Potassium Intake and Bone Health: A Narrative Review
Abstract
:1. Introduction
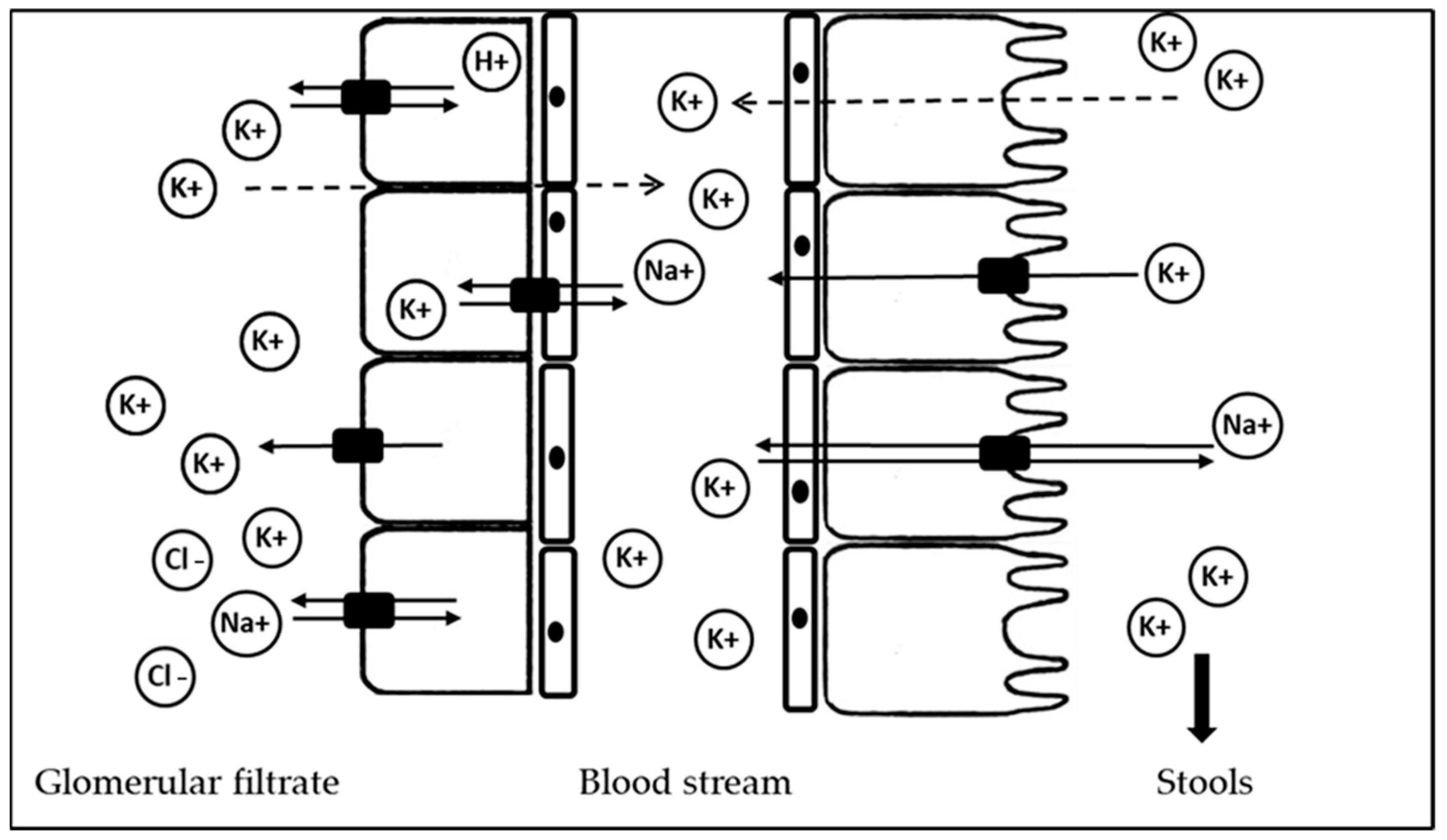
2. Potassium Sources
3. Potassium and Bone
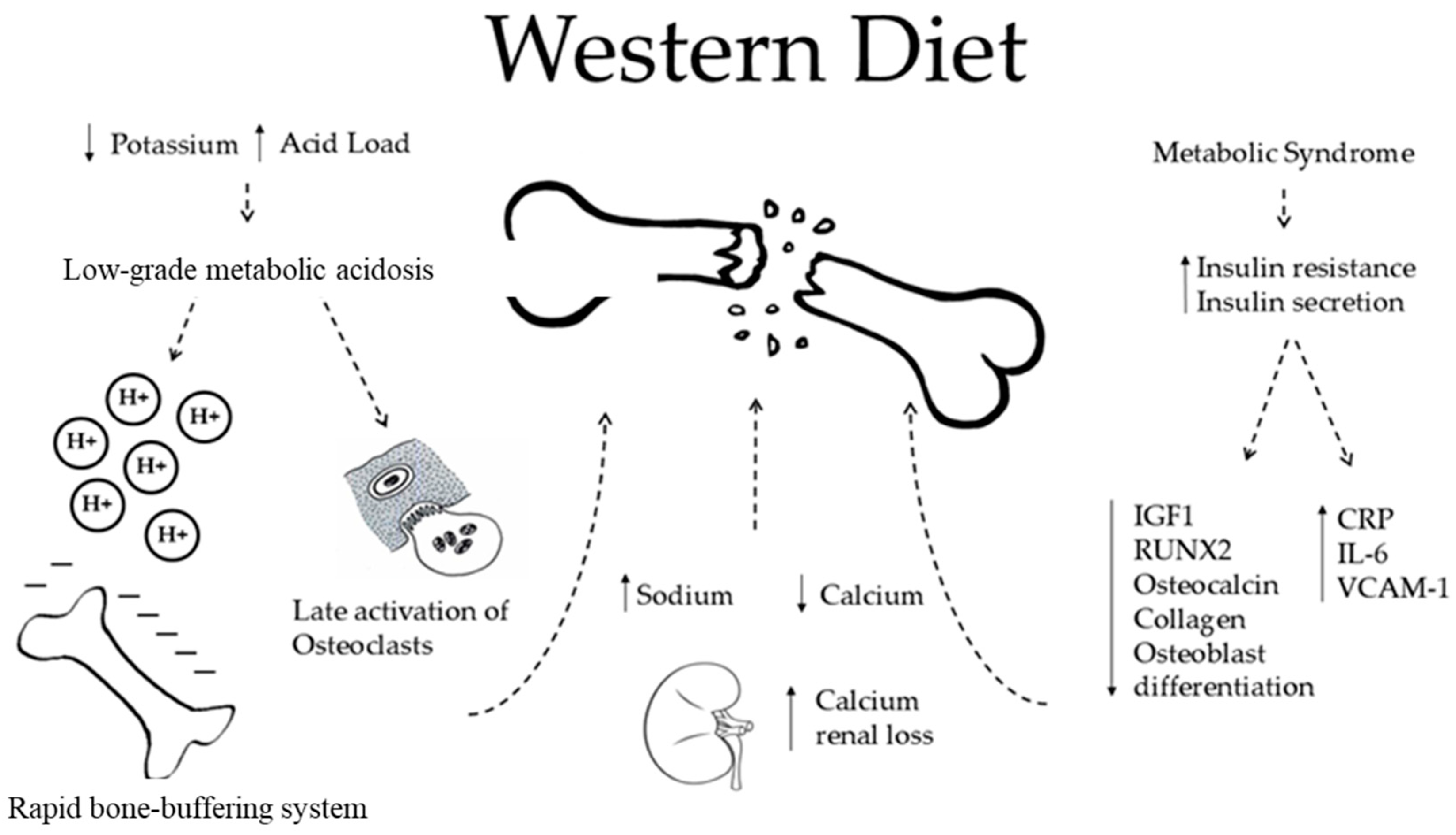
4. Western Diet and Bone
- The WD is characterized by a high acid load and low K intake. The acid excess acts on bone health in two different ways. The first occurs rapidly through an ion exchange, in which bone cells are not involved (see below).
- Also, WD is characterized by high sodium intake, which promotes sodium and calcium kidney loss by a passive mechanism. This is responsible for osteoclastic activation and in the end, higher bone loss.
- The WD is also associated with an increased risk of metabolic syndrome (MetS) and diabetes, which are both characterized by increased insulin resistance and increased insulin secretion. MetS is a risk factor for fractures and low BMD in women examined by DXA for suspected osteoporosis (Op) [41]. Regarding diabetes, there is much evidence about a higher risk of fractures in patients affected by diabetes mellitus type 2, even though their BMD measured by DXA is apparently higher than that of normal controls [41]. Speculations can be made to explain insulin excess. Insulin has been proven to stimulate DNA synthesis, to promote the secretion of osteocalcin, collagen, and insulin grown factor 1 (IGF 1), and to promote the expression of RUNX2 that is involved in the osteoblastic differentiation [42]. All of these processes may decrease with the WD, affecting the BMD. However, the same mechanisms fade in non-diabetic subjects, leaving a gap in the knowledge and forcing one to find other pathways by which the WD may play a role on bone health [43,44,45]. In this regard, the WD is directly associated with increased secretion of markers of inflammation, such as C-reactive protein, Interleukin-6, E-selection, soluble intercellular adhesion molecule 1, and soluble vascular cell adhesion molecule 1, which promotes post-menopausal bone loss [46]. Furthermore, patients with type 2 diabetes mellitus revealed a direct relation between serum insulin grown factor 1 (IGF 1) levels and BMD. Since diabetes is related to lower IGF 1, the study concluded that there was a higher risk of fragility fractures [47].
5. Low-Grade Metabolic Acidosis
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Toft, U.; Riis, N.L.; Jula, A. Potassium—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Extrarenal Effects of Aldosterone on Potassium Homeostasis. Kidney360 2022, 3, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar] [PubMed]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of Hypokalemia and Hyperkalemia. Circ. Arrhythm. Electrophysiol. 2017, 10, e004667. [Google Scholar] [CrossRef]
- Wang, W.H.; Giebisch, G. Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch. 2009, 458, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Linas, S.L.; Wingo, C.S. Disorders of potassium metabolism. In Comprehensive Clinical Nephrology, 4th ed.; Johnson, R., Fluege, J., Feehally, J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2010; pp. 118–129. [Google Scholar]
- Guyton, A.C.; Hall, J.E. Renal regulation of potassium, calcium, phosphate, and magnesium; integration of renal mechanisms for control of blood volume and extracellular fluid volume. In Textbook of Medical, Physiology; Schmitt, W., Gruliow, R., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 365–382. [Google Scholar]
- Bailey, J.L.; Sands, J.M.; Franch, H.A. Water, electrolytes, and acid-based metabolism. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 102–132. [Google Scholar]
- Holbrook, J.T.; Patterson, K.Y.; Bodner, J.E.; Douglas, L.W.; Veillon, C.; Kelsay, J.L.; Mertz, W.; Smith, J.C., Jr. Sodium and potassium intake and balance in adults consuming self-selected diets. Am. J. Clin. Nutr. 1984, 40, 786–793. [Google Scholar] [CrossRef]
- Tasevska, N.; Runswick, S.A.; Bingham, S.A. Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J. Nutr. 2006, 136, 1334–1340. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Yamasaki, M.; Hayabuchi, H.; Goda, T.; Oka, J.; Baba, K.; Ohki, K.; et al. Misreporting of dietary energy, protein, potassium and sodium in relation to body mass index in young Japanese women. Eur. J. Clin. Nutr. 2007, 62, 111–118. [Google Scholar] [CrossRef]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef]
- Reddin, C.; Ferguson, J.; Murphy, R.; Clarke, A.; Judge, C.; Griffith, V.; Alvarez, A.; Smyth, A.; Mente, A.; Yusuf, S.; et al. Global mean potassium intake: A systematic review and Bayesian meta-analysis. Eur. J. Nutr. 2023, 62, 2027–2037. [Google Scholar] [CrossRef]
- D’Elia, L.; Barba, G.; Cappuccio, F.P.; Strazzullo, P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J. Am. Coll. Cardiol. 2011, 57, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [PubMed]
- Available online: https://www.dietaryguidelines.gov/sites/default/files/2024-08/Food-Sources-Potassium-Standard-508C.pdf (accessed on 15 August 2024).
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2000, 9, 121. [Google Scholar] [CrossRef]
- Lanham-New, S.A.; Lambert, H.; Frassetto, L. Potassium. Adv. Nutr. 2012, 3, 820–821. [Google Scholar] [CrossRef]
- Packer, C.D. Chronic hypokalemia due to excessive cola consumption: A case report. Cases J. 2008, 1, 32. [Google Scholar] [CrossRef]
- Styburski, D.; Janda, K.; Baranowska-Bosiacka, I.; Łukomska, A.; Dec, K.; Goschorska, M.; Michalkiewicz, B.; Ziętek, P.; Gutowska, I. Beer as a potential source of macroelements in a diet: The analysis of calcium, chlorine, potassium, and phosphorus content in a popular low-alcoholic drink. Eur. Food Res. Technol. 2018, 244, 1853–1860. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Camajani, E.; Caprio, M.; Armani, A. Health Effects of Red Wine Consumption: A Narrative Review of an Issue That Still Deserves Debate. Nutrients 2023, 15, 1921. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; et al. Scientific opinion on dietary reference values for potassium. EFSA J. 2016, 14, 4592. [Google Scholar]
- Abate, V.; Vergatti, A.; Fiore, A.; Forte, A.; Attanasio, A.; Altavilla, N.; De Filippo, G.; Rendina, D.; D′Elia, L. Low Potassium Intake: A Common Risk Factor for Nephrolithiasis in Patients with High Blood Pressure. High Blood Press. Cardiovasc. Prev. 2023, 30, 343–350. [Google Scholar] [CrossRef]
- Malavolti, M.; Naska, A.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Marchesi, C.; Vinceti, M.; Filippini, T. Sodium and Potassium Content of Foods Consumed in an Italian Population and the Impact of Adherence to a Mediterranean Diet on Their Intake. Nutrients 2021, 13, 2681. [Google Scholar] [CrossRef]
- Bardhi, O.; Clegg, D.J.; Palmer, B.F. The Role of Dietary Potassium in the Cardiovascular Protective Effects of Plant-Based Diets. Semin. Nephrol. 2023, 43, 151406. [Google Scholar] [CrossRef]
- Keys, A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995, 61, 1321S–1323S. [Google Scholar] [CrossRef]
- Harnden, K.E.; Frayn, K.N.; Hodson, L. Dietary Approaches to Stop Hypertension (DASH) diet: Applicability and acceptability to a UK population. J. Hum. Nutr. Diet. 2010, 23, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.A.; Munn, E.A.; Baines, S.K. Protein and vegetarian diets. Med. J. Aust. 2013, 199, S7–S10. [Google Scholar] [CrossRef]
- Khor, B.H.; Tallman, D.A.; Karupaiah, T.; Khosla, P.; Chan, M.; Kopple, J.D. Nutritional Adequacy of Animal-Based and Plant-Based Asian Diets for Chronic Kidney Disease Patients: A Modeling Study. Nutrients 2021, 13, 3341. [Google Scholar] [CrossRef]
- D’Elia, L. Potassium Intake and Human Health. Nutrients 2024, 16, 833. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Iannotta, C.; Sabino, P.; Ippolito, R. Potassium-rich diet and risk of stroke: Updated meta-analysis. Nutr. Met. Cardiovasc. Dis. 2014, 24, 585–587. [Google Scholar] [CrossRef]
- D’Elia, L.; Cappuccio, F.P.; Masulli, M.; La Fata, E.; Rendina, D.; Galletti, F. Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2023, 15, 853. [Google Scholar] [CrossRef]
- D’Elia, L.; Masulli, M.; Cappuccio, F.P.; Zarrella, A.F.; Strazzullo, P.; Galletti, F. Dietary Potassium Intake and Risk of Diabetes: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2022, 14, 4785. [Google Scholar] [CrossRef]
- Lemann, J., Jr.; Worcester, E.M.; Gray, R.W. Hypercalciuria and stones. Am. J. Kidney Dis. 1991, 17, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J., Jr.; Pleuss, J.A.; Gray, R.W.; Hoffmann, R.G. Potassium administration reduces and potassium deprivation increases urinary calcium excretion in healthy adults [corrected]. Kidney Intern. 1991, 39, 973–983. [Google Scholar] [CrossRef]
- Jehle, S.; Zanetti, A.; Muser, J.; Hulter, H.N.; Krapf, R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J. Am. Soc. Nephrol. 2006, 17, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Black, A.J.; Aucott, L.; Duthie, G.; Duthie, S.; Sandison, R.; Hardcastle, A.C.; Lanham New, S.A.; Fraser, W.D.; Reid, D.M. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: A randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Hulter, H.N.; Krapf, R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 207–217. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Rendina, D.; D’Elia, L.; Evangelista, M.; De Filippo, G.; Giaquinto, A.; Abate, V.; Barone, B.; Piccinocchi, G.; Prezioso, D.; Strazzullo, P. Metabolic syndrome is associated to an increased risk of low bone mineral density in free-living women with suspected osteoporosis. J. Endocrinol. Investig. 2021, 44, 1321–1326. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, C.Y.; Man, X.F.; Tang, H.N.; Tang, J.; Zhou, C.L.; Tan, S.W.; Wang, M.; Feng, Y.Z.; Zhou, H.D. Insulin-like growth factor-1 promotes osteogenic differentiation and collagen I alpha 2 synthesis via induction of mRNA-binding protein LARP6 expression. Dev. Growth Differ. 2017, 59, 94–103. [Google Scholar] [CrossRef]
- Napoli, N.; Conte, C.; Pedone, C.; Strotmeyer, E.S.; Barbour, K.E.; Black, D.M.; Samelson, E.J.; Schwartz, A.V. Effect of Insulin Resistance on BMD and Fracture Risk in Older Adults. J. Clin. Endocrinol. Metab. 2019, 104, 3303–3310. [Google Scholar] [CrossRef]
- Pu, B.; Gu, P.; Yue, D.; Xin, Q.; Lu, W.; Tao, J.; Ke, D.; Chen, H.; Ma, Y.; Luo, W. The METS-IR is independently related to bone mineral density, FRAX score, and bone fracture among U.S. non-diabetic adults: A cross-sectional study based on NHANES. BMC Musculoskelet. Disord. 2023, 24, 730. [Google Scholar] [CrossRef]
- Sheu, A.; Blank, R.D.; Tran, T.; Bliuc, D.; Greenfield, J.R.; White, C.P.; Center, J.R. Associations of Type 2 Diabetes, Body Composition, and Insulin Resistance with Bone Parameters: The Dubbo Osteoporosis Epidemiology Study. JBMR Plus 2023, 7, e10780. [Google Scholar] [CrossRef] [PubMed]
- Damani, J.J.; De Souza, M.J.; Strock, N.C.A.; Koltun, K.J.; Williams, N.I.; Weaver, C.; Rogers, C.J. Associations Between Inflammatory Mediators and Bone Outcomes in Postmenopausal Women: A Cross-Sectional Analysis of Baseline Data from the Prune Study. J. Inflamm. Res. 2023, 16, 639–663. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Fu, Z.; Wang, X.; Zhou, P.; Yang, Q.; Jiang, Y.; Zhu, D. A narrative review of diabetic bone disease: Characteristics, pathogenesis, and treatment. Front. Endocrinol. 2022, 13, 1052592. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.E.; Kim, S.; Robert Bruce, W. A western-style diet reduces bone mass and biomechanical bone strength to a greater extent in male compared with female rats during development. Br. J. Nutr. 2003, 90, 589–595. [Google Scholar] [CrossRef]
- Papachristou, N.I.; Blair, H.C.; Kalyvioti, E.S.; Syggelos, S.A.; Karavia, E.A.; Kontogeorgakos, V.; Nikitovic, D.; Tzanakakis, G.N.; Kypreos, K.E.; Papachristou, D.J. Western-type diet differentially modulates osteoblast, osteoclast, and lipoblast differentiation and activation in a background of APOE deficiency. Lab. Investig. 2018, 98, 1516–1526. [Google Scholar] [CrossRef]
- Dawson-Hughes, B. Acid-base balance of the diet-implications for bone and muscle. Eur. J. Clin. Nutr. 2020, 74, 7–13. [Google Scholar] [CrossRef]
- Park, H.J.; Baek, K.; Baek, J.H.; Kim, H.R. TNFα Increases RANKL Expression via PGE₂-Induced Activation of NFATc1. Int. J. Mol. Sci. 2017, 18, 495. [Google Scholar] [CrossRef]
- Weaver, C.M. Potassium and health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef]
- Arnett, T.R.; Spowage, M. Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone 1996, 18, 277–279. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Vu, B.Q.; Lai, T.Q.; Nguyen, N.D.; Nguyen, T.V. Vegetarianism, bone loss, fracture and vitamin D: A longitudinal study in Asian vegans and non-vegans. Eur. J. Clin. Nutr. 2012, 66, 75–82. [Google Scholar] [CrossRef]
- Gunn, C.A.; Weber, J.L.; McGill, A.T.; Kruger, M.C. Increased intake of selected vegetables, herbs and fruit may reduce bone turnover in post-menopausal women. Nutrients 2015, 7, 2499–2517. [Google Scholar] [CrossRef]
- Maurer, M.; Riesen, W.; Muser, J.; Hulter, H.N.; Krapf, R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am. J. Physiol. 2003, 284, F32–F40. [Google Scholar] [CrossRef] [PubMed]
- Gado, M.; Baschant, U.; Hofbauer, L.C.; Henneicke, H. Bad to the Bone: The Effects of Therapeutic Glucocorticoids on Osteoblasts and Osteocytes. Front. Endocrinol. 2022, 13, 835720. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Jia, D.; Plotkin, L.I.; Bellido, T.; Powers, C.C.; Stewart, S.A.; Manolagas, S.C.; Weinstein, R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 2004, 145, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- Song, Y.; Hernandez, N.; Shoag, J.; Goldfarb, D.S.; Eisner, B.H. Potassium citrate decreases urine calcium excretion in patients with hypocitraturic calcium oxalate nephrolithiasis. Urolithiasis 2016, 44, 145–148. [Google Scholar] [CrossRef]
- Lambert, H.; Frassetto, L.; Moore, J.B.; Torgerson, D.; Gannon, R.; Burckhardt, P.; Lanham-New, S. The effect of supplementation with alkaline potassium salts on bone metabolism: A meta-analysis. Osteoporos. Int. 2015, 26, 1311–1318. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Buchanan, L.A.; Ji, C.; Siani, A.; Miller, M.A. Systematic review and meta-analysis of randomised controlled trials on the effects of potassium supplements on serum potassium and creatinine. BMJ 2016, 6, e011716. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]

| Foods | K Content (mg/100 g) |
|---|---|
| Grains and cereal products | |
| Wheat bran | 1160 |
| Buckwheat | 450 |
| Oatmeal | 370 |
| Corn | 287 |
| Brown rice | 214 |
| Pearl barley | 120 |
| Legumes (dried * vs. cooked) | |
| Soy | 1740 vs. 590 |
| Bean | 1445 vs. 1273 |
| Peas | 990 vs. 444 |
| Lentils | 980 vs. 185 |
| Chickpea | 881 vs. 581 |
| Fava beans | 236 vs. 228 |
| Vegetables | |
| Parsley | 670 |
| Potatoes | 600 |
| Garlic | 600 |
| Spinach | 530 |
| Arugula | 468 |
| Brussels sprouts | 450 |
| Fruits | |
| Peaches | 950 |
| Grape | 864 |
| Apples | 730 |
| Avocado | 450 |
| Kiwi | 400 |
| Currant | 370 |
| Banana | 350 |
| Nut | |
| Pistachios | 972 |
| Peanuts | 680 |
| Pecan nuts | 603 |
| Cashew nuts | 565 |
| Hazelnut | 466 |
| Macadamia | 363 |
| Milk and dairy products | |
| Cow milk | 150 |
| Goat yogurt | 251 |
| Dairy yogurt | 185 |
| Sheep milk | 182 |
| Goat milk | 180 |
| Cream cheese | 150 |
| Drinks and Beverages 1 | |
| Coffee [17] | 89 to 154 |
| Orange juice [18] | 200 |
| Soft high-sugar drinks [19] | 4.3 |
| Beer [20] | 50 |
| Wine [21] | 99 |
| Fish and derivates | |
| Stockfish (dried) * | 1500 |
| Anchovies in oil | 700 |
| Trout | 530 |
| Mackerel fish | 360 |
| Cod | 330 |
| Salmon | 310 |
| Meat and derivates | |
| Bresaola | 505 |
| Chicken | 497 |
| Turkey | 475 |
| Ham | 454 |
| Pork | 370 |
| Veal | 360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, V.; Vergatti, A.; Altavilla, N.; Garofano, F.; Salcuni, A.S.; Rendina, D.; De Filippo, G.; Vescini, F.; D’Elia, L. Potassium Intake and Bone Health: A Narrative Review. Nutrients 2024, 16, 3016. https://doi.org/10.3390/nu16173016
Abate V, Vergatti A, Altavilla N, Garofano F, Salcuni AS, Rendina D, De Filippo G, Vescini F, D’Elia L. Potassium Intake and Bone Health: A Narrative Review. Nutrients. 2024; 16(17):3016. https://doi.org/10.3390/nu16173016
Chicago/Turabian StyleAbate, Veronica, Anita Vergatti, Nadia Altavilla, Francesca Garofano, Antonio Stefano Salcuni, Domenico Rendina, Gianpaolo De Filippo, Fabio Vescini, and Lanfranco D’Elia. 2024. "Potassium Intake and Bone Health: A Narrative Review" Nutrients 16, no. 17: 3016. https://doi.org/10.3390/nu16173016





