The Protective Effects of an Aged Black Garlic Water Extract on the Prostate
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Sample Preparation of ABGE
2.2. Ex Vivo Studies
2.3. Cell Culture
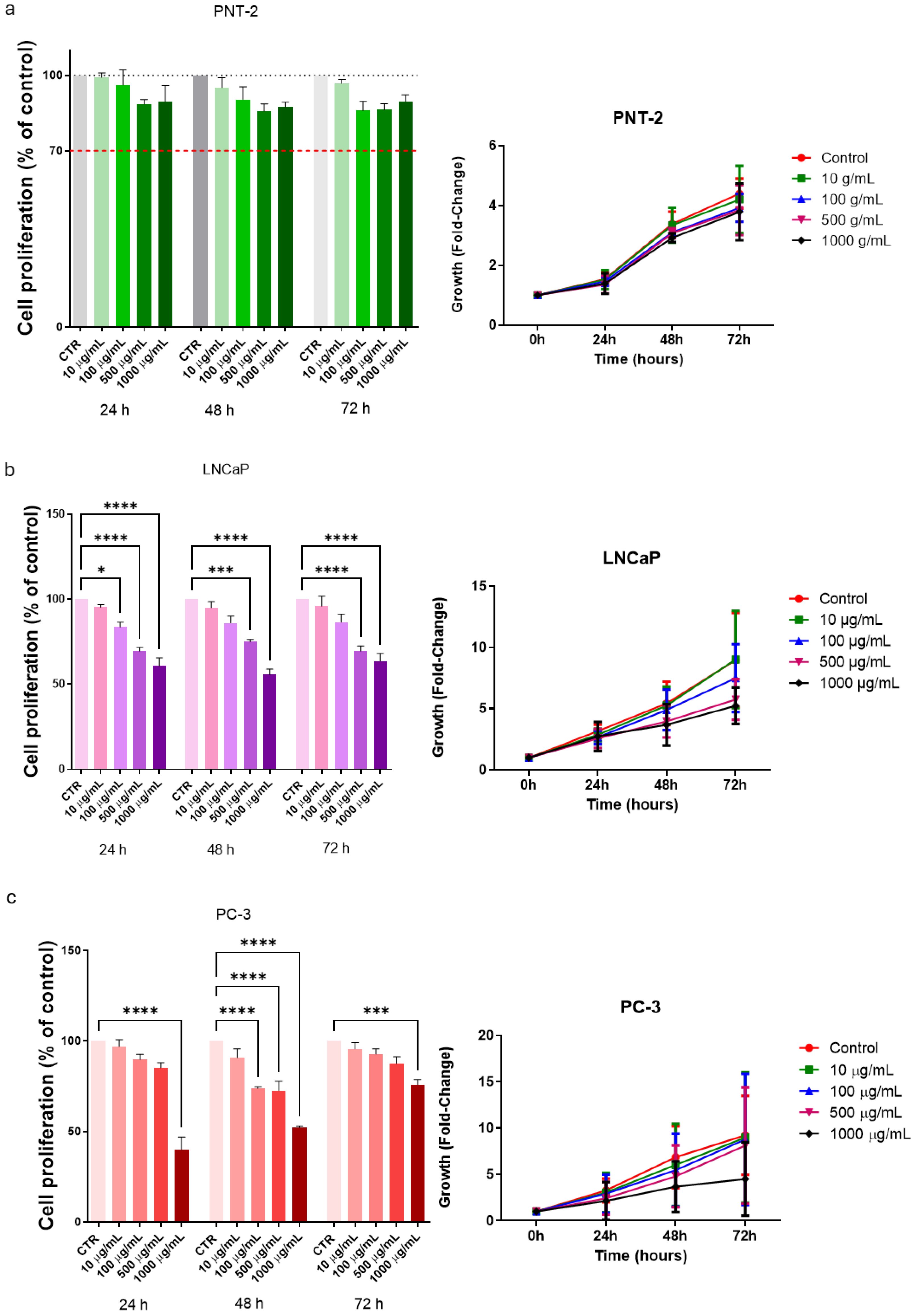
2.4. Cell Proliferation
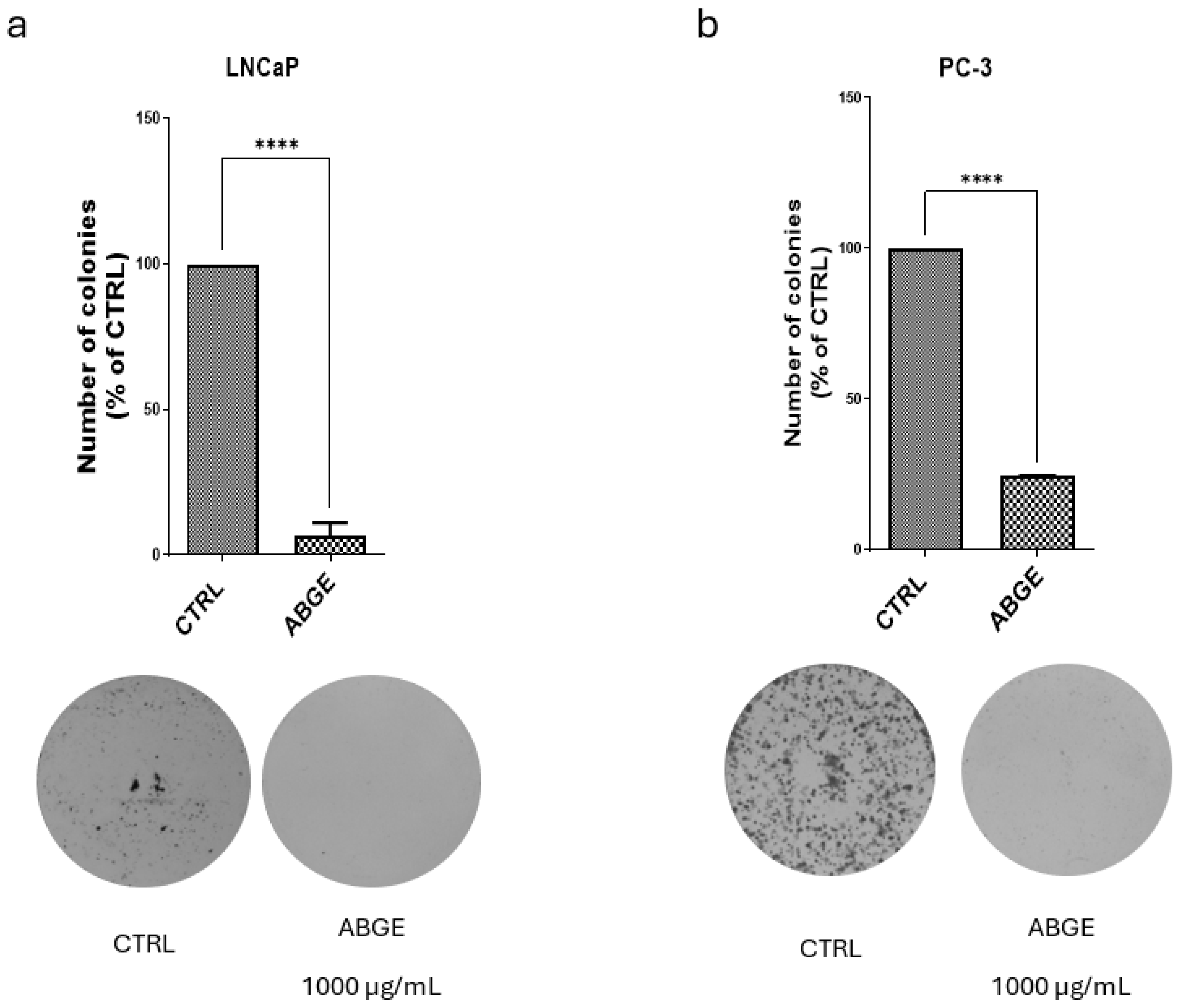
2.5. Clonogenic Assay
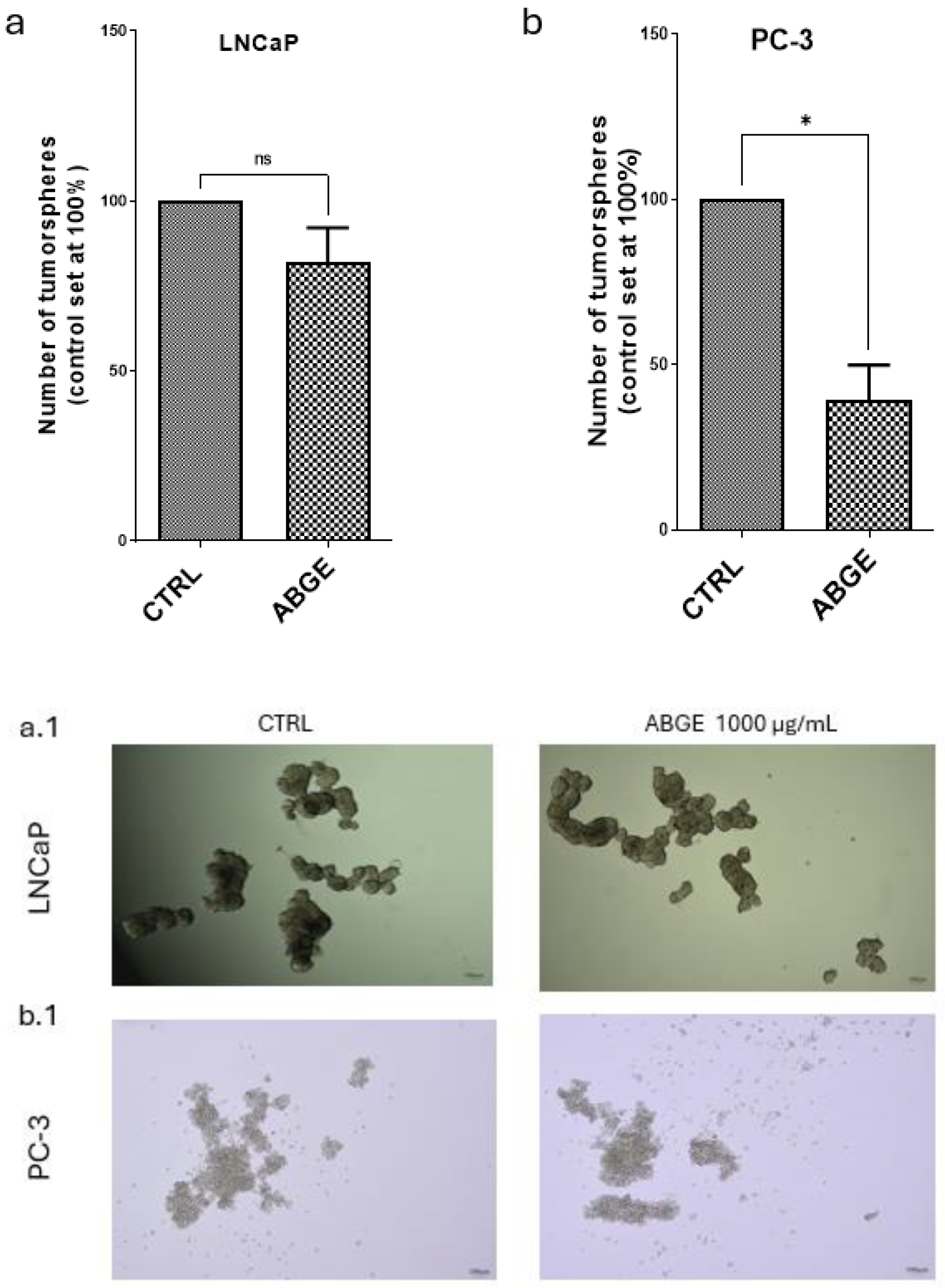
2.6. Tumorsphere Formation
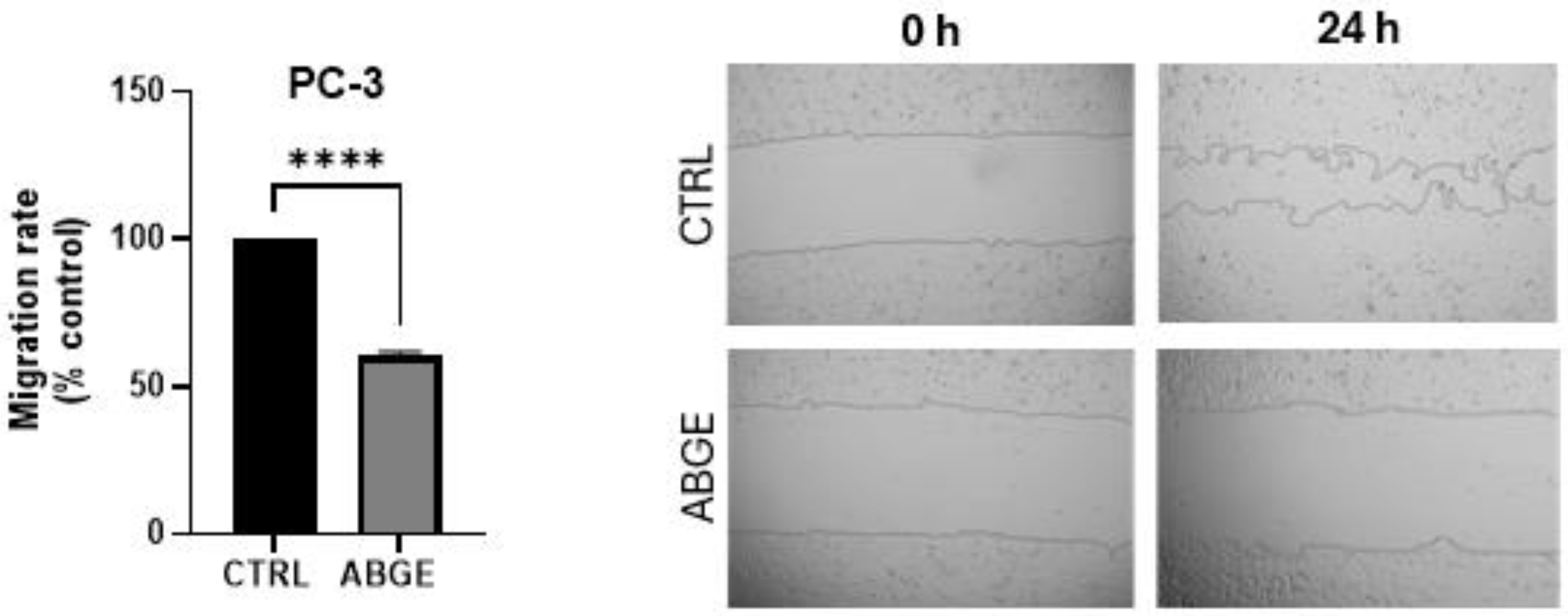
2.7. Cell Migration Assay
2.8. Phosphorylation Array
2.9. Statistical Analysis
3. Results and Discussion
3.1. Ex Vivo Studies
3.2. Cell Proliferation in Basal Conditions
3.3. Colony Formation
3.4. Tumor Spheroid Formation
3.5. Migration Assay
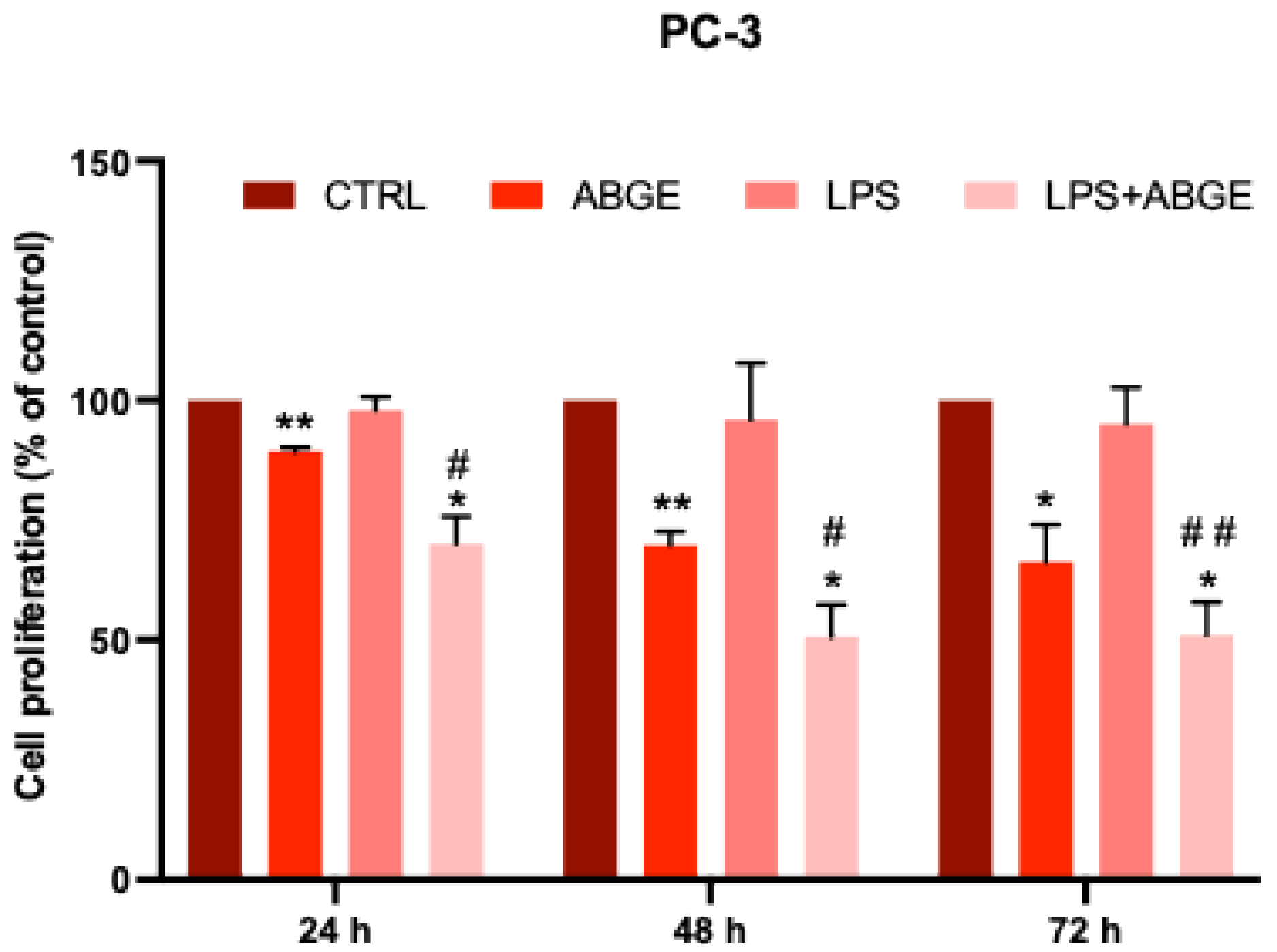
3.6. Cell Proliferation after LPS Pre-Treatment
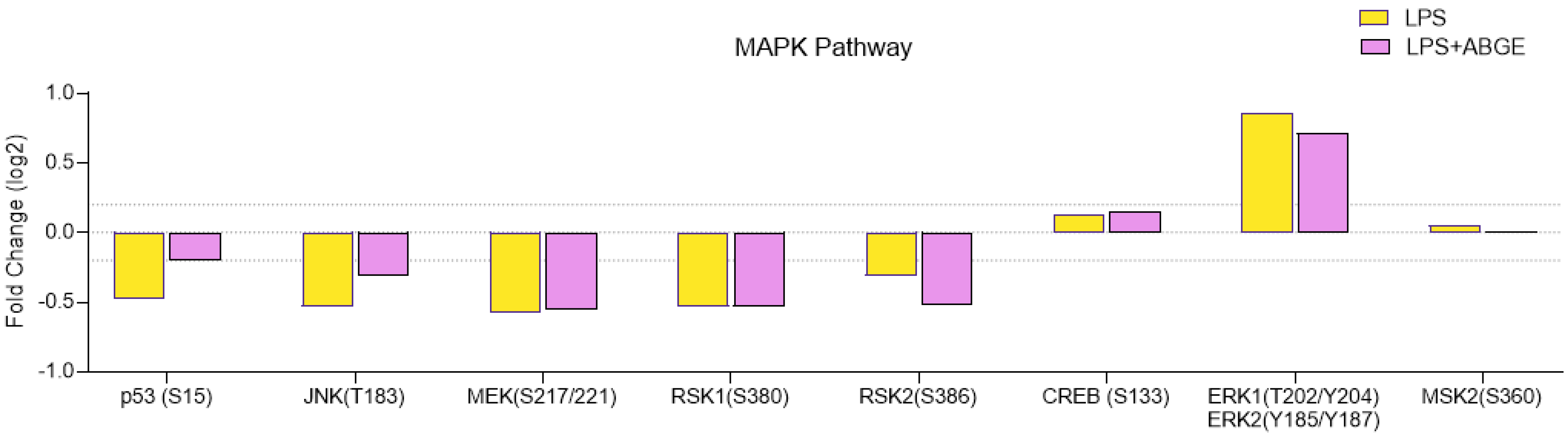
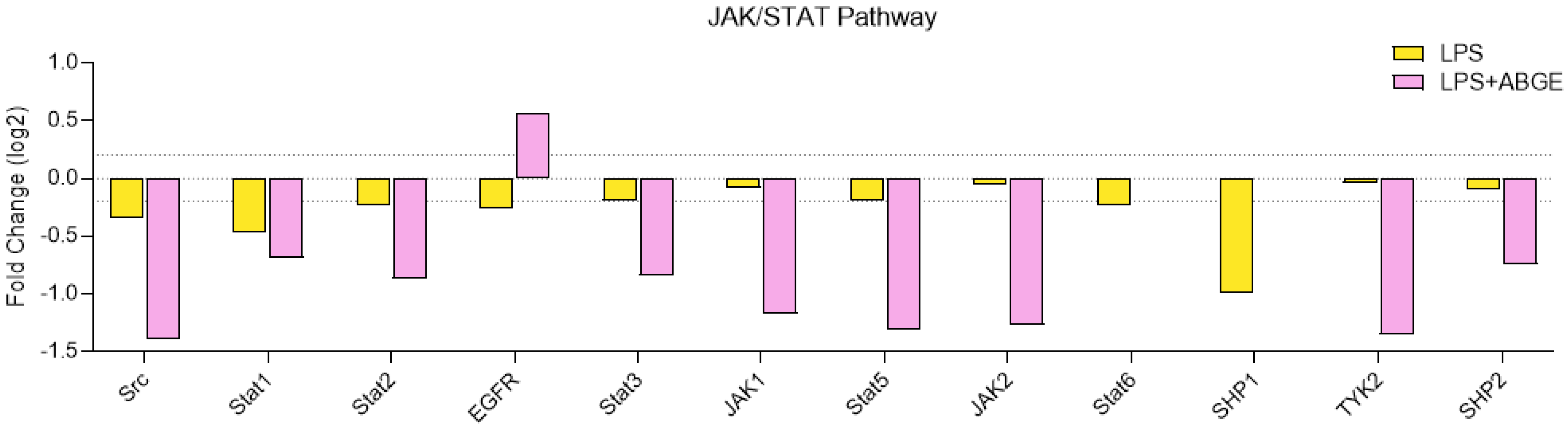
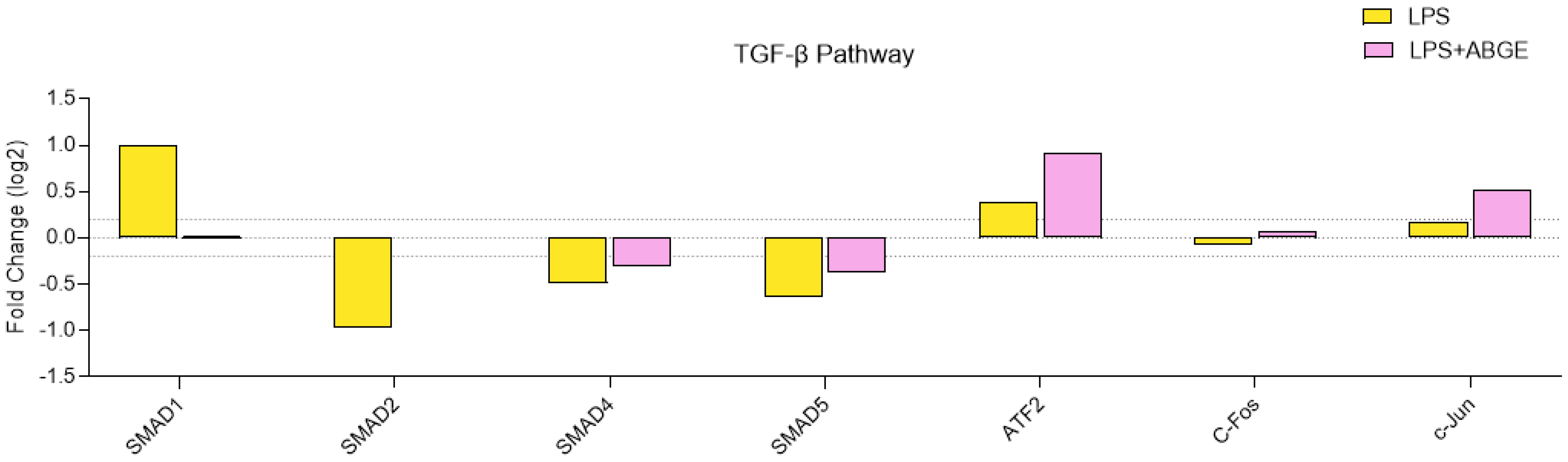
3.7. Phosphorylation Array
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate Carcinogenesis: Inflammatory Storms. Nat. Rev. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, J.M.; Montero-Hidalgo, A.J.; Fuentes-Fayos, A.C.; Sarmento-Cabral, A.; Guzmán-Ruiz, R.; Malagón, M.M.; Herrera-Martínez, A.D.; Gahete, M.D.; Luque, R.M. Exploring the Role of the Inflammasomes on Prostate Cancer: Interplay with Obesity. Rev. Endocr. Metab. Disord. 2023, 24, 1165–1187. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, J.; Hui, J.; Xu, J. Inflammation-Related Indicators Have a Potential to Increase Overall Quality of the Prostate Cancer Management: A Narrative Review. Transl. Androl. Urol. 2023, 12, 809–822. [Google Scholar] [CrossRef]
- Oseni, S.O.; Naar, C.; Pavlović, M.; Asghar, W.; Hartmann, J.X.; Fields, G.B.; Esiobu, N.; Kumi-Diaka, J. The Molecular Basis and Clinical Consequences of Chronic Inflammation in Prostatic Diseases: Prostatitis, Benign Prostatic Hyperplasia, and Prostate Cancer. Cancers 2023, 15, 3110. [Google Scholar] [CrossRef]
- Sessa, F.; Nicoletti, R.; De Nunzio, C.; Porreca, A.; Magrini, S.M.; Mirone, V.; Tubaro, A.; Serni, S.; Gontero, P.; Noale, M.; et al. Inflammation and Prostate Cancer: Pathological Analysis from Pros-IT CNR 2. Cancers 2023, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H.; Qin, Z.; Zhao, F.; Zhou, L.; Xu, L.; Jia, R. NLRP3 Inflammasome Promoted the Malignant Progression of Prostate Cancer via the Activation of Caspase-1. Cell Death Discov. 2021, 7, 399. [Google Scholar] [CrossRef]
- Hou, J.; Karin, M.; Sun, B. Targeting Cancer-Promoting Inflammation—Have Anti-Inflammatory Therapies Come of Age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef]
- Koliaraki, V.; Prados, A.; Armaka, M.; Kollias, G. The Mesenchymal Context in Inflammation, Immunity and Cancer. Nat. Immunol. 2020, 21, 974–982. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Signal Transduction by Tumor Necrosis Factor and Its Relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Sharma, J.; Gray, K.P.; Harshman, L.C.; Evan, C.; Nakabayashi, M.; Fichorova, R.; Rider, J.; Mucci, L.; Kantoff, P.W.; Sweeney, C.J. Elevated IL-8, TNF-α, and MCP-1 in Men with Metastatic Prostate Cancer Starting Androgen-Deprivation Therapy (ADT) Are Associated with Shorter Time to Castration-Resistance and Overall Survival. Prostate 2014, 74, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Thomas, R. Immune Deficiency or Hyperactivity-Nf-Κb Illuminates Autoimmunity. J. Autoimmun. 2008, 31, 245–251. [Google Scholar] [CrossRef]
- Staal, J.; Beyaert, R. Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications. Cells 2018, 7, 122. [Google Scholar] [CrossRef]
- Liu, X.-H.; Kirschenbaum, A.; Lu, M.; Yao, S.; Klausner, A.; Preston, C.; Holland, J.F.; Levine, A.C. Prostaglandin E(2) Stimulates Prostatic Intraepithelial Neoplasia Cell Growth through Activation of the Interleukin-6/GP130/STAT-3 Signaling Pathway. Biochem. Biophys. Res. Commun. 2002, 290, 249–255. [Google Scholar] [CrossRef]
- Zha, S.; Gage, W.R.; Sauvageot, J.; Saria, E.A.; Putzi, M.J.; Ewing, C.M.; Faith, D.A.; Nelson, W.G.; De Marzo, A.M.; Isaacs, W.B. Cyclooxygenase-2 Is up-Regulated in Proliferative Inflammatory Atrophy of the Prostate, but Not in Prostate Carcinoma. Cancer Res. 2001, 61, 8617–8623. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural Products: A Continuing Source of Novel Drug Leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, C.; Chen, J.; Zheng, J. Effects of Anaerobic Fermentation on Black Garlic Extract by Lactobacillus: Changes in Flavor and Functional Components. Front. Nutr. 2021, 8, 645416. [Google Scholar] [CrossRef]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged Black Garlic Extract Inhibits HT29 Colon Cancer Cell Growth via the PI3K/Akt Signaling Pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, F.; Wang, Q.-W.; Wang, J.; Yang, K.; Hu, R.-R.; Liu, H.-C.; Wang, H.-Y.; Wang, Y.-S. Aged Black Garlic Extract Induces Inhibition of Gastric Cancer Cell Growth in Vitro and in Vivo. Mol. Med. Rep. 2012, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Libero, M.L.; Citi, V.; Chiavaroli, A.; Martelli, A.; Foligni, R.; Mannozzi, C.; Acquaviva, A.; Di Simone, S.; Calderone, V.; et al. Anti-Inflammatory and Vasorelaxant Effects Induced by an Aqueous Aged Black Garlic Extract Supplemented with Vitamins D, C, and B12 on Cardiovascular System. Foods 2023, 12, 1558. [Google Scholar] [CrossRef]
- Libero, M.L.; Lucarini, E.; Recinella, L.; Ciampi, C.; Veschi, S.; Piro, A.; Chiavaroli, A.; Acquaviva, A.; Nilofar, N.; Orlando, G.; et al. Anti-Inflammatory and Anti-Hyperalgesic Effects Induced by an Aqueous Aged Black Garlic Extract in Rodent Models of Ulcerative Colitis and Colitis-Associated Visceral Pain. Phytother. Res. 2024, 38, 4177–4188. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Marconi, G.D.; Gesmundo, I.; Granata, R.; Cai, R.; Sha, W.; Schally, A.V.; et al. Antinflammatory, Antioxidant, and Behavioral Effects Induced by Administration of Growth Hormone-Releasing Hormone Analogs in Mice. Sci. Rep. 2020, 10, 732. [Google Scholar] [CrossRef]
- Recinella, L.; Micheli, L.; Chiavaroli, A.; Libero, M.L.; Orlando, G.; Menghini, L.; Acquaviva, A.; Di Simone, S.; Ferrante, C.; Ghelardini, C.; et al. Anti-Inflammatory Effects Induced by a Polyphenolic Granular Complex from Olive (Olea Europaea, Mainly Cultivar Coratina): Results from In Vivo and Ex Vivo Studies in a Model of Inflammation and MIA-Induced Osteoarthritis. Nutrients 2022, 14, 1487. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of Allicin Determined by Chemical and Biological Assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and Chemical Stability of Garlic-Derived Allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective Effects Induced by Two Polyphenolic Liquid Complexes from Olive (Olea Europaea, Mainly Cultivar Coratina) Pressing Juice in Rat Isolated Tissues Challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef]
- Recinella, L.; Libero, M.L.; Veschi, S.; Piro, A.; Marconi, G.D.; Diomede, F.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Florio, R.; et al. Effects of GHRH Deficiency and GHRH Antagonism on Emotional Disorders in Mice. Cells 2023, 12, 2615. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hormaechea-Agulla, D.; Gahete, M.D.; Jiménez-Vacas, J.M.; Gómez-Gómez, E.; Ibáñez-Costa, A.; L-López, F.; Rivero-Cortés, E.; Sarmento-Cabral, A.; Valero-Rosa, J.; Carrasco-Valiente, J.; et al. The Oncogenic Role of the In1-Ghrelin Splicing Variant in Prostate Cancer Aggressiveness. Mol. Cancer 2017, 16, 146. [Google Scholar] [CrossRef]
- Fuentes-Fayos, A.C.; G-García, M.E.; Pérez-Gómez, J.M.; Montero-Hidalgo, A.J.; Martín-Colom, J.; Doval-Rosa, C.; Blanco-Acevedo, C.; Torres, E.; Toledano-Delgado, Á.; Sánchez-Sánchez, R.; et al. Metformin and Simvastatin Exert Additive Antitumour Effects in Glioblastoma via Senescence-State: Clinical and Translational Evidence. eBioMedicine 2023, 90, 104484. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Gómez-Gómez, E.; León-González, A.J.; Sáez-Martínez, P.; Alors-Pérez, E.; Fuentes-Fayos, A.C.; Martínez-López, A.; Sánchez-Sánchez, R.; González-Serrano, T.; et al. Spliceosome Component SF3B1 as Novel Prognostic Biomarker and Therapeutic Target for Prostate Cancer. Transl. Res. 2019, 212, 89–103. [Google Scholar] [CrossRef]
- Del Rio-Moreno, M.; Alors-Perez, E.; Borges de Souza, P.; Prados-Gonzalez, M.E.; CastaÑo, J.P.; Luque, R.M.; Gahete, M.D. Peptides Derived from the Extracellular Domain of the Somatostatin Receptor Splicing Variant SST5TMD4 Increase Malignancy in Multiple Cancer Cell Types. Transl. Res. 2019, 211, 147–160. [Google Scholar] [CrossRef]
- León-González, A.J.; Sáez-Martínez, P.; Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Madrona, A.; Castaño, J.P.; Espartero, J.L.; Gahete, M.D.; et al. Comparative Cytotoxic Activity of Hydroxytyrosol and Its Semisynthetic Lipophilic Derivatives in Prostate Cancer Cells. Antioxidants 2021, 10, 1348. [Google Scholar] [CrossRef]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling Pathway of MAPK/ERK in Cell Proliferation, Differentiation, Migration, Senescence and Apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Ko, E.-B.; Choi, K.-C. Gallic Acid, a Phenolic Acid, Hinders the Progression of Prostate Cancer by Inhibition of Histone Deacetylase 1 and 2 Expression. J. Nutr. Biochem. 2020, 84, 108444. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The LNCaP Cell Line--a New Model for Studies on Human Prostatic Carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar] [PubMed]
- Sramkoski, R.M.; Pretlow, T.G.; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A New Human Prostate Carcinoma Cell Line, 22Rv1. In Vitro Cell Dev. Biol. Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef]
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged Garlic Extract Inhibits 1,2-Dimethylhydrazine-Induced Colon Tumor Development by Suppressing Cell Proliferation. Oncol. Rep. 2015, 33, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica Granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 Is a Cell Line Characteristic of Prostatic Small Cell Carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Malik, A.; Adhami, V.M.; Asim, M.; Hafeez, B.B.; Sarfaraz, S.; Mukhtar, H. Green Tea Polyphenol EGCG Sensitizes Human Prostate Carcinoma LNCaP Cells to TRAIL-Mediated Apoptosis and Synergistically Inhibits Biomarkers Associated with Angiogenesis and Metastasis. Oncogene 2008, 27, 2055–2063. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective Effects Induced by a Hydroalcoholic Allium Sativum Extract in Isolated Mouse Heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The Role of JAK/STAT Signaling Pathway and Its Inhibitors in Diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Senturk, S.; Mumcuoglu, M.; Gursoy-Yuzugullu, O.; Cingoz, B.; Akcali, K.C.; Ozturk, M. Transforming Growth Factor-Beta Induces Senescence in Hepatocellular Carcinoma Cells and Inhibits Tumor Growth. Hepatology 2010, 52, 966–974. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, Y. The Cell Death Machinery Governed by the P53 Tumor Suppressor in Response to DNA Damage. Cancer Sci. 2010, 101, 831–835. [Google Scholar] [CrossRef]
- Yu, G.; Lee, Y.-C.; Cheng, C.-J.; Wu, C.-F.; Song, J.H.; Gallick, G.E.; Yu-Lee, L.-Y.; Kuang, J.; Lin, S.-H. RSK Promotes Prostate Cancer Progression in Bone through ING3, CKAP2, and PTK6-Mediated Cell Survival. Mol. Cancer Res. 2015, 13, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.-P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR Coordinates Protein Synthesis, Mitochondrial Activity and Proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, K.; Ma, Q. The Expression and Significance of p4E-BP1/4E-BP1 in Prostate Cancer. J. Clin. Lab. Anal. 2022, 36, e24332. [Google Scholar] [CrossRef]
- Ougolkov, A.V.; Billadeau, D.D. Targeting Gsk-3: A Promising Approach For Cancer Therapy? Future Oncol. 2006, 2, 91–100. [Google Scholar] [CrossRef]
- González-García, A.; Garrido, A.; Carrera, A.C. Targeting PTEN Regulation by Post Translational Modifications. Cancers 2022, 14, 5613. [Google Scholar] [CrossRef]
- Nemoto, K.; Vogt, A.; Oguri, T.; Lazo, J.S. Activation of the Raf-1/MEK/Erk Kinase Pathway by a Novel Cdc25 Inhibitor in Human Prostate Cancer Cells. Prostate 2004, 58, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wheeler, T.M.; Dai, H.; Sayeeduddin, M.; Scardino, P.T.; Frolov, A.; Ayala, G.E. Biological Correlates of P27 Compartmental Expression in Prostate Cancer. J. Urol. 2006, 175, 528–532. [Google Scholar] [CrossRef]
- Keerthana, C.K.; Rayginia, T.P.; Shifana, S.C.; Anto, N.P.; Kalimuthu, K.; Isakov, N.; Anto, R.J. The Role of AMPK in Cancer Metabolism and Its Impact on the Immunomodulation of the Tumor Microenvironment. Front. Immunol. 2023, 14, 1114582. [Google Scholar] [CrossRef]
- Nam, S.; Kim, D.; Cheng, J.Q.; Zhang, S.; Lee, J.-H.; Buettner, R.; Mirosevich, J.; Lee, F.Y.; Jove, R. Action of the Src Family Kinase Inhibitor, Dasatinib (BMS-354825), on Human Prostate Cancer Cells. Cancer Res. 2005, 65, 9185–9189. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Bai, L.; Liu, S.; Yang, J.C.; Kung, H.-J.; Evans, C.P. Src Family Kinase Oncogenic Potential and Pathways in Prostate Cancer as Revealed by AZD0530. Oncogene 2008, 27, 6365–6375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, J.; Wang, J.; Zhang, W. Inhibition of CDKL3 Downregulates STAT1 Thus Suppressing Prostate Cancer Development. Cell Death Dis. 2023, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Canar, J.; Darling, K.; Dadey, R.; Gamero, A.M. The Duality of STAT2 Mediated Type I Interferon Signaling in the Tumor Microenvironment and Chemoresistance. Cytokine 2023, 161, 156081. [Google Scholar] [CrossRef]
- Gu, L.; Vogiatzi, P.; Puhr, M.; Dagvadorj, A.; Lutz, J.; Ryder, A.; Addya, S.; Fortina, P.; Cooper, C.; Leiby, B.; et al. Stat5 Promotes Metastatic Behavior of Human Prostate Cancer Cells in Vitro and in Vivo. Endocr. Relat. Cancer 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Mendoza-Rodríguez, M.G.; Sánchez-Barrera, C.Á.; Callejas, B.E.; García-Castillo, V.; Beristain-Terrazas, D.L.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; León-Cabrera, S.A.; Rodríguez-Sosa, M.; Gutierrez-Cirlos, E.B.; et al. Use of STAT6 Phosphorylation Inhibitor and Trimethylglycine as New Adjuvant Therapies for 5-Fluorouracil in Colitis-Associated Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 2130. [Google Scholar] [CrossRef]
- Tam, L.; McGlynn, L.M.; Traynor, P.; Mukherjee, R.; Bartlett, J.M.S.; Edwards, J. Expression Levels of the JAK/STAT Pathway in the Transition from Hormone-Sensitive to Hormone-Refractory Prostate Cancer. Br. J. Cancer 2007, 97, 378–383. [Google Scholar] [CrossRef]
- Gu, L.; Talati, P.; Vogiatzi, P.; Romero-Weaver, A.L.; Abdulghani, J.; Liao, Z.; Leiby, B.; Hoang, D.T.; Mirtti, T.; Alanen, K.; et al. Pharmacologic Suppression of JAK1/2 by JAK1/2 Inhibitor AZD1480 Potently Inhibits IL-6-Induced Experimental Prostate Cancer Metastases Formation. Mol. Cancer Ther. 2014, 13, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Nakagawa, T.; Terado, Y.; Kamiyama, Y.; Muto, S.; Horie, S. Tyk2 Expression and Its Signaling Enhances the Invasiveness of Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2008, 369, 292–296. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Niu, R. Functions of Shp2 in Cancer. J. Cell Mol. Med. 2015, 19, 2075–2083. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, M.; Faruq, O.; Zacksenhaus, E.; Chen, W.; Liu, A.; Chang, H. SMAD1 as a Biomarker and Potential Therapeutic Target in Drug-Resistant Multiple Myeloma. Biomark. Res. 2021, 9, 48. [Google Scholar] [CrossRef]
- Miyaki, M.; Kuroki, T. Role of Smad4 (DPC4) Inactivation in Human Cancer. Biochem. Biophys. Res. Commun. 2003, 306, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chang, K.; Peng, J.; Shi, Q.; Gan, H.; Gao, K.; Feng, K.; Xu, F.; Zhang, H.; Dai, B.; et al. SPOP Promotes ATF2 Ubiquitination and Degradation to Suppress Prostate Cancer Progression. J. Exp. Clin. Cancer Res. 2018, 37, 145. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.K.; Bakiri, L.; Hasenfuss, S.C.; Wu, H.; Morente, M.; Wagner, E.F. Loss of JUNB/AP-1 Promotes Invasive Prostate Cancer. Cell Death Differ. 2015, 22, 574–582. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libero, M.L.; Montero-Hidalgo, A.J.; Recinella, L.; Luque, R.M.; Generali, D.; Acquaviva, A.; Orlando, G.; Ferrante, C.; Menghini, L.; Di Simone, S.C.; et al. The Protective Effects of an Aged Black Garlic Water Extract on the Prostate. Nutrients 2024, 16, 3025. https://doi.org/10.3390/nu16173025
Libero ML, Montero-Hidalgo AJ, Recinella L, Luque RM, Generali D, Acquaviva A, Orlando G, Ferrante C, Menghini L, Di Simone SC, et al. The Protective Effects of an Aged Black Garlic Water Extract on the Prostate. Nutrients. 2024; 16(17):3025. https://doi.org/10.3390/nu16173025
Chicago/Turabian StyleLibero, Maria Loreta, Antonio J. Montero-Hidalgo, Lucia Recinella, Raúl M. Luque, Daniele Generali, Alessandra Acquaviva, Giustino Orlando, Claudio Ferrante, Luigi Menghini, Simonetta Cristina Di Simone, and et al. 2024. "The Protective Effects of an Aged Black Garlic Water Extract on the Prostate" Nutrients 16, no. 17: 3025. https://doi.org/10.3390/nu16173025
APA StyleLibero, M. L., Montero-Hidalgo, A. J., Recinella, L., Luque, R. M., Generali, D., Acquaviva, A., Orlando, G., Ferrante, C., Menghini, L., Di Simone, S. C., Nilofar, N., Chiavaroli, A., Brunetti, L., & Leone, S. (2024). The Protective Effects of an Aged Black Garlic Water Extract on the Prostate. Nutrients, 16(17), 3025. https://doi.org/10.3390/nu16173025
















