The Role of Vitamin D in Hematopoietic Stem Cell Transplantation: Implications for Graft-versus-Host Disease—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definition of Research Question
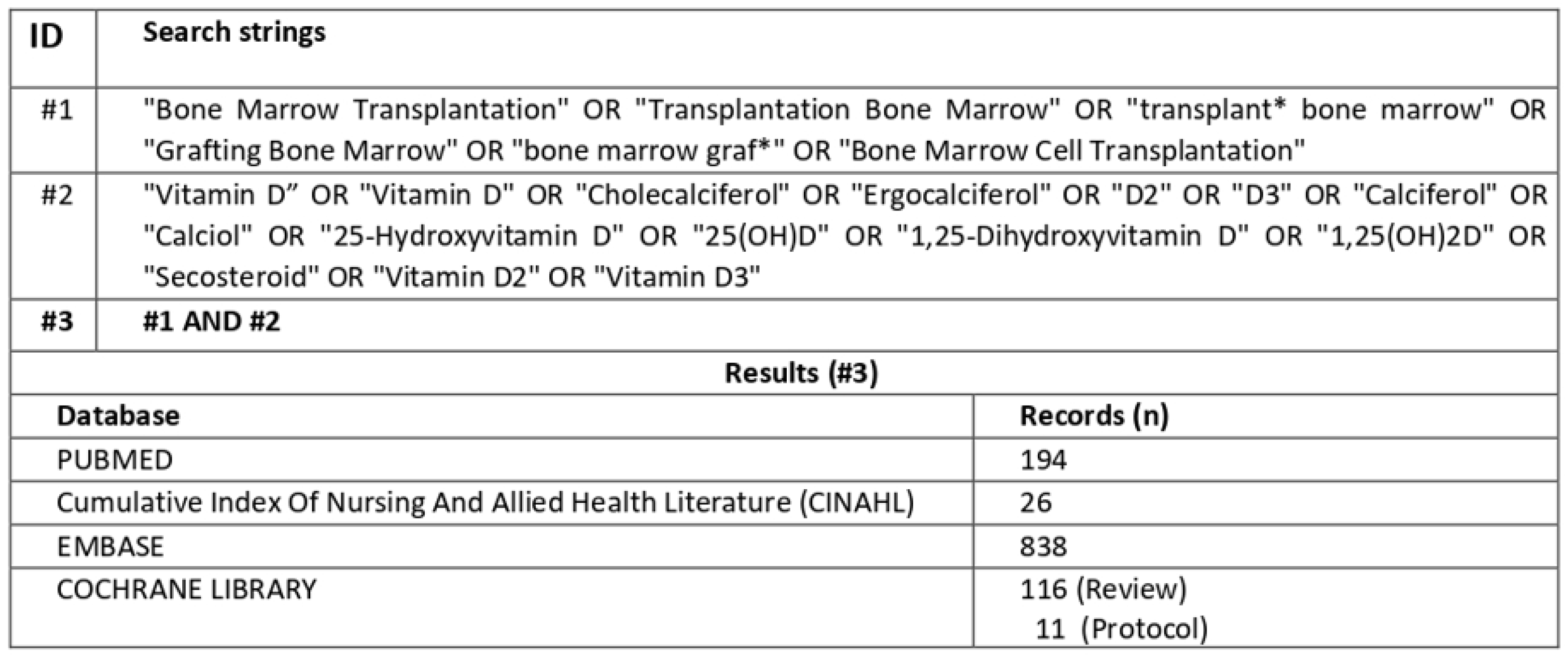
2.3. Literature Search and Criteria
2.4. Data Extraction and Synthesis
3. Results
3.1. General Characteristics of the Studies Included
3.2. Vitamin D Levels and Supplementation during Different Phases of HSCT
3.3. Vitamin D Levels and GvHD
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Echeverry, G.; Dalton, A. Hematologic Disorders. Anesthesiol. Clin. 2018, 36, 553–565. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- The Leukemia & Lymphoma Society. Facts 2022–2023. Updated Data on Blood Cancers. 2023. Available online: https://www.lls.org/booklet/facts-updated-data-blood-cancers (accessed on 2 August 2024).
- Khaddour, K.; Hana, C.K.; Mewawalla, P. Hematopoietic Stem Cell Transplantation; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Moreno, D.F.; Cid, J. Enfermedad del injerto contra el receptor. Med. Clin. 2019, 152, 22–28. [Google Scholar] [CrossRef]
- Arai, S.; Arora, M.; Wang, T.; Spellman, S.R.; He, W.; Couriel, D.R.; Urbano-Ispizua, A.; Cutler, C.S.; Bacigalupo, A.A.; Battiwalla, M.; et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation: A Report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 2015, 21, 266–274. [Google Scholar] [CrossRef]
- Funke, V.A.M.; Moreira, M.C.R.; Vigorito, A.C. Acute and chronic Graft-versus-host disease after hematopoietic stem cell transplantation. Front. Public Health 2016, 62, 44–50. [Google Scholar] [CrossRef]
- Arora, M.; Cutler, C.S.; Jagasia, M.H.; Pidala, J.; Chai, X.; Martin, P.J.; Flowers, M.E.; Inamoto, Y.; Chen, G.L.; Wood, W.A.; et al. Late Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 449–455. [Google Scholar] [CrossRef]
- Cuvelier, G.D.; Schoettler, M.; Buxbaum, N.P.; Pinal-Fernandez, I.; Schmalzing, M.; Distler, J.H.; Penack, O.; Santomasso, B.D.; Zeiser, R.; Angstwurm, K.; et al. Toward a Better Understanding of the Atypical Features of Chronic Graft-Versus-Host Disease: A Report from the 2020 National Institutes of Health Consensus Project Task Force. Biol. Blood Marrow Transplant. 2022, 28, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Ferraro, C.S.; Hamilton, B.K.; Majhail, N.S. To D or not to D: Vitamin D in hematopoietic cell transplantation. Bone Marrow Transplant. 2020, 55, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Body, J.J.; Brandi, M.L.; Broady, R.; Cannata-Andia, J.; Cannata-Ortiz, M.J.; El Maghraoui, A.; Guglielmi, G.; Hadji, P.; Pierroz, D.D.; et al. Osteoporosis management in hematologic stem cell transplant recipients: Executive summary. J. Bone Oncol. 2021, 28, 100361. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Baek, K.H.; Kim, H.-J.; Lee, S.; Lee, J.W.; Kang, M.-I. Changes in trabecular bone score and bone mineral density following allogeneic hematopoietic stem cell transplantation. Bone 2019, 124, 40–46. [Google Scholar] [CrossRef]
- Pirsl, F.; Curtis, L.M.; Steinberg, S.M.; Tella, S.H.; Katić, M.; Dobbin, M.; Hsu, J.; Hakim, F.T.; Mays, J.W.; Im, A.P.; et al. Characterization and Risk Factor Analysis of Osteoporosis in a Large Cohort of Patients with Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2016, 22, 1517–1524. [Google Scholar] [CrossRef]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7–13. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Abo-Zaid, M.A.; Hamdi, H.A.; Elashmawy, N.F. Vitamin D and Immunity: A comprehensive review of its impact on autoimmunity, allergy suppression, antimicrobial defense, and cancer inhibition. Egypt. J. Immunol. 2023, 30, 47–66. [Google Scholar] [CrossRef]
- Arain, A.; Matthiesen, C. Vitamin D deficiency and graft-versus-host disease in hematopoietic stem cell transplant population. Hematol. Stem Cell Ther. 2019, 12, 133–139. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Calendo, G.; Bui, T.; Tian, Y.; Lee, C.Y.; Zhou, Y.; Zhao, X.; Abraham, C.; Mo, W.; et al. Tissue-infiltrating alloreactive T cells require Id3 to deflect PD-1–mediated immune suppression during GVHD. Blood 2024, 143, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Beres, A.J.; Drobyski, W.R. The Role of Regulatory T Cells in the Biology of Graft Versus Host Disease. Front. Immunol. 2013, 4, 54650. [Google Scholar] [CrossRef] [PubMed]
- Ros-Soto, J.; Anthias, C.; Madrigal, A.; Snowden, J.A. Vitamin D: Is it important in haematopoietic stem cell transplantation? A review. Bone Marrow Transplant. 2019, 54, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Pira, G.L.; Di Cecca, S.; Montanari, M.; Moretta, L.; Manca, F. Specific removal of alloreactive T-cells to prevent GvHD in hemopoietic stem cell transplantation: Rationale, strategies and perspectives. Blood Rev. 2016, 30, 297–307. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Bissonnette, A.; Ahmad, R.; Wu, Z.; Vasir, B.; Stevenson, K.; Zarwan, C.; Keefe, W.; Glotzbecker, B.; Mills, H.; et al. Immunomodulatory effects of vitamin D: Implications for GVHD. Bone Marrow Transplant. 2010, 45, 1463–1468. [Google Scholar] [CrossRef]
- Toenges, R.; Greinix, H.; Lawitschka, A.; Halter, J.; Baumgartner, A.; Simon, A.; Arends, J.; Jäger, P.; Middeke, M.; Hilgendorf, I.; et al. Current practice in nutrition after allogeneic hematopoietic stem cell transplantation—Results from a survey among hematopoietic stem cell transplant centers. Clin. Nutr. 2021, 40, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Ros-Soto, J.; Snowden, J.A.; Salooja, N.; Gilleece, M.; Parker, A.; Greenfield, D.; Anthias, C.; Alfred, A.; Harrington, A.; Peczynski, C.; et al. Current practice in vitamin D management across adult and paediatric allogeneic haematopoietic stem cell transplant centres: A survey by transplant complications working party of EBMT. Bone Marrow Transplant. 2019, 54, 242. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Mastaglio, S.; Wong, E.; Perera, T.; Lim, A.; Mason, K.; Collins, J.; Szer, J.; Grigg, A.; Koldej, R.; Ritchie, D. The impact of vitamin d replacement on survival, relapse and GvHD after allogeneic stem cell transplantation: A matched cohort analysis. Bone Marrow Transplant. 2019, 53, 639–640. [Google Scholar]
- von Bahr, L.; Blennow, O.; Alm, J.; Björklund, A.; Malmberg, K.-J.; Mougiakakos, D.; Le Blanc, A.; Oefner, P.J.; Labopin, M.; Ljungman, P.; et al. Increased incidence of chronic GvHD and CMV disease in patients with vitamin D deficiency before allogeneic stem cell transplantation. Bone Marrow Transplant. 2015, 50, 1217–1223. [Google Scholar] [CrossRef]
- Glotzbecker, B.; Ho, V.T.; Aldridge, J.; Kim, H.T.; Horowitz, G.; Ritz, J.; Soiffer, R.; Avigan, D.; Rosenblatt, J. Low levels of 25-hydroxyvitamin D before allogeneic hematopoietic SCT correlate with the development of chronic GVHD. Bone Marrow Transplant. 2013, 48, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.L.; Zhang, G.; Wallace, G.; McLean, S.; Myers, K.C.; Teusink-Cross, A.; Taggart, C.; Patel, B.; Davidson, R.; Davies, S.M.; et al. Optimized vitamin D repletion with oral thin film cholecalciferol in patients undergoing stem cell transplant. Blood Adv. 2023, 7, 4555–4562. [Google Scholar] [CrossRef]
- Quillinan, C.; Murray, J. Vitamin D Deficiency: Does it have an impact on GvHD and infection? In Bone Marrow Transplantation; Macmillan: New York, NY, USA, 2018; Volume 53, p. 845. [Google Scholar]
- Ros-Soto, J.; Matthews, N.C.; Burton, C.; Szydlo, R.; Alfred, A.; Andrews, R.; Anthias, C.; Mehra, V.; Potter, V.; Madrigal, A.; et al. Association between vitamin d and GVHD biomarkers with response to immunosuppression and survival in acute GVHD: An exploratory study. In Bone Marrow Transplantation; Springer Nature: London, UK, 2022; Volume 57, pp. 100–416. [Google Scholar]
- Gjærde, L.K.; Ostrowski, S.R.; Andersen, N.S.; Friis, L.S.; Kornblit, B.; Petersen, S.L.; Schjødt, I.; Sengeløv, H. Pre-Transplantation Plasma Vitamin D Levels and Acute Graft-Versus-Host Disease after Myeloablative Allogeneic Hematopoietic Cell Transplantation. Transpl. Immunol. 2021, 68, 101437. [Google Scholar] [CrossRef]
- Jindal, N.; Saroha, M.; Mirgh, S.; Chichra, A.; Nayak, L.; Bonda, A.; Gokarn, A.; Punatar, S.; Bagal, B.; Chavan, P.; et al. Relevance of vitamin d in patients undergoing HLA matched allogenic stem cell transplant for acute leukaemia. Transpl. Immunol. 2023, 81, 101925. [Google Scholar] [CrossRef]
- Dikyar, A.; Hocaoǧlu, E.; Kaynar, L.A.; Özkurt, Z.N.; Yeǧin, Z.A. The eventual role of donor and recipient vitamin D levels in allogeneic hematopoetic stem cell transplant setting. In Proceedings of the 46th Annual Meeting of the European Society for Blood and Marrow Transplantation, Virtual, 29 August–1 September 2020; Volume 55, pp. 422–423. [Google Scholar] [CrossRef]
- Shan, R.; Zhang, Q.; Ding, Y.; Zhang, L.; Dong, Y.; Gao, W. Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights. Open Life Sci. 2024, 19, 20220787. [Google Scholar] [CrossRef]
- Charoenngam, N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 10659. [Google Scholar] [CrossRef]
- Webb, A.R.; Kazantzidis, A.; Kift, R.C.; Farrar, M.D.; Wilkinson, J.; Rhodes, L.E. Colour Counts: Sunlight and Skin Type as Drivers of Vitamin D Deficiency at UK Latitudes. Nutrients 2018, 10, 457. [Google Scholar] [CrossRef]
- Giustina, A.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Lazaretti-Castro, M.; Lips, P.; Marcocci, C.; Bilezikian, J.P. Vitamin D in the older population: A consensus statement. Endocrine 2022, 79, 31–44. [Google Scholar] [CrossRef] [PubMed]
- de Souza de Santana, K.V.; Oliver, S.L.; Mendes, M.M.; Lanham-New, S.; Charlton, K.E.; Ribeiro, H. Association between vitamin D status and lifestyle factors in Brazilian women: Implications of Sun Exposure Levels, Diet, and Health. EClinicalMedicine 2022, 47, 101400. [Google Scholar] [CrossRef]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2017, 7, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Ther. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Papadopoulou, S.K.; Detopoulou, P.; Tsoumana, D.; Giaginis, C.; Kondyli, F.S.; Lymperaki, E.; Pritsa, A. Vitamin D and Calcium in Osteoporosis, and the Role of Bone Turnover Markers: A Narrative Review of Recent Data from RCTs. Diseases 2023, 11, 29. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef]
- Malard, F.; Bossard, C.; Brissot, E.; Chevallier, P.; Guillaume, T.; Delaunay, J.; Mosnier, J.-F.; Moreau, P.; Grégoire, M.; Gaugler, B.; et al. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant. 2014, 49, 539–544. [Google Scholar] [CrossRef]
- Flamann, C.; Peter, K.; Kreutz, M.; Bruns, H. Regulation of the Immune Balance During Allogeneic Hematopoietic Stem Cell Transplantation by Vitamin D. Front. Immunol. 2019, 10, 2586. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, Country | Type of Study | Sample | Objective | Intervention | Results |
|---|---|---|---|---|---|
| Bartlett et al. 2023 USA [9] | Observational study | 20 (Allo-SCT) | Increase vitamin D levels | Cholecalciferol supplementation | Improvement in vitamin D levels; no toxicity observed |
| Ros-Soto et al. 2022 UK [31] | Observational study | 16 (Allo-SCT) | Vitamin D levels and GvHD | Measurement of VitD and GvHD biomarkers | Association between patients vitamin D levels and GvHD |
| Jindal et al. 2022 India [33] | Retrospective Observational study | 162 (Allo-SCT) | Vitamin D levels and GvHD | Cholecalciferol supplementation | High incidence of vitamin D deficiency; no association between GvHD and vitamin D levels |
| Gjærde et al. 2021 Denmark [32] | Observational study | 116 (Allo-SCT) | Vitamin D levels and GvHD | Measurement of VitD levels | No significant association between vitamin D levels and acute GvHD |
| Dikyar et al. 2020 Turkey [34] | Retrospective observational study | 123 donor; 123 recipents (Allo-SCT) | Vitamin D levels and GvHD | Evaluate the possible impact of donor and recipient VitD levels on HSCT outcome | Association between patients vitamin D levels and GvHD |
| Mastaglio et al. 2019 Australia [26] | Retrospective matched cohort study | IG 78 CG 156 (Allo-SCT) | Vitamin D levels and GvHD | Cholecalciferol supplementation | No association between patients vitamin D levels and GvHD |
| Quillinan & Murray 2019 UK [30] | Observational study | 102 (Allo-SCT) | Vitamin D levels and GvHD | Measurement of VitD levels | Clear link between VitD deficiency and GvHD; 25% of patients developed GvHD; need for VitD supplementation |
| Von Bahr et al. 2015 Sweden [27] | Retrospective cohort study | 166 (Allo-SCT) | Vitamin D levels and GvHD | Measurement of VitD levels | Low baseline vitamin D levels |
| Glotzbecker et al. 2013 USA [28] | Retrospective cohort study | 53 (Allo-SCT) | Vitamin D levels and GvHD | Measurement of VitD levels | Low vitamin D levels associated with increased risk of chronic GVHD |
| Author | Sample (n) | Cholecalciferol Start Indication | Cholecalciferol Dosage (Mean) | Method of Administration | Time of Administration | Vitamin D Levels(ng/mL) [Timing] |
|---|---|---|---|---|---|---|
| Bartlett et al. [9] | Allo-SCT (n = 20) | ≤35 ng/mL | 40,000 IU/week | OTF | From day 21–428 post-transplant | t0: 29.2 [+21] t1: 53 [+51] * t2: 58 [+428] |
| Jindal et al. [33] | Allo-SCT (n = 162) | ≤20 ng/mL | 60,000 IU/week | Oral | For 8 weeks followed by maintenance with 800 IU/day | t0: ≤20 [pre-HSCT] (86.9%) t1: N.R t2: 34 [+120] |
| Mastaglio et al. [26] | Allo-SCT (n = 78) | N.R | 1000 IU/day or 0.25 mg calcitriol/day | Oral | Before HSCT until 1 year post-transplant | N.R |
| Gjærde et al. [32] | Allo-SCT (n = 116) | N.R | N.R | N.R | N.R | t0: 25.6 |
| Dikyar et al. [34] | Donors a (n = 123); Allo-SCT b (n = 123) | N.R | N.R | N.R | N.R | t0: 16 a 12.8 b |
| Quillinan & Murray [30] | Allo-SCT (n = 102) | N.R | N.R | N.R | N.R | t0: ≤20 [pre-HSCT] (73.5%) |
| Von Bahr et al. [27] | Allo-SCT (n = 166) | t0: 15.6 [pre-HSCT] | ||||
| Glotzbecker et al. [28] | Allo-SCT (n = 116) | N.R | N.R | N.R | N.R | t0: 21.9 |
| Author, Year | Sample | aGvHD Results | cGvHD Results |
|---|---|---|---|
| Ros-Soto et al. [31] | Allo-SCT (n = 16) | Patients with grade 0–II had a higher concentration of 25(OH)D3 compared to those with grade III–IV (41.6 vs. 23.3 nmol/L; p = 0.032) | N.R |
| Mastaglio et al. [26] | Allo-SCT (n = 78) | No association between baseline vitamin D levels and GVHD | |
| Gjærde et al. [32] | Allo-SCT (n = 116) | No association between baseline vitamin D levels or vitamin D insufficiency and acute GvHD | N.R |
| Dikyar et al. [34] | Donors (n = 123) Allo-SCT Recipients (n = 123) | N.R | Negative correlation between baseline recipient VitD levels and cGvHD (p = 0.011, r = −0.235) |
| Quillinan & Murray [30] | Allo-SCT (n = 102) | Association between GvHD and baseline vitamin D deficiency with approximately 25% of patients developing acute or chronic GvHD | |
| Glotzbecker et al. [28] | Allo-SCT (n = 116) | aGvHD grades II–IV at 100 days was 53.1% in patients with vitamin D < 25, versus 33.3% in patients with vitamin D ≥ 25 ng/mL (p = 0.13) | Low baseline vitamin D levels are associated with cGVHD (hazard ratio = 5.26, p = 0.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancin, S.; Cangelosi, G.; Matteucci, S.; Palomares, S.M.; Parozzi, M.; Sandri, E.; Sguanci, M.; Piredda, M. The Role of Vitamin D in Hematopoietic Stem Cell Transplantation: Implications for Graft-versus-Host Disease—A Narrative Review. Nutrients 2024, 16, 2976. https://doi.org/10.3390/nu16172976
Mancin S, Cangelosi G, Matteucci S, Palomares SM, Parozzi M, Sandri E, Sguanci M, Piredda M. The Role of Vitamin D in Hematopoietic Stem Cell Transplantation: Implications for Graft-versus-Host Disease—A Narrative Review. Nutrients. 2024; 16(17):2976. https://doi.org/10.3390/nu16172976
Chicago/Turabian StyleMancin, Stefano, Giovanni Cangelosi, Sofia Matteucci, Sara Morales Palomares, Mauro Parozzi, Elena Sandri, Marco Sguanci, and Michela Piredda. 2024. "The Role of Vitamin D in Hematopoietic Stem Cell Transplantation: Implications for Graft-versus-Host Disease—A Narrative Review" Nutrients 16, no. 17: 2976. https://doi.org/10.3390/nu16172976
APA StyleMancin, S., Cangelosi, G., Matteucci, S., Palomares, S. M., Parozzi, M., Sandri, E., Sguanci, M., & Piredda, M. (2024). The Role of Vitamin D in Hematopoietic Stem Cell Transplantation: Implications for Graft-versus-Host Disease—A Narrative Review. Nutrients, 16(17), 2976. https://doi.org/10.3390/nu16172976








