Efficacy of Food Supplement Based on Monacolins, γ-Oryzanol, and γ-Aminobutyric Acid in Mild Dyslipidemia: A Randomized, Double-Blind, Parallel-Armed, Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Supplement and Placebo
2.2. Study Design
2.3. Participants and Recruiting
2.4. Study Outcomes
2.5. Safety and Tolerability
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller-Nordhorn, J.; Binting, S.; Roll, S.; Willich, S.N. An update on regional variation in cardiovascular mortality within Europe. Eur. Heart J. 2008, 29, 1316–1326. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar] [PubMed]
- Buzzelli, L.; Segreti, A.; Di Gioia, D.; Lemme, E.; Squeo, M.R.; Nenna, A.; Di Gioia, G. Alternative lipid lowering strategies: State-of-the-art review of red yeast rice. Fitoterapia 2024, 172, 105719. [Google Scholar] [CrossRef] [PubMed]
- Laffin, L.J.; Bruemmer, D.; Garcia, M.; Brennan, D.M.; McErlean, E.; Jacoby, D.S.; Michos, E.D.; Ridker, P.M.; Wang, T.Y.; Watson, K.E.; et al. Comparative effects of low-dose rosuvastatin, placebo, and dietary supplements on lipids and inflammatory biomarkers. J. Am. Coll. Cardiol. 2023, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Catapano, A.L.; Cicero, A.F.; Escobar, C.; Foger, B.; Katsiki, N.; Latkovskis, G.; Rakowski, M.; Reiner, Z.; Sahebkar, A.; et al. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: A position paper of the International Lipid Expert Panel. Pharmacol. Res. 2022, 183, 106370. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red yeast rice for hypercholesterolemia: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef]
- Kokumai, T.; Ito, J.; Kobayashi, E.; Shimizu, N.; Hashimoto, H.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Comparison of blood profiles of γ -oryzanol and ferulic acid in rats after oral intake of γ-oryzanol. Nutrients 2019, 11, 1174. [Google Scholar] [CrossRef]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Saari, N.; Mustafa, S.; Yusof, R.M.; Hussin, A.S.M.; Hamid, A.; Manap, M.Y.A. Effect of pre-germination time of brown rice on serum cholesterol levels of hypercholesterolaemic rats. J. Sci. Food. Agric. 2010, 90, 245–251. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Russo, R. Effects of a nutraceutical combination of monacolin, gamma-oryzanol and gamma-aminobutyric acid on lipid profile and C-reactive protein in mice. Arch. Med. Sci. 2019, 15, 792–796. [Google Scholar] [CrossRef]
- Wang, T.J.; Lien, A.S.; Chen, J.L.; Lin, C.H.; Yang, Y.S.; Yang, S.H. A randomized clinical efficacy trial of red yeast rice (Monascus pilosus) against hyperlipidemia. Am. J. Chin. Med. 2019, 47, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2022/860 of 1 June 2022 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Monacolins from Red Yeast Rice (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32022R0860&qid=1719245311125 (accessed on 24 June 2024).
- Baldi, A. The Beneficial Effect on Cardiovascular Function of a Food Supplement Based on Red Yeast Rice (Monacolin K), Gamma-Oryzanol from Rice Bran, and Gamma-Aminobutyric Acid. Available online: https://www.isrctn.com/ISRCTN90678255 (accessed on 6 July 2024).
- Sanità, the Italian National Institute of Health (ISD). Il Progetto Cuore. Available online: https://www.cuore.iss.it/ (accessed on 6 July 2024).
- Appel, L.J. The effects of dietary factors on blood pressure. Cardiol. Clin. 2017, 35, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelic, D.; Kahleova, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH dietary pattern and cardiometabolic outcomes: An umbrella review of systematic reviews and meta-analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Sanità, the Italian Ministry of Health (MDS) and The Italian National Institute of Health (ISD). VigiErbe. Available online: www.vigierbe (accessed on 7 April 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- R.C. Team. R: A Language and Environment for Statistical Computing. 2022. Available online: http://www.r-project.org/index.html (accessed on 4 June 2024).
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D.; Group, C.P. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Acosta, S.; Johansson, A.; Drake, I. Diet and lifestyle factors and risk of atherosclerotic cardiovascular disease—A prospective cohort study. Nutrients 2021, 13, 3822. [Google Scholar] [CrossRef]
- Ferrari, R.; Lüscher, T.F. Reincarnated medicines: Using out-dated drugs for novel indications. Eur. Heart J. 2016, 37, 2571–2576. [Google Scholar] [CrossRef][Green Version]
- Lawrence, G.D. Dietary fats and health: Dietary recommendations in the context of scientific evidence. Adv. Nutr. 2013, 4, 294–302. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated fats and health: A reassessment and proposal for food-based recommendations: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Gidding, S.S.; Allen, N.B. Cholesterol and atherosclerotic cardiovascular disease: A lifelong problem. J. Am. Heart Assoc. 2019, 8, e012924. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.M.; Defina, L.F.; Leonard, D.; Barlow, C.E.; Radford, N.B.; Willis, B.L.; Rohatgi, A.; McGuire, D.K.; de Lemos, J.A.; Grundy, S.M.; et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: Results from the Cooper Center Longitudinal Study. Circulation 2018, 138, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Lee, F.; Astor, B.C. Association of high-density lipoprotein cholesterol with coronary heart disease risk across categories of low-density lipoprotein cholesterol: The atherosclerosis risk in communities study. Am. J. Med. Sci. 2011, 341, 173–180. [Google Scholar] [CrossRef]
- Danese, M.D.; Gleeson, M.; Kutikova, L.; Griffiths, R.I.; Khunti, K.; Seshasai, S.R.K.; Ray, K.K. Management of lipid-lowering therapy in patients with cardiovascular events in the UK: A retrospective cohort study. BMJ Open 2017, 7, e013851. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri, N.; Banach, M.; Sirtori, C.R.; Corsini, A. Side effects of statins: From pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc. Res. 2022, 118, 3288–3304. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.M.; Hegele, R.A. Functional foods and dietary supplements for the management of dyslipidaemia. Nat. Rev. Endocrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- Cicero, A.F.; Ferroni, A.; Ertek, S. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid-lowering effects. Expert Opin. Drug Saf. 2012, 11, 753–766. [Google Scholar] [CrossRef]
- Verhoeven, V.; Lopez Hartmann, M.; Remmen, R.; Wens, J.; Apers, S.; Van Royen, P. Red yeast rice lowers cholesterol in physicians—A double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2013, 13, 178. [Google Scholar] [CrossRef]
- Cicero, A.F.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 2013, 33, 622–628. [Google Scholar] [CrossRef]
- Minamizuka, T.; Koshizaka, M.; Shoji, M.; Yamaga, M.; Hayashi, A.; Ide, K.; Ide, S.; Kitamoto, T.; Sakamoto, K.; Hattori, A.; et al. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in Japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435. [Google Scholar] [PubMed]
- Xiong, X.; Wang, P.; Li, X.; Zhang, Y.; Li, S. The effects of red yeast rice dietary supplement on blood pressure, lipid profile, and C-reactive protein in hypertension: A systematic review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1831–1851. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel; Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368. [Google Scholar]
- Ding, J.; Ulanov, A.V.; Dong, M.; Yang, T.; Nemzer, B.V.; Xiong, S.; Zhao, S.; Feng, H. Enhancement of gama-aminobutyric acid (GABA) and other health-related metabolites in germinated red rice (Oryza sativa L.) by ultrasonication. Ultrason. Sonochem. 2018, 40, 791–797. [Google Scholar] [CrossRef]
- Li, X.-M.; Shen, X.-H.; Duan, Z.-W.; Guo, S.-R. Advances on the pharmacological effects of red yeast rice. Chin. J. Nat. Med. 2011, 9, 161–166. [Google Scholar]
- Legesse, D.H.; Fan, C.; Teng, J.; Zhuang, Y.; Howard, R.J.; Noviello, C.M.; Lindahl, E.; Hibbs, R.E. Structural insights into opposing actions of neurosteroids on GABA(A) receptors. Nat. Commun. 2023, 14, 5091. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]

| Characteristic | Placebo Group (n = 44) | Food Supplement Group (n = 44) |
|---|---|---|
| Age (years) | 57 ± 7 | 53 ± 5 |
| Sex: | ||
| Females | 26 | 20 |
| Males | 18 | 24 |
| Ethnicity: Caucasian | 44 | 44 |
| BMI (kg/m2): | ||
| Females | 23.33 ± 3.56 | 23.30 ± 3.07 |
| males | 23.42 ± 3.84 | 24.08 ± 3.63 |
| Waist circumference (cm) | ||
| females | 99.46 ± 6.15 | 97.85 ± 5.47 |
| males | 99.50 ± 4.59 | 97.66 ± 4.91 |
| Variable | Placebo Group | Food Supplement Group | ||
|---|---|---|---|---|
| t0 | t1 | t0 | t1 | |
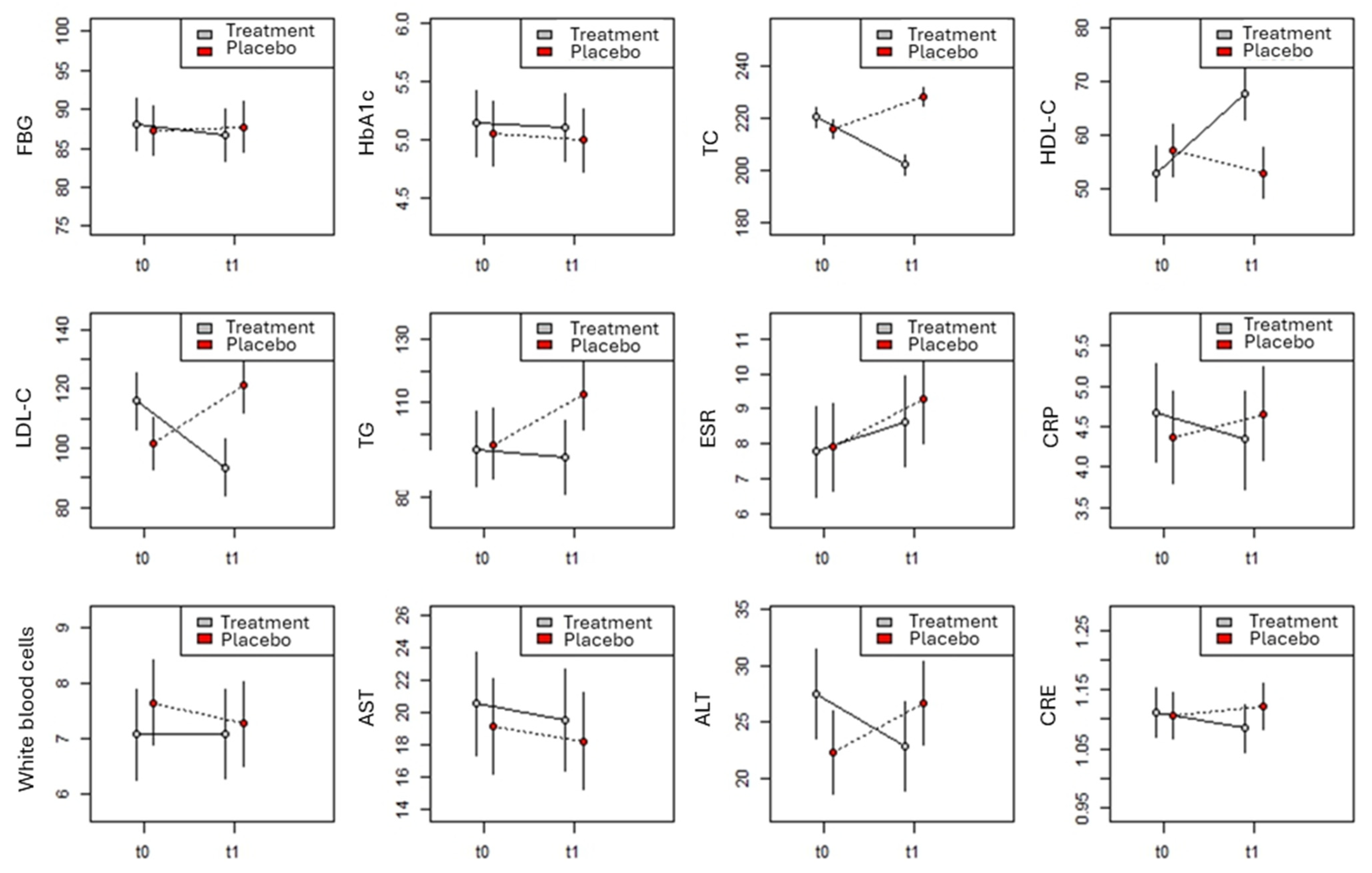
| FBG | 87.3 ± 10.4 | 87.9 ± 9.3 | 88.0 ± 10.2 | 86.8 ± 9.1 |
| (70–104) | (70–105) | (71–105) | (71–104) | |
| HbA1c | 5.0 ± 0.8 | 5.0 ± 0.9 | 5.1 ± 0.8 | 5.0 ± 0.9 |
| (4–6) | (4–6) | (4–6) | (4–6) | |
| TC | 217.5 ± 10.7 | 229.6 ± 12.7 | 222.7 ± 9.4 | 204.3 ± 11.3 |
| (201–239) | (210–250) | (202–238) | (184–224) | |
| HDL-C | 56.2 ± 14.6 | 51.8 ± 11.5 | 51.6 ± 13.1 | 66.7 ± 18.6 |
| (30–80) | (30–70) | (30–103) | (36–130) | |
| LDL-C | 101.6 ± 31.0 | 122.4 ± 27.4 | 115.7 ± 28.5 | 93.4 ± 23.8 |
| (56–159) | (72–163) | (57–165) | (55–140) | |
| TG | 98.6 ± 37.5 | 112.9 ± 38.0 | 100.4 ± 36.3 | 97.3 ± 25.9 |
| (40–165) | (62–177) | (40–163) | (42–152) | |
| ESR | 7.9 ± 4.2 | 9.1 ± 3.8 | 7.9 ± 3.7 | 8.8 ± 3.6 |
| (2–15) | (2–15) | (2–14) | (2–15) | |
| CRP | 4.3 ± 1.8 | 4.6 ± 1.9 | 4.5 ± 1.6 | 4.2 ± 1.7 |
| (2–7) | (2–7) | (2–7) | (2–7) | |
| WBCs | 7.5 ± 2.4 | 7.2 ± 2.3 | 7.0 ± 2.4 | 7.0 ± 2.4 |
| (4–11) | (4–11) | (4–11) | (4–11) | |
| AST | 19.6 ± 9.4 | 18.5 ± 8.9 | 21.5 ± 9.5 | 20.4 ± 8.5 |
| (5–35) | (6–37) | (6–37) | (6–37) | |
| ALT | 23.8 ± 11.7 | 28.2 ± 11.9 | 29.4 ± 11 | 24.8 ± 11.3 |
| (4–44) | (5–45) | (9–45) | (5–45) | |
| CRE | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| (0.91–1.28) | (0.9–1.3) | (0.9–1.3) | (0.9–1.29) | |
| Variable | F-Value | DF | p |
|---|---|---|---|
| TC | |||
| Measure | 3.162 | 1.88 | 0.08 |
| Group | 38.899 | 1.83 | <0.001 |
| Sex | 5.169 | 1.82 | 0.026 |
| Age | 0.114 | 1.82 | 0.74 |
| BMI | 0.002 | 1.90 | 0.97 |
| Waist circumference | 0.955 | 1.99 | 0.33 |
| Measure × group | 85.597 | 1.87 | <0.001 |
| HDL-C | |||
| Measure | 5.809 | 1.88 | 0.018 |
| Group | 5.144 | 1.83 | 0.026 |
| Sex | 0.915 | 1.82 | 0.34 |
| Age | 0.174 | 1.83 | 0.68 |
| BMI | 0.146 | 1.90 | 0.70 |
| Waist circumference | 0.000 | 1.99 | 0.99 |
| Measure × group | 18.866 | 1.88 | <0.001 |
| LDL-C | |||
| Measure | 0.144 | 1.88 | 0.71 |
| Group | 2.059 | 1.83 | 0.16 |
| Sex | 0.006 | 1.82 | 0.94 |
| Age | 1.270 | 1.83 | 0.26 |
| BMI | 0.157 | 1.92 | 0.69 |
| Waist circumference | 2.935 | 1.102 | 0.09 |
| Measure × group | 27.617 | 1.88 | <0.001 |
| TG | |||
| Measure | 1.589 | 1.168 | 0.21 |
| Group | 3.865 | 1.168 | 0.051 |
| Sex | 1.261 | 1.168 | 0.26 |
| Age | 1.995 | 1.168 | 0.16 |
| BMI | 0.275 | 1.168 | 0.60 |
| Waist circumference | 3.282 | 1.168 | 0.07 |
| Measure × group | 3.166 | 1.168 | 0.08 |
| Variable | F | Gdl | p |
| FBG | |||
| Measure | 0.097 | 1.88 | 0.76 |
| Group | 0.002 | 1.83 | 0.97 |
| Sex | 0.001 | 1.82 | 0.97 |
| Age | 0.067 | 1.82 | 0.80 |
| BMI | 0.081 | 1.92 | 0.78 |
| Waist circumference | 0.312 | 1.103 | 0.58 |
| Measure × group | 0.378 | 1.87 | 0.54 |
| HbA1c | |||
| Measure | 0.129 | 1.87 | 0.72 |
| Group | 0.549 | 1.83 | 0.46 |
| Sex | 0.618 | 1.82 | 0.43 |
| Age | 0.130 | 1.82 | 0.72 |
| BMI | 0.731 | 1.90 | 0.39 |
| Waist circumference | 0.024 | 1.99 | 0.88 |
| Measure × group | 0.004 | 1.87 | 0.95 |
| CRP | |||
| Measure | 0.010 | 1.88 | 0.92 |
| Group | 0.000 | 1.83 | 0.99 |
| Sex | 0.350 | 1.82 | 0.56 |
| Age | 0.080 | 1.82 | 0.78 |
| BMI | 0.037 | 1.91 | 0.85 |
| Waist circumference | 0.972 | 1.101 | 0.33 |
| Measure × group | 1.449 | 1.87 | 0.23 |
| WBCs | |||
| Measure | 0.266 | 1.168 | 0.61 |
| Group | 1.072 | 1.168 | 0.30 |
| Sex | 0.317 | 1.168 | 0.57 |
| Age | 0.043 | 1.168 | 0.84 |
| BMI | 0.949 | 1.168 | 0.33 |
| Waist circumference | 0.180 | 1.168 | 0.67 |
| Measure × group | 0.296 | 1.168 | 0.59 |
| ESR | |||
| Measure | 3.688 | 1.168 | 0.06 |
| Group | 0.425 | 1.168 | 0.52 |
| Sex | 0.002 | 1.168 | 0.97 |
| Age | 0.002 | 1.168 | 0.96 |
| BMI | 5.060 | 1.168 | 0.026 |
| Waist circumference | 1.652 | 1.168 | 0.20 |
| Measure × group | 0.201 | 1.168 | 0.65 |
| Variable | F | Gdl | p |
|---|---|---|---|
| AST | |||
| Measure | 0.460 | 1.168 | 0.50 |
| Group | 0.913 | 1.168 | 0.34 |
| Sex | 0.961 | 1.168 | 0.33 |
| Age | 0.012 | 1.168 | 0.91 |
| BMI | 0.003 | 1.168 | 0.96 |
| Waist circumference | 0.981 | 1.168 | 0.32 |
| Measure × group | 0.000 | 1.168 | 0.98 |
| ALT | |||
| Measure | 0.010 | 1.168 | 0.92 |
| Group | 0.151 | 1.168 | 0.70 |
| Sex | 4.165 | 1.168 | 0.043 |
| Age | 0.085 | 1.168 | 0.77 |
| BMI | 0.012 | 1.168 | 0.91 |
| Waist circumference | 0.165 | 1.168 | 0.68 |
| Measure × group | 6.546 | 1.168 | 0.011 |
| CRE | |||
| Measure | 0.104 | 1.168 | 0.75 |
| Group | 0.810 | 1.168 | 0.37 |
| Sex | 0.599 | 1.168 | 0.44 |
| Age | 0.270 | 1.168 | 0.60 |
| BMI | 0.138 | 1.168 | 0.71 |
| Waist circumference | 0.862 | 1.168 | 0.35 |
| Measure × group | 1.377 | 1.168 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lellis, L.F.; Morone, M.V.; Buccato, D.G.; Cordara, M.; Larsen, D.S.; Ullah, H.; Piccinocchi, R.; Piccinocchi, G.; Balaji, P.; Baldi, A.; et al. Efficacy of Food Supplement Based on Monacolins, γ-Oryzanol, and γ-Aminobutyric Acid in Mild Dyslipidemia: A Randomized, Double-Blind, Parallel-Armed, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 2983. https://doi.org/10.3390/nu16172983
De Lellis LF, Morone MV, Buccato DG, Cordara M, Larsen DS, Ullah H, Piccinocchi R, Piccinocchi G, Balaji P, Baldi A, et al. Efficacy of Food Supplement Based on Monacolins, γ-Oryzanol, and γ-Aminobutyric Acid in Mild Dyslipidemia: A Randomized, Double-Blind, Parallel-Armed, Placebo-Controlled Clinical Trial. Nutrients. 2024; 16(17):2983. https://doi.org/10.3390/nu16172983
Chicago/Turabian StyleDe Lellis, Lorenza Francesca, Maria Vittoria Morone, Daniele Giuseppe Buccato, Marcello Cordara, Danaè S. Larsen, Hammad Ullah, Roberto Piccinocchi, Gaetano Piccinocchi, Paulraj Balaji, Alessandra Baldi, and et al. 2024. "Efficacy of Food Supplement Based on Monacolins, γ-Oryzanol, and γ-Aminobutyric Acid in Mild Dyslipidemia: A Randomized, Double-Blind, Parallel-Armed, Placebo-Controlled Clinical Trial" Nutrients 16, no. 17: 2983. https://doi.org/10.3390/nu16172983
APA StyleDe Lellis, L. F., Morone, M. V., Buccato, D. G., Cordara, M., Larsen, D. S., Ullah, H., Piccinocchi, R., Piccinocchi, G., Balaji, P., Baldi, A., Di Minno, A., El-Seedi, H. R., Sacchi, R., & Daglia, M. (2024). Efficacy of Food Supplement Based on Monacolins, γ-Oryzanol, and γ-Aminobutyric Acid in Mild Dyslipidemia: A Randomized, Double-Blind, Parallel-Armed, Placebo-Controlled Clinical Trial. Nutrients, 16(17), 2983. https://doi.org/10.3390/nu16172983









