Extract of Araçá-Boi and Its Major Phenolic Compound, Trans-Cinnamic Acid, Reduce Viability and Inhibit Migration of Human Metastatic Melanoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Phenolic Extract Obtantion
2.3. Chemical Characterization of Araçá-Boi Extract
2.3.1. Determination of Total Phenolic Content (TPC)
2.3.2. Determination of Condensed Tannin Content (CTC)
2.3.3. Chromatographic Analysis of Phenolic Compounds by HPLC-DAD
2.3.4. Trolox Equivalent Antioxidant Capacity (TEAC) Assay Using ABTS•+ Radical
2.3.5. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.3.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.4. Cellular Assays
2.4.1. Cell Culture and Treatments
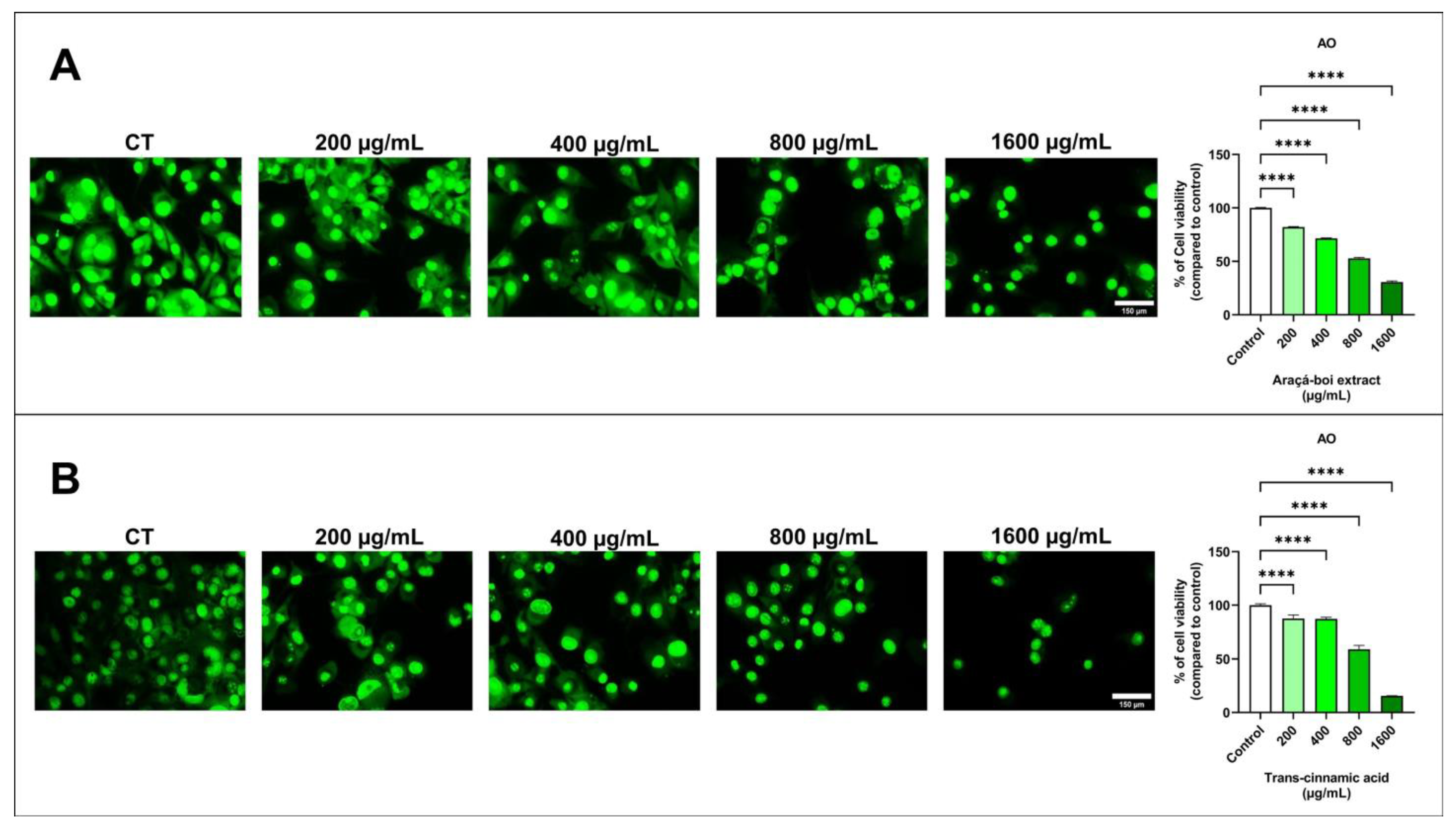
2.4.2. Cell Viability by Fluorescence Microscopy Assay
2.4.3. Measurement of Mitochondrial Transmembrane Potential (ΔΨm)
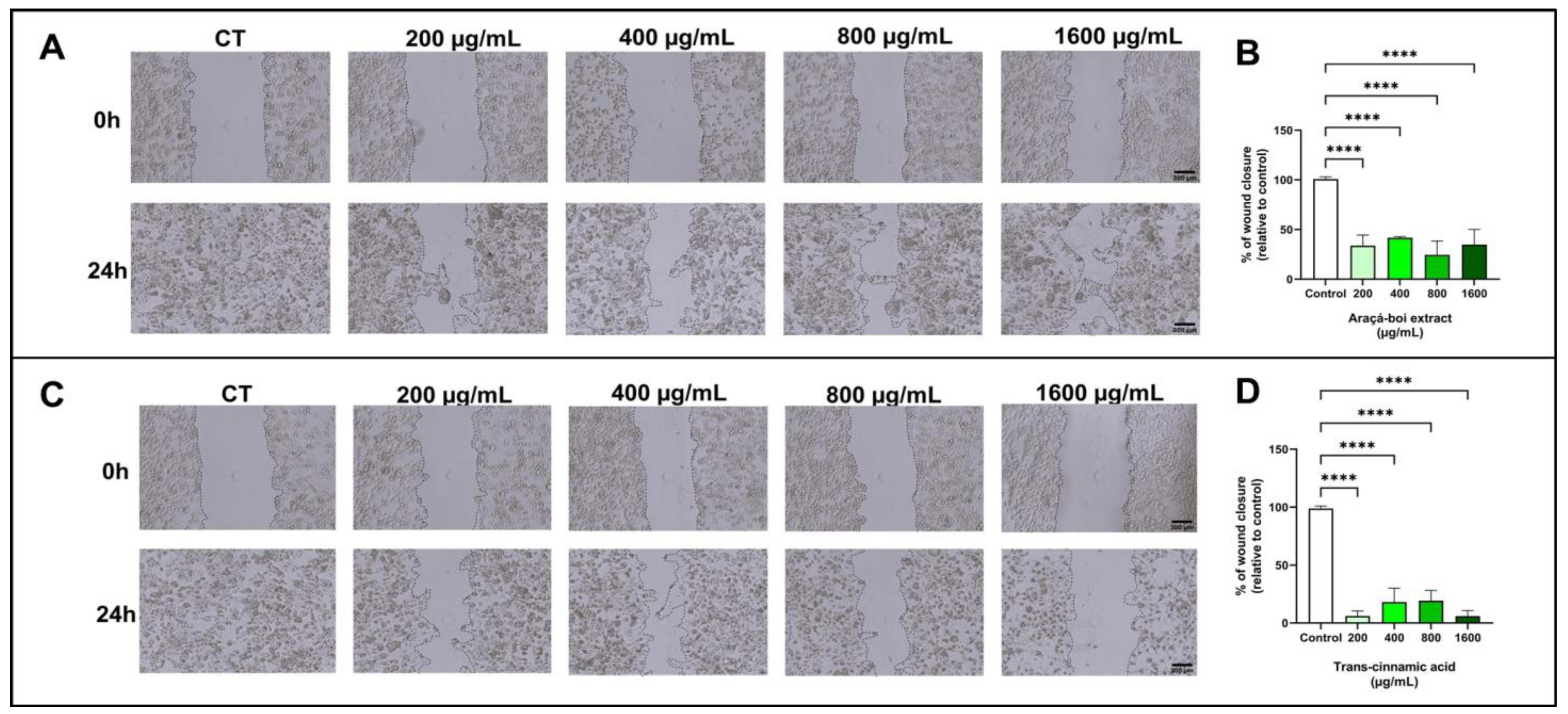
2.4.4. Cell Migration Assay
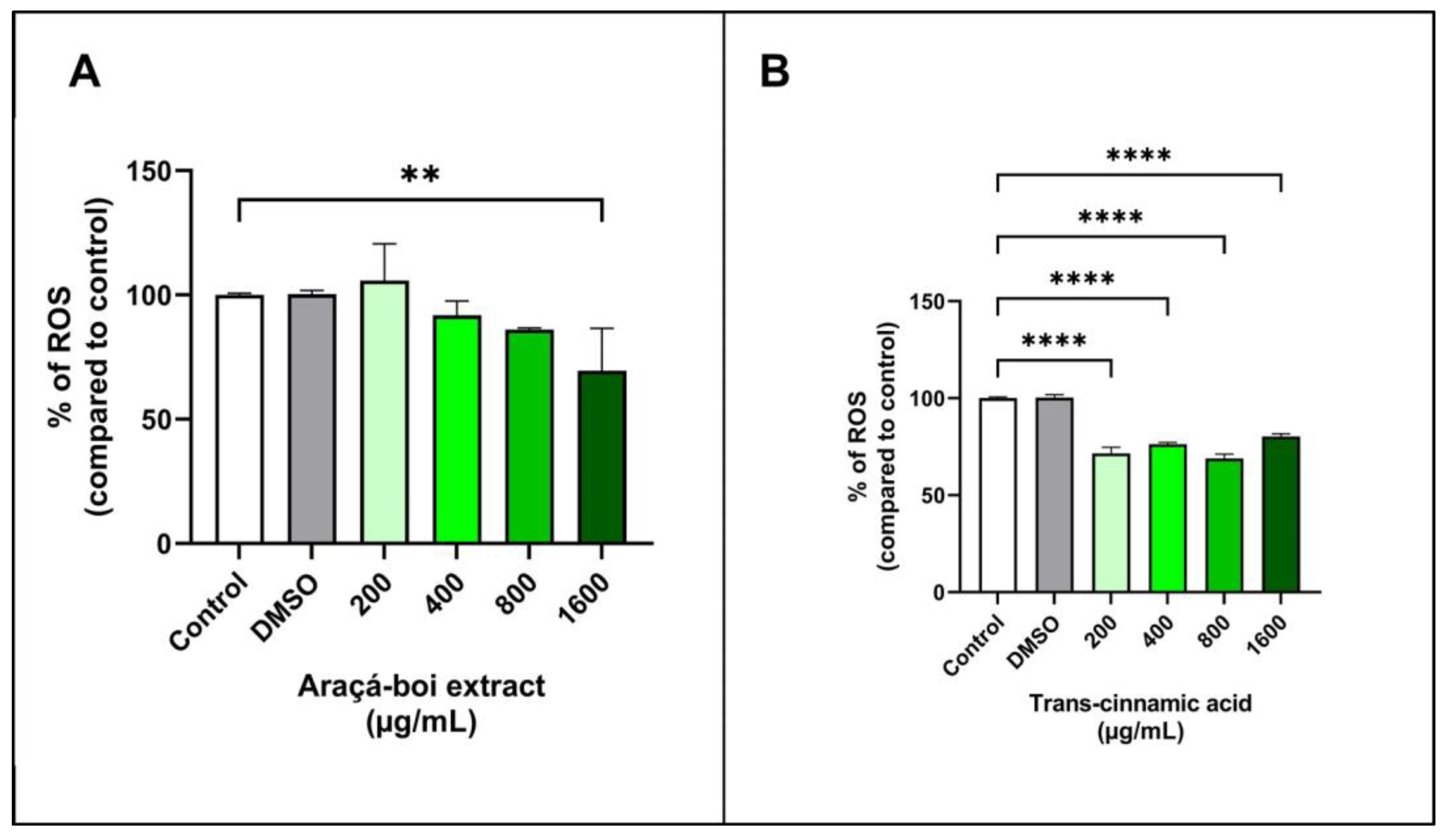
2.4.5. Detection of Cellular Reactive Oxygen Species (ROS)
2.4.6. Assessment of Caspase-3 and NLRP3 Expression
2.5. Statistical Analysis
3. Results
3.1. Chemical Characterization of Araçá-Boi Extract
3.1.1. Total Phenolic Content, Condensed Tannin Content, and Antioxidant Activity
3.1.2. Content of Individual Phenolic Compounds by HPLC-DAD Method
3.2. Effects of Araçá-Boi Extract and Trans-Cinnamic Acid on Human Metastatic Melanoma Cells
3.2.1. Cell Viability in Melanoma Cells
3.2.2. Transmembrane Potential of Mitochondria of Melanoma Cells (ΔΨm)
3.2.3. Cell Migration of Melanoma Cells
3.2.4. Detection of Cellular Reactive Oxygen Species (ROS)
3.2.5. Expression of Caspase-3 and NLRP3 in Melanoma Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, J.; Rodrigues, C.M.P.; Gaspar, M.M.; Reis, C.P. Melanoma Management: From Epidemiology to Treatment and Latest Advances. Cancers 2022, 14, 4652. [Google Scholar] [CrossRef]
- Switzer, B.; Puzanov, I.; Skitzki, J.J.; Hamad, L.; Ernstoff, M.S. Managing Metastatic Melanoma in 2022: A Clinical Review. JCO Oncol. Pract. 2022, 18, 335–351. [Google Scholar] [CrossRef]
- GLOBOCAN World Heatlh Organization. Available online: https://gco.iarc.fr/ (accessed on 19 June 2024).
- Dzwierzynski, W.W. Melanoma Risk Factors and Prevention. Clin. Plast. Surg. 2021, 48, 543–550. [Google Scholar] [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Liang, C.; Wang, P.; Li, M.; Li, R.; Lai, K.P.; Chen, J. Anti-Cancer Mechanisms of Natural Isoflavones against Melanoma. Heliyon 2024, 10, e28616. [Google Scholar] [CrossRef]
- de Carvalho Braga, G.; Coiado, J.V.; de Melo, V.C.; Loureiro, B.B.; Bagatini, M.D. Cutaneous Melanoma and Purinergic Modulation by Phenolic Compounds. Purinergic Signal. 2024, 1–13. [Google Scholar] [CrossRef]
- Tabolacci, C.; De Vita, D.; Facchiano, A.; Bozzuto, G.; Beninati, S.; Failla, C.M.; Di Martile, M.; Lintas, C.; Mischiati, C.; Stringaro, A.; et al. Phytochemicals as Immunomodulatory Agents in Melanoma. Int. J. Mol. Sci. 2023, 24, 2657. [Google Scholar] [CrossRef]
- Baloghová, J.; Michalková, R.; Baranová, Z.; Mojžišová, G.; Fedáková, Z.; Mojžiš, J. Spice-Derived Phenolic Compounds: Potential for Skin Cancer Prevention and Therapy. Molecules 2023, 28, 6251. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Arruda, H.S.; Borsoi, F.T.; Andrade, A.C.; Pastore, G.M.; Marostica Junior, M.R. Scientific Advances in the Last Decade on the Recovery, Characterization, and Functionality of Bioactive Compounds from the Araticum Fruit (Annona crassiflora Mart.). Plants 2023, 12, 1536. [Google Scholar] [CrossRef]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Neri-Numa, I.A.; de Oliveira, W.Q.; de Araújo, F.F.; Pastore, G.M. Dietary Polyphenols and Their Relationship to the Modulation of Non-Communicable Chronic Diseases and Epigenetic Mechanisms: A Mini-Review. Food Chem. Mol. Sci. 2023, 6, 100155. [Google Scholar] [CrossRef]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera indica L.): A Magnificent Plant with Cancer Preventive and Anticancer Therapeutic Potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 2125–2151. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Chemical Characterization of Eugenia stipitata: A Native Fruit from the Amazon Rich in Nutrients and Source of Bioactive Compounds. Food Res. Int. 2021, 139, 109904. [Google Scholar] [CrossRef]
- Soares, J.C.; Rosalen, P.L.; Lazarini, J.G.; Massarioli, A.P.; da Silva, C.F.; Nani, B.D.; Franchin, M.; de Alencar, S.M. Comprehensive Characterization of Bioactive Phenols from New Brazilian Superfruits by LC-ESI-QTOF-MS, and Their ROS and RNS Scavenging Effects and Anti-Inflammatory Activity. Food Chem. 2019, 281, 178–188. [Google Scholar] [CrossRef]
- Goncalves, A.E.S.S.; Lajolo, F.M.; Genovese, M.I. Chemical Composition and Antioxidant/Antidiabetic Potential of Brazilian Native Fruits and Commercial Frozen Pulps. J. Agric. Food Chem. 2010, 58, 4666–4674. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Carvalho-Silva, L.B.; Morales, J.P.; Malta, L.G.; Muramoto, M.T.; Ferreira, J.E.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Maróstica Junior, M.R.; Pastore, G.M. Evaluation of the Antioxidant, Antiproliferative and Antimutagenic Potential of Araçá-Boi Fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest. Food Res. Int. 2013, 50, 70–76. [Google Scholar] [CrossRef]
- Baldini, T.; Neri-Numa, I.; Do Sacramento, C.; Schmiele, M.; Bolini, H.; Pastore, G.; Bicas, J. Elaboration and Characterization of Apple Nectars Supplemented with Araçá-Boi (Eugenia stipitata Mac Vaugh—Myrtaceae). Beverages 2017, 3, 59. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of High-Intensity Ultrasound Process Parameters on the Phenolic Compounds Recovery from Araticum Peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef]
- Pereira, G.A.; Arruda, H.S.; Pastore, G.M. Modification and Validation of Folin-Ciocalteu Assay for Faster and Safer Analysis of Total Phenolic Content in Food Samples. Braz. J. Food Res. 2018, 9, 125. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of Free, Esterified, Glycosylated and Insoluble-Bound Phenolics Composition in the Edible Part of Araticum Fruit (Annona crassiflora Mart.) and Its by-Products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Silva, J.D.R.; Arruda, H.S.; Andrade, A.C.; Berilli, P.; Borsoi, F.T.; Monroy, Y.M.; Rodrigues, M.V.N.; Sampaio, K.A.; Pastore, G.M.; Marostica Junior, M.R. Eugenia calycina and Eugenia stigmatosa as Promising Sources of Antioxidant Phenolic Compounds. Plants 2024, 13, 2039. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ramírez, D.; González-García, K.E.; Medrano-Hernández, J.M.; Famiani, F.; Cruz-Castillo, J.G. Antioxidants in Processed Fruit, Essential Oil, and Seed Oils of Feijoa. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 11988. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC–Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- McGahon, A.J.; Martin, S.J.; Bissonnette, R.P.; Mahboubi, A.; Shi, Y.; Mogil, R.J.; Nishioka, W.K.; Green, D.R. The End of the (Cell) Line: Methods for the Study of Apoptosis in vitro. In Methods in Cell Biology; Schwartz, L.M., Osborne, B.A., Eds.; Academic Press: San Diego, CA, USA, 1995; Volume 46, pp. 153–185. [Google Scholar]
- Joshi, D.C.; Bakowska, J.C. Determination of Mitochondrial Membrane Potential and Reactive Oxygen Species in Live Rat Cortical Neurons. J. Vis. Exp. 2011, 51, e2704. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In Vitro Cell Migration and Invasion Assays. J. Vis. Exp. 2014, 88, 51046. [Google Scholar] [CrossRef]
- Wu, T.; Qiang, L.; Chen, F.-H.; Zhao, Q.; Yang, Z.; Zou, M.-J.; Sun, Y.-J.; Li, Z.-Y.; Guo, Q.-L. LFG-500, a Newly Synthesized Flavonoid, Induced a Reactive Oxygen Species-Mitochondria-Mediated Apoptosis in Hepatocarcinoma Cells. Biomed. Prev. Nutr. 2011, 1, 132–138. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Popescu, D.I.; Botoran, O.R.; Cristea, R.; Mihăescu, C.; Șuțan, N.A. Effects of Geographical Area and Harvest Times on Chemical Composition and Antibacterial Activity of Juniperus communis L. Pseudo-Fruits Extracts: A Statistical Approach. Horticulturae 2023, 9, 325. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389. [Google Scholar] [CrossRef]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of Action and Determination of Antioxidant Activity in the Dietary Sources: An Overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, F.A.; Ariza, E.; Anzola, C.; Restrepo, P. Estudio de La Capacidad Antioxidante Del Arazá (Eugenia stipitata MC Vaugh) Durante La Maduración. Rev. Colomb. Química 2013, 42, 21–28. [Google Scholar]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, J.; Su, W.; Li, H.; Xiao, T.; Chen, D.; Zhang, Z. Cinnamic Acid Hybrids as Anticancer Agents: A Mini-review. Arch. Pharm. 2022, 355, 2200052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ge, S.; Li, S.; Lin, H.; Lin, S. Anti-Obesity Effect of Trans-Cinnamic Acid on HepG2 Cells and HFD-Fed Mice. Food Chem. Toxicol. 2020, 137, 111148. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential Implications of Quercetin and Its Derivatives in Cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. O-Substituted Quercetin Derivatives: Structural Classification, Drug Design, Development, and Biological Activities, a Review. J. Mol. Struct. 2022, 1254, 132392. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin Derivatives: Drug Design, Development, and Biological Activities, a Review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Dumitraș, D.-A.; Andrei, S. Recent Advances in the Antiproliferative and Proapoptotic Activity of Various Plant Extracts and Constituents against Murine Malignant Melanoma. Molecules 2022, 27, 2585. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Cianca, S.I.; Romero-Castillo, C.; Gálvez-Romero, J.L.; Juárez, Z.N.; Hernández, L.R. Antioxidant and Anti-Inflammatory Compounds from Edible Plants with Anti-Cancer Activity and Their Potential Use as Drugs. Molecules 2023, 28, 1488. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, N.; Sharma, M.C. Anticancer Properties of Some Selected Plant Phenolic Compounds: Future Leads for Therapeutic Development. J. Herb. Med. 2023, 42, 100801. [Google Scholar] [CrossRef]
- Kardorff, M.; Mahler, H.C.; Huwyler, J.; Sorret, L. Comparison of Cell Viability Methods for Human Mesenchymal/Stromal Stem Cells and Human A549 Lung Carcinoma Cells after Freeze-Thaw Stress. J. Pharmacol. Toxicol. Methods 2023, 124, 107474. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087361. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Zhou, W.; Qu, J.; Xie, S.; Sun, Y.; Yao, H. Mitochondrial Dysfunction in Chronic Respiratory Diseases: Implications for the Pathogenesis and Potential Therapeutics. Oxid. Med. Cell Longev. 2021, 2021, 5188306. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- da Silva, G.B.; Manica, D.; da Silva, A.P.; Marafon, F.; Moreno, M.; Bagatini, M.D. Rosmarinic Acid Decreases Viability, Inhibits Migration and Modulates Expression of Apoptosis-Related CASP8/CASP3/NLRP3 Genes in Human Metastatic Melanoma Cells. Chem. Biol. Interact. 2023, 375, 110427. [Google Scholar] [CrossRef]
- Manica, D.; da Silva, G.B.; da Silva, A.P.; Marafon, F.; Maciel, S.F.V.O.; Bagatini, M.D.; Moreno, M. Curcumin Promotes Apoptosis of Human Melanoma Cells by Caspase 3. Cell Biochem. Funct. 2023, 41, 1295–1304. [Google Scholar] [CrossRef]
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as Mitochondria-Targeted Anticancer Drugs. Cancer Lett. 2015, 366, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Chavarria, D.; Borges, F.; Wojtczak, L.; Wieckowski, M.R.; Karkucinska-Wieckowska, A.; Oliveira, P.J. Dietary Polyphenols and Mitochondrial Function: Role in Health and Disease. Curr. Med. Chem. 2019, 26, 3376–3406. [Google Scholar] [CrossRef]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid. Med. Cell Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef]
- da Silva, G.B.; Yamauchi, M.A.; Zanini, D.; Bagatini, M.D. Novel Possibility for Cutaneous Melanoma Treatment by Means of Rosmarinic Acid Action on Purinergic Signaling. Purinergic Signal. 2022, 18, 61–81. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Barradas, Y.M.; Borsoi, F.T.; Dacoreggio, M.V.; Moroni, L.S.; Bonadiman, B.S.R.; Marafon, F.; Giacobbo, C.L.; Bagatini, M.D.; Kempka, A.P. Phytochemical Profiling, Antidiabetic, Antitumoral and Cytotoxic Potential of Psidium cattleianum Afzel. Ex Sabine Leaves of Red Variety. Nat. Prod. Res. 2023, 37, 608–612. [Google Scholar] [CrossRef]
- Gambin, L.B.; Cavali, M.; Dresch, A.P.; Fuhr, J.F.; Marafon, F.; Bonadiman, B.S.R.; Bilibio, D.; Araujo, L.; Mibielli, G.M.; Priamo, W.L.; et al. Phenolic Compounds from Feijoa (Acca sellowiana) Fruits: Ultrasound-Assisted Extraction and Antiproliferative Effect on Cutaneous Melanoma Cells (SK-MEL-28). Food Biosci. 2023, 55, 103078. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Bonadiman, B.S.R.; Marafon, F.; Fischer, D.L.O.; Bagatini, M.D.; Kempka, A.P. Eugenia uniflora L. Seed and Pulp Extracts: Phytochemical Profile, Cytotoxic Potential, Antitumoral Activity, and α-Amylase and α-Glucosidase Inhibition Capacity. Nat. Prod. Res. 2023, 37, 3862–3867. [Google Scholar] [CrossRef]
- Isacescu, E.; Chiroi, P.; Zanoaga, O.; Nutu, A.; Budisan, L.; Pirlog, R.; Atanasov, A.G.; Berindan-Neagoe, I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.D.; Diaconeasa, Z. Recent Advances in Phenolic Metabolites and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 9707. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Measurement of Reactive Species. In Free Radicals in Biology and Medicine; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 284–353. [Google Scholar]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Maya-Cano, D.A.; Arango-Varela, S.; Santa-Gonzalez, G.A. Phenolic Compounds of Blueberries (Vaccinium spp.) as a Protective Strategy against Skin Cell Damage Induced by ROS: A Review of Antioxidant Potential and Antiproliferative Capacity. Heliyon 2021, 7, e06297. [Google Scholar] [CrossRef]
- Lv, Q.; Long, J.; Gong, Z.; Nong, K.; Liang, X.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X2110277. [Google Scholar] [CrossRef]
- Yang, R.; Tian, J.; Liu, Y.; Zhu, L.; Sun, J.; Meng, D.; Wang, Z.; Wang, C.; Zhou, Z.; Chen, L. Interaction Mechanism of Ferritin Protein with Chlorogenic Acid and Iron Ion: The Structure, Iron Redox, and Polymerization Evaluation. Food Chem. 2021, 349, 129144. [Google Scholar] [CrossRef]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of Phenolic Acids on Free Radical Scavenging and Heavy Metal Bioavailability in Kandelia obovata under Cadmium and Zinc Stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.; Conterno, P.; da Silva, L.D.; de Lima, V.A.; Oldoni, T.L.C.; Thomé, G.R.; Carpes, S.T. Extraction of Phenolic Compounds from Tabernaemontana catharinensis Leaves and Their Effect on Oxidative Stress Markers in Diabetic Rats. Molecules 2020, 25, 2391. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, W.; Zhang, J.; Song, D.; Zhuang, L.; Ma, Q.; Yang, X.; Liu, X.; Zhang, J.; Zhang, H.; et al. Antioxidant Capacity of Phenolic Compounds Separated from Tea Seed Oil In Vitro and In Vivo. Food Chem. 2022, 371, 131122. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wirtz, S. Does Pyroptosis Play a Role in Inflammasome-Related Disorders? Int. J. Mol. Sci. 2022, 23, 10453. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and Their Substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Do, B.H.; Hoang, N.S.; Nguyen, T.P.T.; Ho, N.Q.C.; Le, T.L.; Doan, C.C. Phenolic Extraction of Moringa oleifera Leaves Induces Caspase-Dependent and Caspase-Independent Apoptosis through the Generation of Reactive Oxygen Species and the Activation of Intrinsic Mitochondrial Pathway in Human Melanoma Cells. Nutr. Cancer 2021, 73, 869–888. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 Inflammasome: Activation and Regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 Inflammasome Activation and Cell Death. Cell Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Moossavi, M.; Parsamanesh, N.; Bahrami, A.; Atkin, S.L.; Sahebkar, A. Role of the NLRP3 Inflammasome in Cancer. Mol. Cancer 2018, 17, 158. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front. Immunol. 2020, 11, 538030. [Google Scholar] [CrossRef]
- Özenver, N.; Efferth, T. Phytochemical Inhibitors of the NLRP3 Inflammasome for the Treatment of Inflammatory Diseases. Pharmacol. Res. 2021, 170, 105710. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of Dietary Polyphenols on Cancer Cell Pyroptosis and the Tumor Immune Microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Sarkar, C.; Rawat, V.S.; Kalita, D.; Deka, S.; Agnihotri, A. Promise of the NLRP3 Inflammasome Inhibitors in In vivo Disease Models. Molecules 2021, 26, 4996. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Rao, Z.; Peng, L.; Lei, Z.; Zeng, J.; Peng, X.; Yang, R.; Liu, R.; Zeng, N. Cinnamic Acid Preserves against Myocardial Ischemia/Reperfusion Injury via Suppression of NLRP3/Caspase-1/GSDMD Signaling Pathway. Phytomedicine 2022, 100, 154047. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 Inflammasome in Cancer and Metabolic Diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]


| Assay | Araçá-Boi Extract |
|---|---|
| TPC (mg GAE/g extract dw) | 12.16 ± 0.38 |
| Condensed tannins (mg CE/g extract dw) | 5.76 ± 0.07 |
| TEAC (μmol TE/g extract dw) | 102.51 ± 1.16 |
| FRAP (μmol TE/g extract dw) | 150.77 ± 4.37 |
| ORAC (μmol TE/g extract dw) | 583.81 ± 17.67 |
| Class | Compound | Araçá-Boi Extract (µg/g Extract dw) |
|---|---|---|
| Phenolic acids | 2,5-Dihydroxybenzoic acid (gentisic acid) | 54.94 ± 0.86 |
| 3,4-Dihydroxybenzoic acid (protocatechuic acid) | n.d. | |
| 3,5-Dihydroxybenzoic acid (α-resorcylic acid) | n.d. | |
| 4-Hydroxybenzoic acid | 2.56 ± 0.11 | |
| Benzoic acid | n.d. | |
| Caffeic acid | n.d. | |
| Chlorogenic acid | n.d. | |
| Ferulic acid | 6.71 ± 0.19 | |
| Gallic acid | 73.93 ± 0.18 | |
| p-Coumaric acid | 28.09 ± 0.49 | |
| Sinapic acid | n.d. | |
| Syringic acid | 113.07 ± 0.74 | |
| Trans-cinnamic acid | 155.79 ± 0.32 | |
| Vanillic acid | n.d. | |
| Total phenolic acids | 435.09 ± 2.19 | |
| Flavonoids | Apigenin | n.d. |
| Apigenin-7-O-glucoside (apigetrin) | n.d. | |
| Apigenin-8-C-glucoside (vitexin) | n.d. | |
| Catechin | n.d. | |
| Epicatechin | n.d. | |
| Hesperetin | 1.15 ± 0.07 | |
| Kaempferol | n.d. | |
| Kaempferol-3-O-glucoside (astragalin) | 62.61 ± 0.63 | |
| Luteolin | n.d. | |
| Myricetin | n.d. | |
| Naringenin | n.d. | |
| Procyanidin A2 | n.d. | |
| Procyanidin B1 | n.d. | |
| Procyanidin B2 | n.d. | |
| Quercetin | n.d | |
| Quercetin-3-O-galactoside (hyperoside) | 151.32 ± 1.86 | |
| Quercetin-3-O-rhamnoside (quercetrin) | 116.27 ± 0.27 | |
| Quercetin-3-O-rutinoside (rutin) | n.d. | |
| Vitexin-2″-O-rhamnoside | n.d. | |
| Total flavonoids | 331.35 ± 2.49 | |
| Total phenolic compounds | 766.44 ± 4.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsoi, F.T.; da Silva, G.B.; Manica, D.; Bagatini, M.D.; Pastore, G.M.; Arruda, H.S. Extract of Araçá-Boi and Its Major Phenolic Compound, Trans-Cinnamic Acid, Reduce Viability and Inhibit Migration of Human Metastatic Melanoma Cells. Nutrients 2024, 16, 2929. https://doi.org/10.3390/nu16172929
Borsoi FT, da Silva GB, Manica D, Bagatini MD, Pastore GM, Arruda HS. Extract of Araçá-Boi and Its Major Phenolic Compound, Trans-Cinnamic Acid, Reduce Viability and Inhibit Migration of Human Metastatic Melanoma Cells. Nutrients. 2024; 16(17):2929. https://doi.org/10.3390/nu16172929
Chicago/Turabian StyleBorsoi, Felipe Tecchio, Gilnei Bruno da Silva, Daiane Manica, Margarete Dulce Bagatini, Glaucia Maria Pastore, and Henrique Silvano Arruda. 2024. "Extract of Araçá-Boi and Its Major Phenolic Compound, Trans-Cinnamic Acid, Reduce Viability and Inhibit Migration of Human Metastatic Melanoma Cells" Nutrients 16, no. 17: 2929. https://doi.org/10.3390/nu16172929







