Antihypertensive Effects of Lindera erythrocarpa Makino via NO/cGMP Pathway and Ca2+ and K+ Channels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract
2.2. Animals
2.3. Chemicals and Solution Preparation
2.4. Ex Vivo Vasorelaxant Evaluation
2.4.1. General Experimental Procedures
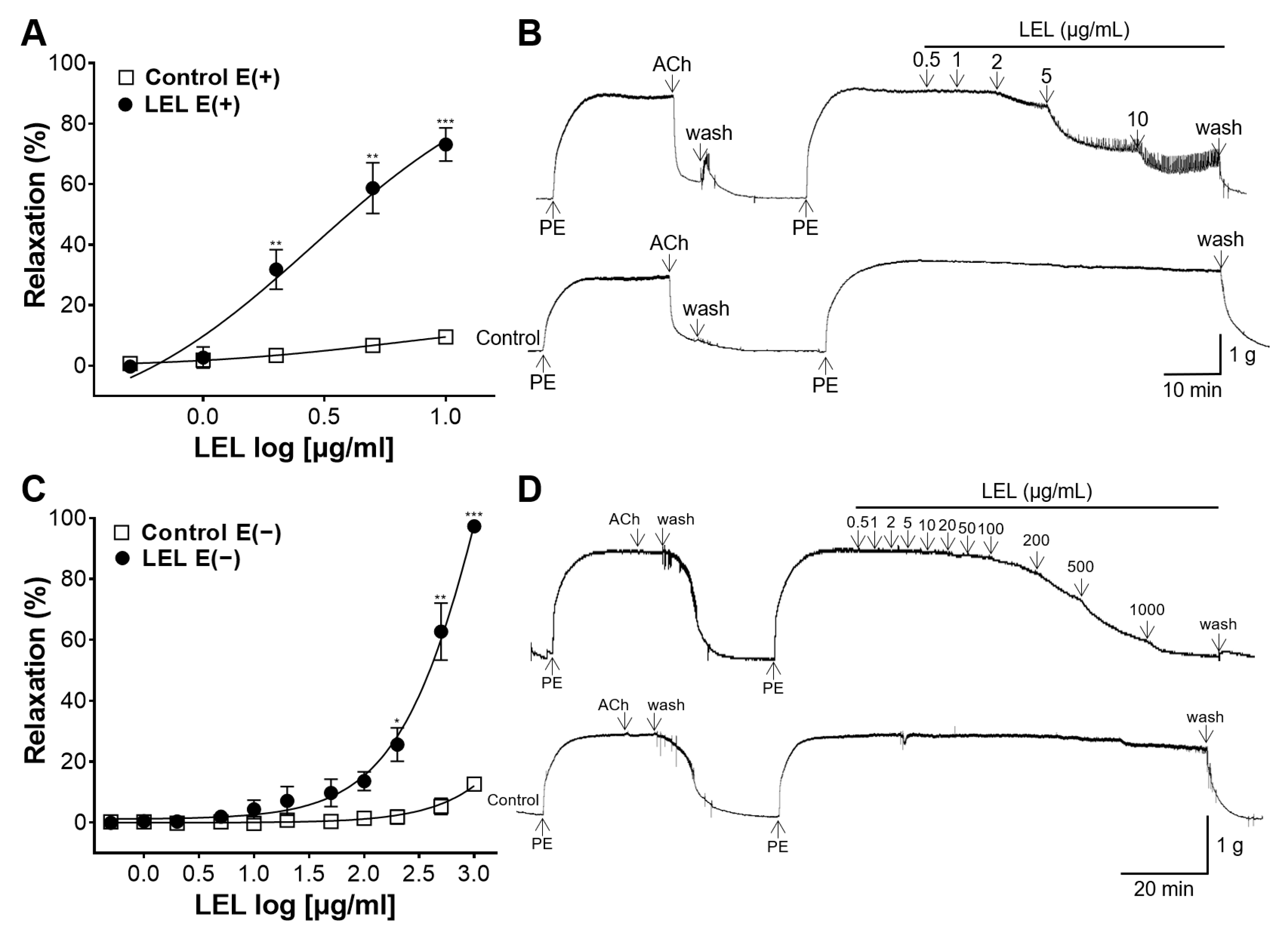
2.4.2. Role of Endothelium in LEL-Induced Vasorelaxant Activity
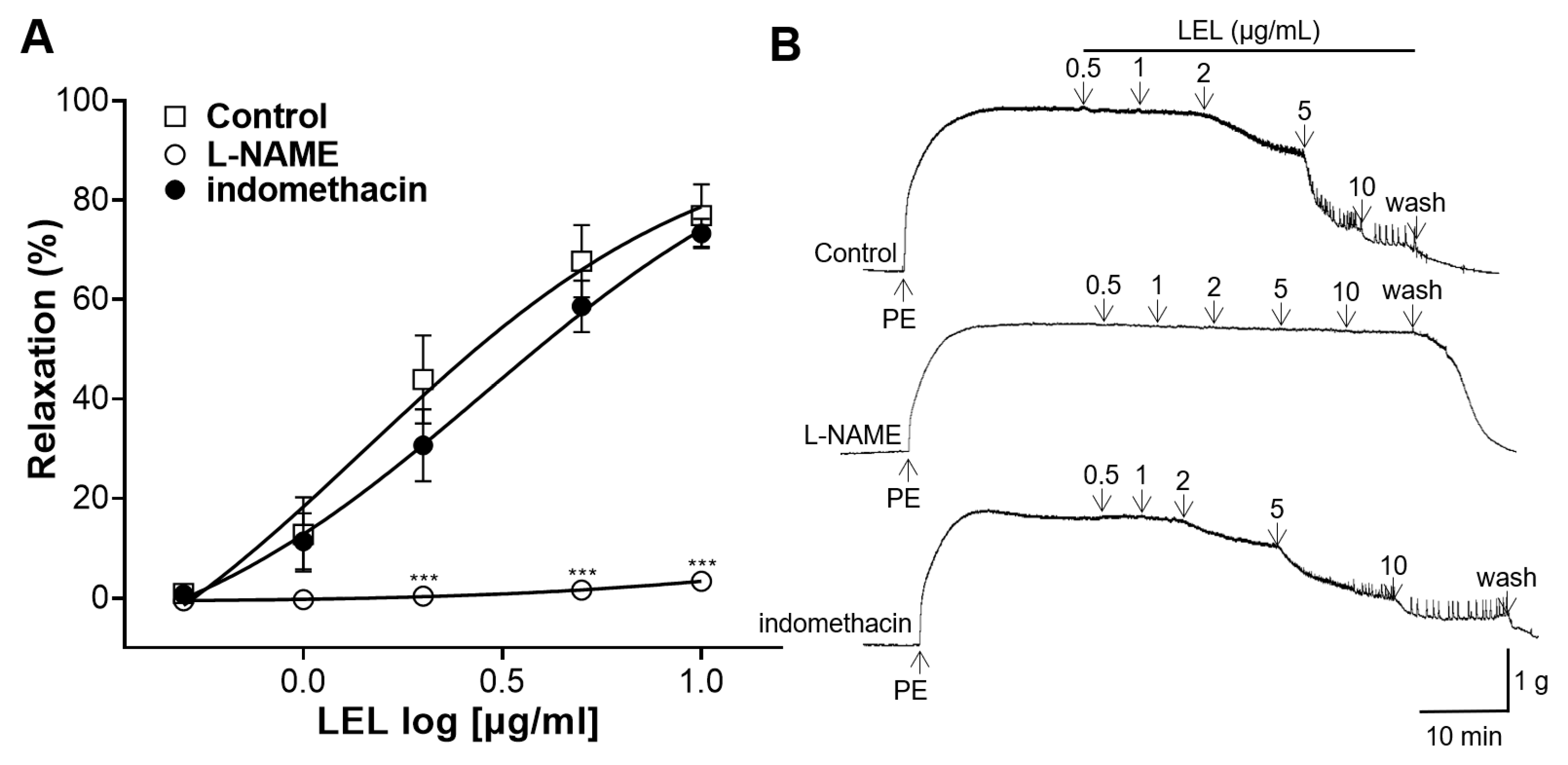
2.4.3. Role of NO Synthase and Cyclooxygenase (COX) in LEL-Induced Vasorelaxant Activity
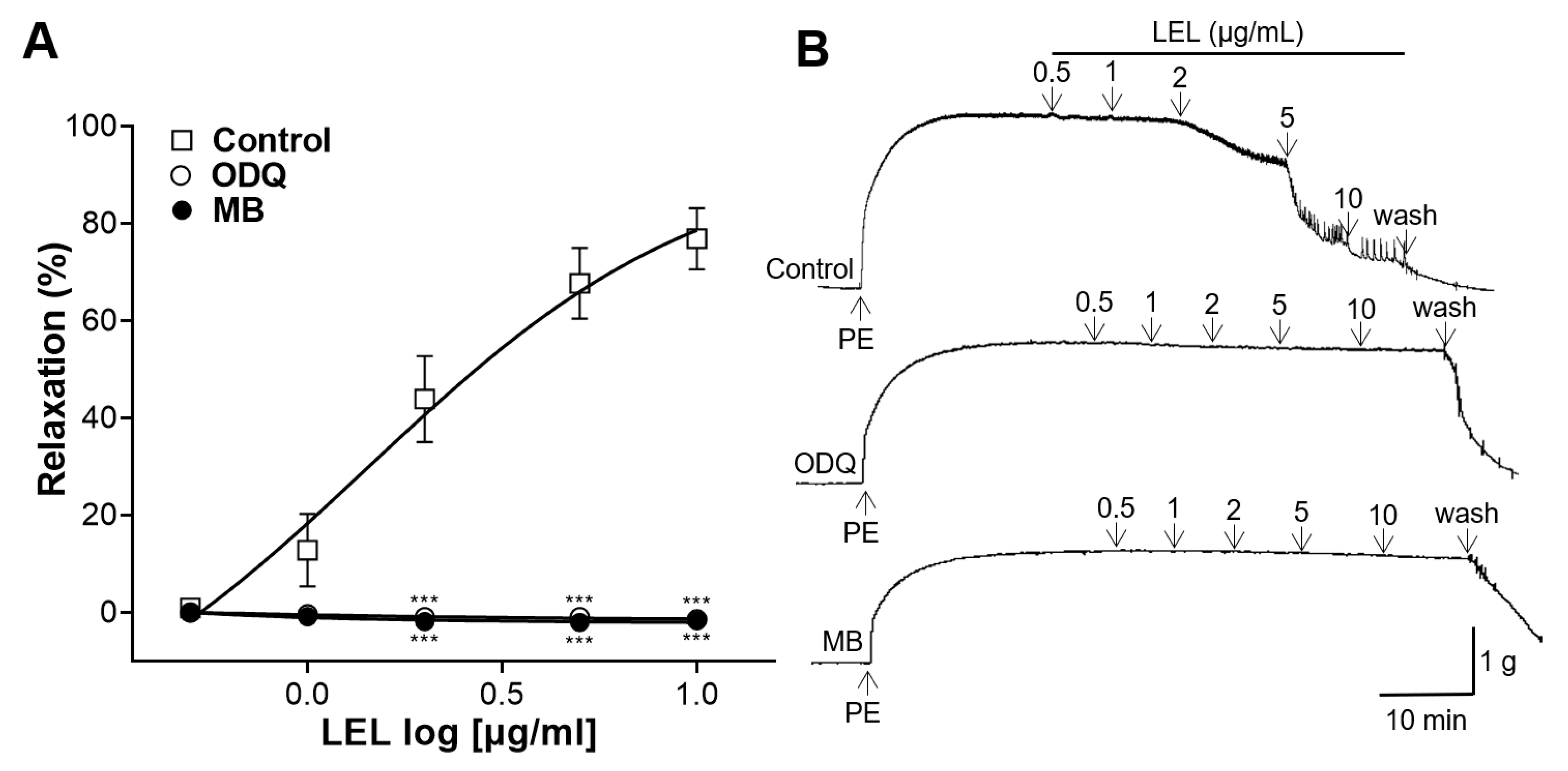
2.4.4. Role of Soluble Guanylate Cyclase (sGC) and cGMP in LEL-Induced Vasorelaxant Activity
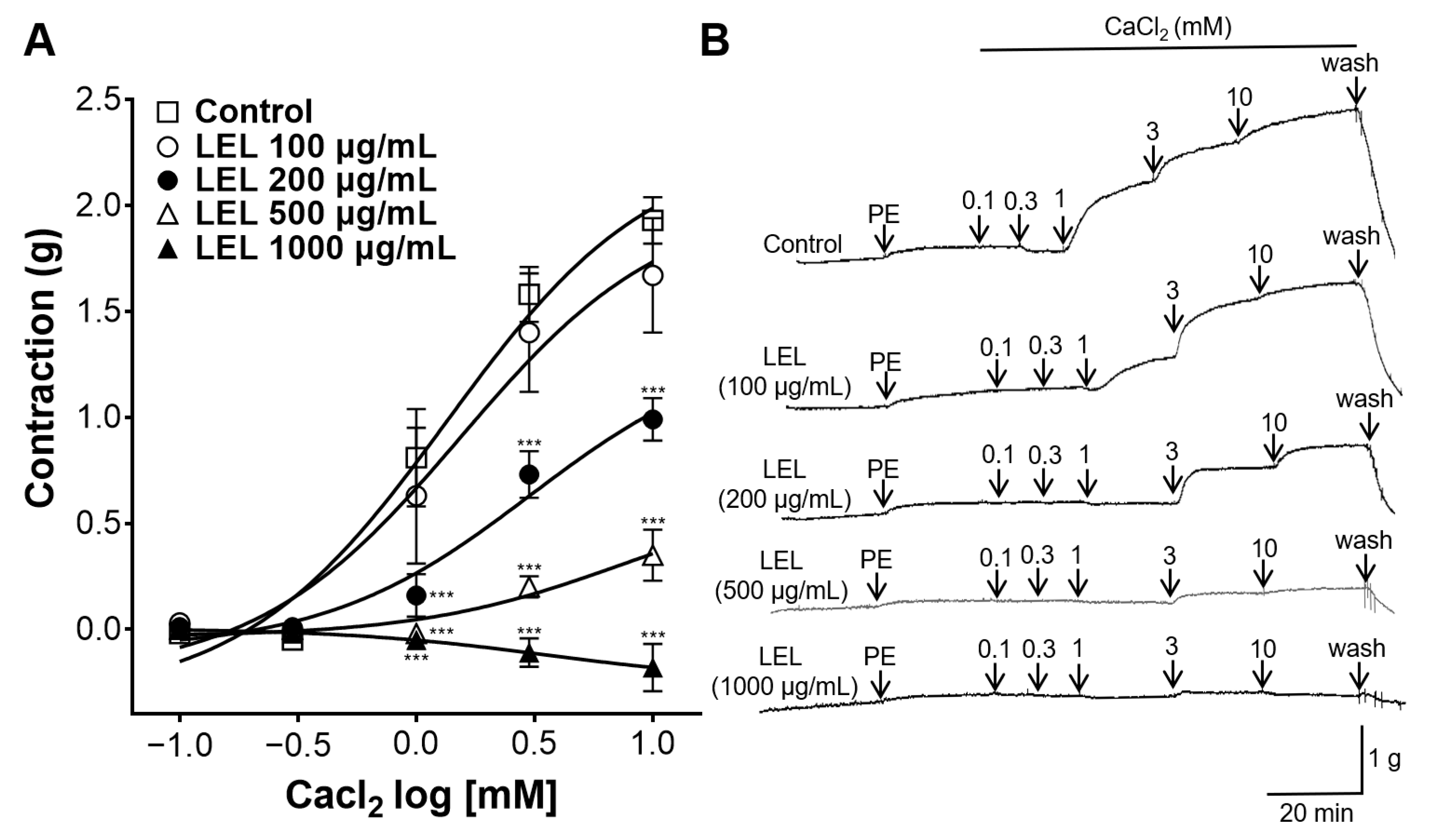
2.4.5. Effect of LEL on Ca2+ Influx through Ca2+ Channels
2.4.6. Effect of LEL on Rings Constricted by Bay K8644
2.4.7. Role of K+ Channels in LEL-Induced Vasorelaxant Activity
2.4.8. Effect of LEL on Rings Constricted by Angiotensin II
2.5. Blood Pressure Measurement
2.6. Statistical Analysis
3. Results
3.1. Role of Endothelium in LEL-Induced Vasorelaxant Activity
3.2. Role of NO Synthase and COX in LEL-Induced Vasorelaxant Activity
3.3. Role of sGC and cGMP in LEL-Induced Vasorelaxant Activity
3.4. Effect of LEL on Ca2+ Influx
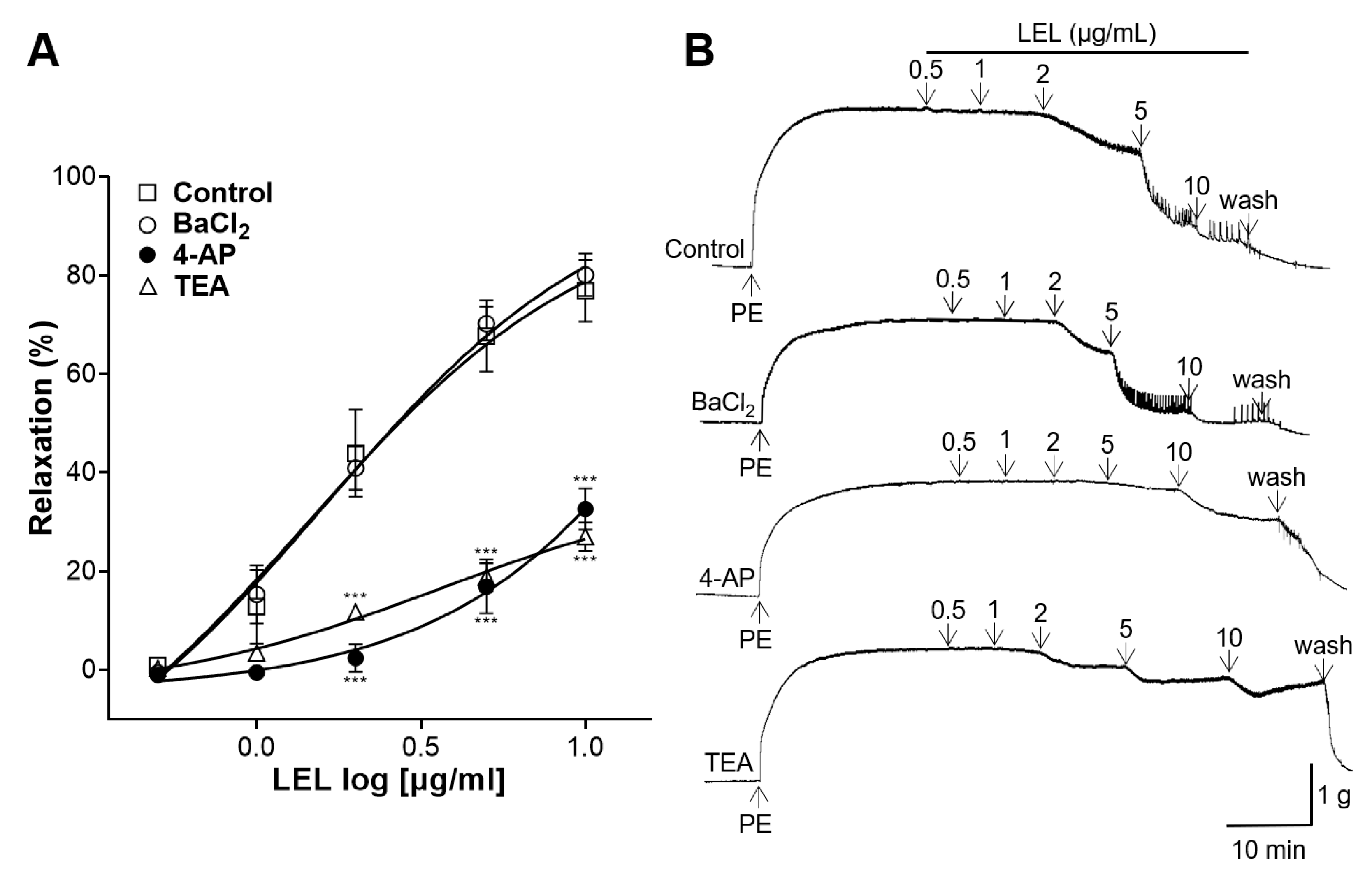
3.5. Role of K+ Channels in LEL-Induced Vasorelaxant Activity
3.6. Effect of LEL on Rings Constricted by Angiotensin II
3.7. Hypotensive Effect of LEL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diabetes Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Niiranen, T.J.; Lyass, A.; Larson, M.G.; Hamburg, N.M.; Benjamin, E.J.; Mitchell, G.F.; Vasan, R.S. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension 2017, 70, 267–274. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: An analysis of 123 nationally representative surveys. Lancet 2019, 394, 639–651. [Google Scholar] [CrossRef]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2020, 43, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Hardy, S.T.; Fine, L.J.; Jaeger, B.C.; Wozniak, G.; Levitan, E.B.; Colantonio, L.D. Trends in Blood Pressure Control Among US Adults with Hypertension, 1999–2000 to 2017–2018. JAMA 2020, 324, 1190–1200. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Girão, H.; Salgueiro, L. Natural Products in Cardiovascular Diseases: The Potential of Plants from the Allioideae Subfamily (Ex-Alliaceae Family) and Their Sulphur-Containing Compounds. Plants 2022, 11, 1920. [Google Scholar] [CrossRef]
- Rodríguez, L.; Mendez, D.; Montecino, H.; Carrasco, B.; Arevalo, B.; Palomo, I.; Fuentes, E. Role of Phaseolus vulgaris L. in the Prevention of Cardiovascular Diseases-Cardioprotective Potential of Bioactive Compounds. Plants 2022, 11, 186. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.d.; Silva, R.d.C.V.d.; Mariano, L.N.B.; Dick, S.L.; Ventura, G.C.; Cechinel-Filho, V. Diuretic and Natriuretic Effects of Hesperidin, a Flavanone Glycoside, in Female and Male Hypertensive Rats. Plants 2023, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Kamyab, R.; Namdar, H.; Torbati, M.; Ghojazadeh, M.; Araj-Khodaei, M.; Fazljou, S.M.B. Medicinal Plants in the Treatment of Hypertension: A Review. Adv. Pharm. Bull. 2021, 11, 601–617. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Traditional herbs: A remedy for cardiovascular disorders. Phytomedicine 2016, 23, 1082–1089. [Google Scholar] [CrossRef]
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Ihm, S.-H.; Park, S.-H.; Lee, J.-O.; Kim, O.-R.; Park, E.-H.; Kim, K.-R.; Kim, J.-H.; Hwang, B.-H.; Youn, H.-J.; Oak, M.-H.; et al. A Standardized Lindera obtusiloba Extract Improves Endothelial Dysfunction and Attenuates Plaque Development in Hyperlipidemic ApoE-Knockout Mice. Plants 2021, 10, 2493. [Google Scholar] [CrossRef]
- Shin, S.; Park, J.; Choi, H.-Y.; Lee, K. Hypotensive and Endothelium-Dependent Vasorelaxant Effects of Grayblue Spicebush Ethanol Extract in Rats. Foods 2023, 12, 4282. [Google Scholar] [CrossRef]
- Lee, J.-O.; Oak, M.-H.; Jung, S.H.; Park, D.H.; Auger, C.; Kim, K.R.; Lee, S.-W.; Schini-Kerth, V.B. An ethanolic extract of Lindera obtusiloba stems causes NO-mediated endothelium-dependent relaxations in rat aortic rings and prevents angiotensin II-induced hypertension and endothelial dysfunction in rats. N-S Arch. Pharmacol. 2011, 383, 635–645. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Ahn, G.; Ham, Y.-M.; Song, S.-M.; Ko, E.-Y.; Cho, S.-H.; Yoon, W.-J.; Kim, K.-N. Anti-Inflammatory Effect and Mechanism of Action of Lindera erythrocarpa Essential Oil in Lipopolysaccharide-Stimulated Raw264.7 Cells. Excli J. 2017, 16, 1103–1113. [Google Scholar]
- Yoon, C.-S.; Lee, H.; Liu, Z.; Lee, H.-K.; Lee, D.-S. Effects of Compounds Isolated from Lindera erythrocarpa on Anti-Inflammatory and Anti-Neuroinflammatory Action in BV2 Microglia and RAW264.7 Macrophage. Int. J. Mol. Sci. 2022, 23, 7122. [Google Scholar] [CrossRef]
- Jung, J.; Shin, S.; Park, J.; Lee, K.; Choi, H.-Y. Hypotensive and Vasorelaxant Effects of Sanguisorbae Radix Ethanol Extract in Spontaneously Hypertensive and Sprague Dawley Rats. Nutrients 2023, 15, 4510. [Google Scholar] [CrossRef]
- Shin, S.; Park, J.; Choi, H.-Y.; Bu, Y.; Lee, K. Vasorelaxant and Hypotensive Effects of Galla chinensis in Rats. Int. J. Mol. Sci. 2024, 25, 7962. [Google Scholar] [CrossRef]
- Durand, M.J.; Gutterman, D.D. Diversity in Mechanisms of Endothelium-Dependent Vasodilation in Health and Disease. Microcirculation 2013, 20, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Sign 2008, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, J.-I.; Ushikubi, F.; Hasebe, N. Prostacyclin in Vascular Diseases—Recent Insights and Future Perspectives. Circ. J. 2010, 74, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Cuíñas, A.; García-Morales, V.; Viña, D.; Gil-Longo, J.; Campos-Toimil, M. Activation of PKA and Epac proteins by cyclic AMP depletes intracellular calcium stores and reduces calcium availability for vasoconstriction. Life Sci. 2016, 155, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Hill-Eubanks, D.C.; Werner, M.E.; Heppner, T.J.; Nelson, M.T. Calcium Signaling in Smooth Muscle. Cold Spring Harb. Perspect. Biol. 2011, 3, a004549. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Poburko, D.; Sahota, P.; Sandhu, J.; Ruehlmann, D.O.; Breemen, C.V. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. 2001, 534, 641–650. [Google Scholar] [CrossRef]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintrón, M.; Navedo, M.F. Calcium Channels in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar]
- Shah, K.; Seeley, S.; Schulz, C.; Fisher, J.; Rao, S.G. Calcium Channels in the Heart: Disease States and Drugs. Cells 2022, 11, 943. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv. Pharmacol. 2017, 78, 89–144. [Google Scholar]
- Werner, M.E.; Ledoux, J. K+ channels in biological processes: Vascular K+ channels in the regulation of blood pressure. J. Receptor. Ligand Channel Res. 2014, 7, 51–60. [Google Scholar] [CrossRef]
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fund. Clin. Pharmacol. 2019, 33, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Cat, A.N.D.; Touyz, R.M. Cell Signaling of Angiotensin II on Vascular Tone: Novel Mechanisms. Curr. Hypertens. Rep. 2011, 13, 122–128. [Google Scholar]
- Doris, P.A. Genetics of hypertension: An assessment of progress in the spontaneously hypertensive rat. Physiol. Genom. 2017, 49, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yan, H.-L.; Wang, L.-X.; Xu, J.-F.; Peng, C.; Ao, H.; Tan, Y.-Z. Review of Natural Resources with Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 2021, 12, 627458. [Google Scholar] [CrossRef]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladi, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
- Rizzoni, D.; Agabiti-Rosei, E. Structural abnormalities of small resistance arteries in essential hypertension. Intern. Emerg. Med. 2012, 7, 205–212. [Google Scholar] [CrossRef]
- Odukoya, J.O.; Odukoya, J.O.; Mmutlane, E.M.; Ndinteh, D.T. Ethnopharmacological Study of Medicinal Plants Used for the Treatment of Cardiovascular Diseases and Their Associated Risk Factors in sub-Saharan Africa. Plants 2022, 11, 1387. [Google Scholar] [CrossRef]
- Uy, N.P.; Kim, J.H.; Kim, D.Y.; Ku, J.; Lee, S. Analysis of Marker Compounds in Lindera erythrocarpa from Diverse Geographical Regions of Korea. Separations 2024, 11, 252. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Park, J.; Choi, H.-Y.; Bu, Y.; Lee, K. Antihypertensive Effects of Lindera erythrocarpa Makino via NO/cGMP Pathway and Ca2+ and K+ Channels. Nutrients 2024, 16, 3003. https://doi.org/10.3390/nu16173003
Shin S, Park J, Choi H-Y, Bu Y, Lee K. Antihypertensive Effects of Lindera erythrocarpa Makino via NO/cGMP Pathway and Ca2+ and K+ Channels. Nutrients. 2024; 16(17):3003. https://doi.org/10.3390/nu16173003
Chicago/Turabian StyleShin, Sujin, Junkyu Park, Ho-Young Choi, Youngmin Bu, and Kyungjin Lee. 2024. "Antihypertensive Effects of Lindera erythrocarpa Makino via NO/cGMP Pathway and Ca2+ and K+ Channels" Nutrients 16, no. 17: 3003. https://doi.org/10.3390/nu16173003




