Solianik, R.; Žlibinaitė, L.; Drozdova-Statkevičienė, M.; Sujeta, A. Forty-eight-hour fasting declines mental flexibility but improves balance in overweight and obese older women. Physiol. Behav. 2020, 223, 112995. [Google Scholar] [CrossRef]
- Redfern, M.S.; Yardley, L.; Bronstein, A.M. Visual influences on balance. J. Anxiety Disord. 2001, 15, 81–94. [Google Scholar] [CrossRef]

| Parameters | TRE | CG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | F(1, 29) | p (T) | ηp2 | F(1, 29) | p (T × G) | ηp2 | |
| Weight (kg) | 88.31 ± 13.37 | 84.48 ± 13.37 | 90.87 ± 19.01 | 92.03 ± 19.50 | 13.53 | 0.001 | 0.31 | 46.97 | <0.0005 | 0.61 |
| FM (kg) | 38.52 ± 5.37 | 37.75 ± 5.36 | 40.66 ± 7.14 | 40.99 ± 6.41 | 0.06 | 0.80 | 0.002 | 0.38 | 0.53 | 0.01 |
| FM (%) | 44.02 ± 5.71 | 45.25 ± 6.91 | 46.12 ± 11.07 | 45.97 ± 10.54 | 0.23 | 0.63 | 0.008 | 0.38 | 0.54 | 0.01 |
| FFM (kg) | 49.79 ± 11.29 | 46.73 ± 12.11 | 50.21 ± 19.17 | 51.03 ± 19.13 | 1.57 | 0.22 | 0.05 | 4.74 | 0.04 | 0.14 |
| Steps (nb) | 9388.1 ± 3422.9 | 9913.8 ± 3697.4 | 11,878.7 ± 1356.5 | 7619.9 ± 2192.3 | 0.92 | 0.34 | 0.03 | 1.52 | 0.22 | 0.05 |
| METs/h | 1.39 ± 0.34 | 1.61 ± 0.30 | 1.59 ± 0.22 | 1.25 ± 0.30 | 0.53 | 0.46 | 0.01 | 0.30 | 0.001 | 0.30 |
| EE (kcal) | 339.02 ± 174.18 | 457.26 ± 430.07 | 258.85 ± 75.24 | 255.21 ± 75.22 | 0.85 | 0.36 | 0.02 | 0.96 | 0.33 | 0.03 |
| Parameters | TRE | CG | F | p (T) | ηp2 | F | p (G) | ηp2 | F | p (T × G) | ηp2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | ||||||||||
| Efficiency (%) | 94.80 + 2.77 | 94.64 + 2.47 | 95.04 + 1.88 | 94.39 + 3.21 | 0.41 | 0.52 | 0.01 | 0.1 | 0.99 | 0.004 | 0.15 | 0.70 | 0.005 |
| Latency (min) | 0.13 + 0.23 | 0.37 + 0.66 | 0.41 + 0.45 | 0.38 + 0.40 | 0.69 | 0.41 | 0.02 | 0.18 | 0.66 | 0.006 | 9.44 | 0.005 | 0.24 |
| WASO (min) | 25.28 + 18.11 | 26.66 + 10.91 | 24.17 + 6.64 | 21.67 + 14.19 | 0.02 | 0.87 | 0.001 | 0.90 | 0.35 | 0.03 | 0.31 | 0.57 | 0.01 |
| TIB (min) | 509.34 + 115.88 | 510.85 + 65.73 | 517.14 + 101.79 | 463.17 + 119.97 | 1.39 | 0.24 | 0.04 | 0.44 | 0.50 | 0.01 | 1.56 | 0.22 | 0.05 |
| TST (min) | 485.26 + 103.14 | 485.82 + 68.63 | 494.94 + 102.50 | 438.35 + 114.61 | 1.83 | 0.18 | 0.05 | 0.42 | 0.51 | 0.01 | 1.90 | 0.17 | 0.06 |
| Parameters | TRE | CG | F (G × P) | p (G × P) | ηp2 (G × P) | F (G × T × P) | p (G × T × P) | ηp2 (G × T × P) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | |||||||
| FVC in sitting position (L) | 3.89 ± 0.67 | 3.76 ± 0.59 | 3.58 ± 0.53 | 3.61 ± 0.65 | 0.14 | 0.70 | 0.001 | 0.06 | 0.79 | 0.001 |
| FVC in supine position (L) | 3.66 ± 0.79 | 3.84 ± 0.38 | 3.49 ± 0.60 | 3.72 ± 0.45 | ||||||
| FEV1 in sitting position (L) | 3.11 ± 0.52 | 3.76 ± 0.59 | 3.00 ± 0.47 | 2.95 ± 0.56 | 0.07 | 0.79 | 0.001 | 0.05 | 0.81 | 0.000 |
| FEV1 in supine position (L) | 2.96 ± 0.63 | 3.50 ± 0.73 | 2.85 ± 0.47 | 2.80 ± 0.48 | ||||||
| FEV1/FVC in sitting position (%) | 82.02 ± 5.89 | 82.20 ± 5.19 | 82.57 ± 4.43 | 81.90 ± 5.32 | 0.49 | 0.48 | 0.007 | 0.18 | 0.67 | 0.004 |
| FEV1/FVC in supine position (%) | 79.16 ± 5.75 | 81.40 ± 5.81 | 81.85 ± 4.69 | 81.62 ± 4.88 | ||||||
| Parameters | TRE | CG | F | p (T) | ηp2 | F | p (L) | ηp2 | F | p (G × T × L) | ηp2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | ||||||||||
| Anterior R (cm) | 69.86 ± 6.87 | 73.4 ± 6.64 | 66.87 ± 3.73 | 64.18 ± 3.85 | 0.20 | 0.65 | 0.002 | 4.48 | 0.03 | 0.037 | 0.32 | 0.57 | 0.003 |
| Anterior L (cm) | 67.93 ± 6.45 | 64.68 ± 3.85 | 70.40 ± 6.18 | 63.12 ± 4.01 | |||||||||
| Postero-Medial R (cm) | 67.53 ± 6.68 | 70.46 ± 6.33 | 67.18 ± 7.64 | 65.12 ± 6.99 | 0.33 | 0.56 | 0.003 | 0.001 | 0.97 | 0.000 | 0.06 | 0.80 | 0.001 |
| Postero-Medial L (cm) | 67.00 ± 6.45 | 71.06 ± 5.68 | 67.25 ± 6.98 | 65.12 ± 7.39 | |||||||||
| Postero-Lateral R (cm) | 64.93 ± 6.70 | 67.86 ± 6.09 | 63.93 ± 6.86 | 63.00 ± 7.54 | 0.75 | 0.38 | 0.007 | 0.06 | 0.8 | 0.001 | 0.02 | 0.87 | 0.000 |
| Postero-Lateral L (cm) | 65.00 ± 6.08 | 68.40 ± 6.17 | 68.40 ± 6.17 | 63.12 ± 6.92 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miladi, S.; Hammouda, O.; Ameur, R.; Miladi, S.C.; Feki, W.; Driss, T. Time-Restricted Eating Benefits on Pulmonary Function and Postural Balance in Overweight or Obese Women. Nutrients 2024, 16, 2919. https://doi.org/10.3390/nu16172919
Miladi S, Hammouda O, Ameur R, Miladi SC, Feki W, Driss T. Time-Restricted Eating Benefits on Pulmonary Function and Postural Balance in Overweight or Obese Women. Nutrients. 2024; 16(17):2919. https://doi.org/10.3390/nu16172919
Chicago/Turabian StyleMiladi, Sarra, Omar Hammouda, Ranya Ameur, Sirine C. Miladi, Walid Feki, and Tarak Driss. 2024. "Time-Restricted Eating Benefits on Pulmonary Function and Postural Balance in Overweight or Obese Women" Nutrients 16, no. 17: 2919. https://doi.org/10.3390/nu16172919
APA StyleMiladi, S., Hammouda, O., Ameur, R., Miladi, S. C., Feki, W., & Driss, T. (2024). Time-Restricted Eating Benefits on Pulmonary Function and Postural Balance in Overweight or Obese Women. Nutrients, 16(17), 2919. https://doi.org/10.3390/nu16172919








