Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods
Abstract
:1. Introduction
2. Effect of Heat Stress on Sexual Hormonal Synthesis in Male Adolescents
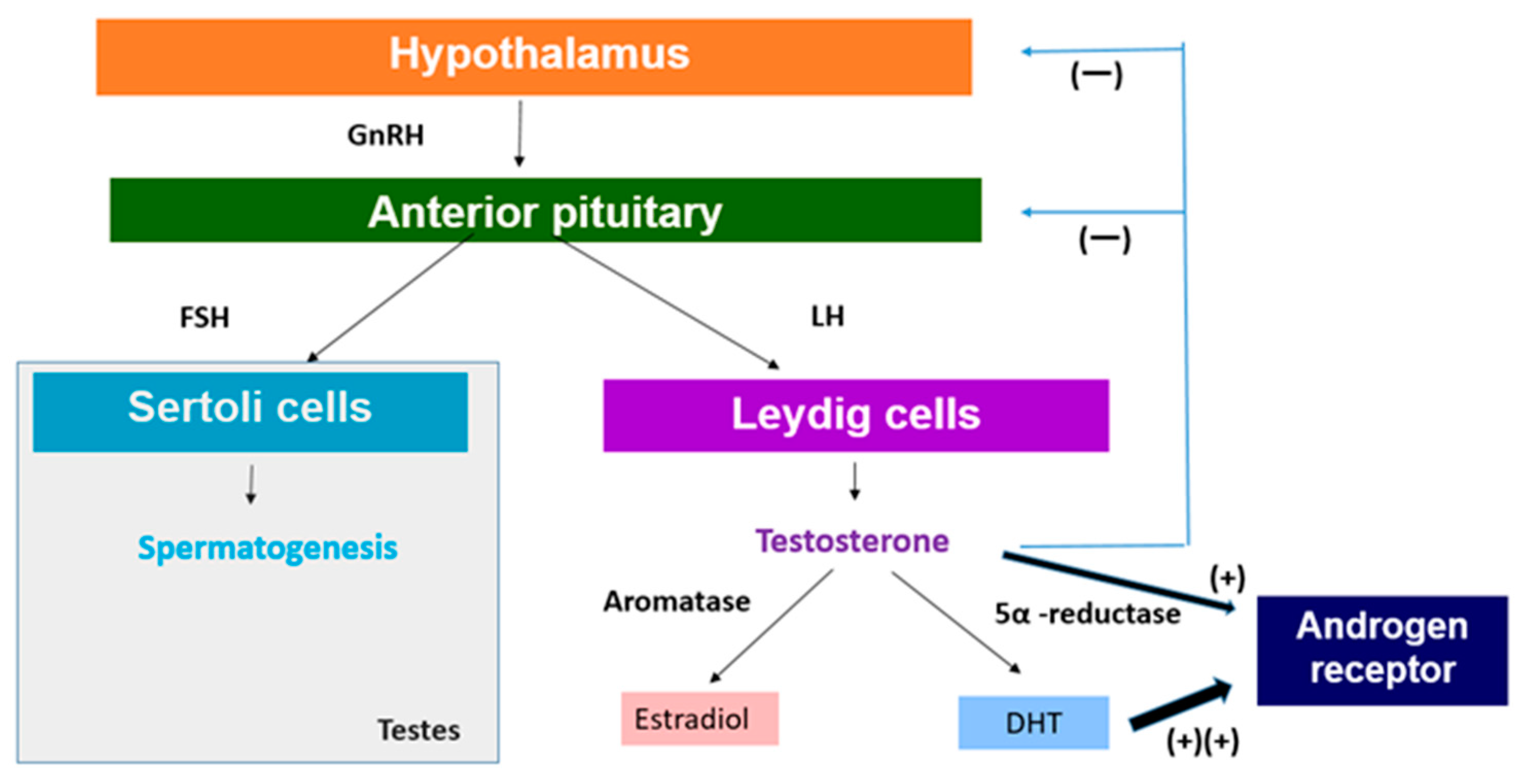
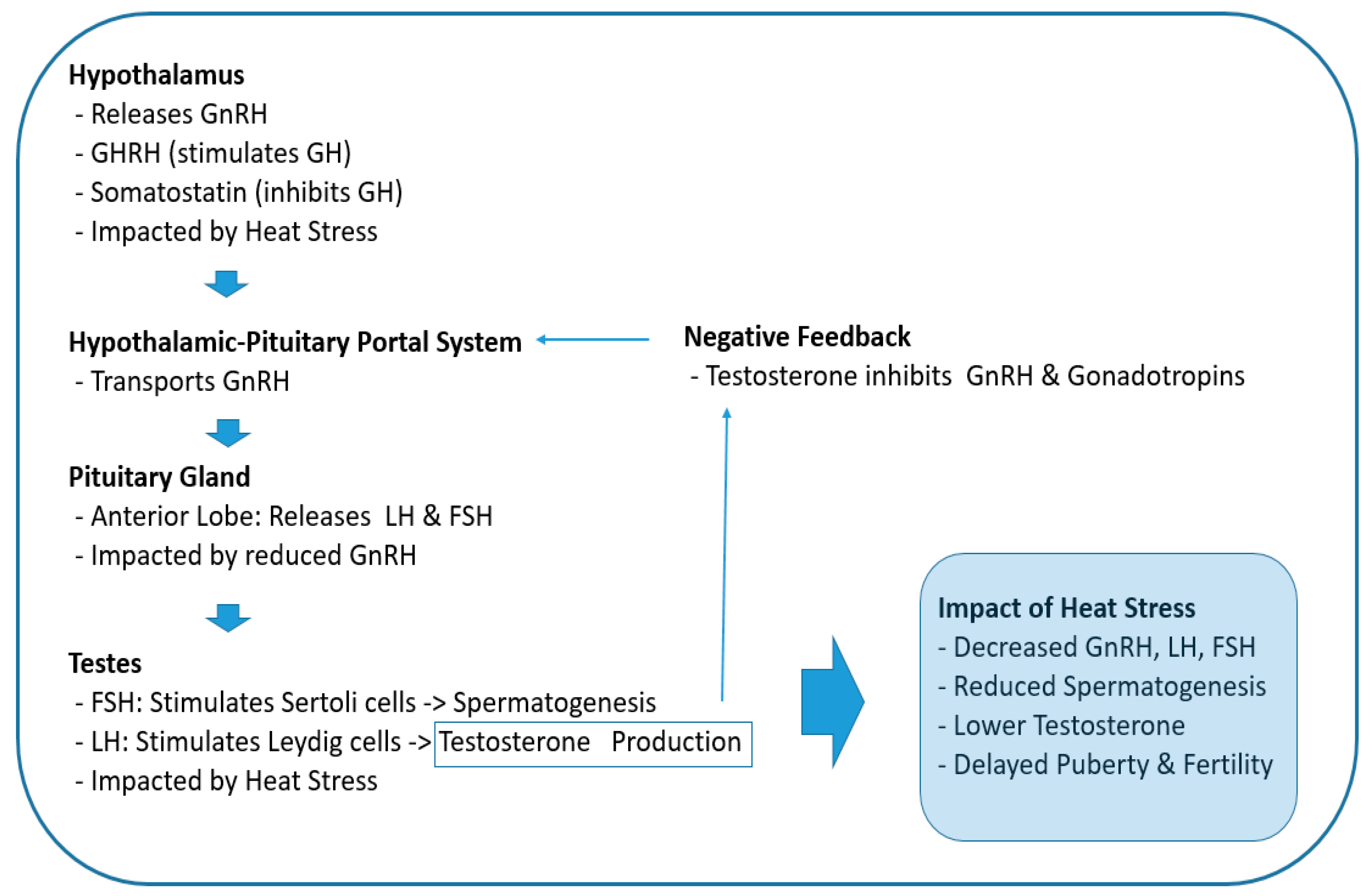
2.1. Impact on the Hypothalamic–Pituitary–Gonadal (HPG) Axis
2.2. Direct Effects on Testicular Function
2.3. Chronic Heat Stress Can Lead to an Imbalance of Testosterone and Estradiol
3. Effects of Dysregulated Sexual Hormone during Adolescence on Reproductive System and Growth
3.1. Low Levels of Testosterone and Reproductive System
3.2. Low Levels of Testosterone Impact on Height and Bone Mineral Density in Adolescence
3.3. Low Testosterone Influence Body Composition in Adolescence
4. Beneficial Foods That Help Prevent or Recover from Low Testosterone and Infertility Caused by Heat Stress
4.1. Metabolism and Energy Requirement for Male Adolescence
4.2. The Role of Melatonin in Preventing Reproductive Damage Caused by Heat Stress
4.3. Supplements to Repair Reproductive Damage Caused by Heat Stress
4.3.1. Tanshinone IIA
4.3.2. Melatonin
4.3.3. Lycium barbarum Polysaccharide
4.3.4. Zinc Sulfate and Folic Acid
4.3.5. Flavonoids
5. Conclusions
Funding
Conflicts of Interest
References
- Zhou, J.; Zhao, J.; Li, Y.; Bai, Y.; Wu, Y.; Xiang, B.; Zhu, H. The hottest center: Characteristics of high temperatures in midsummer of 2022 in Chongqing and its comparison with 2006. Theor. Appl. Climatol. 2024, 155, 151–162. [Google Scholar] [CrossRef]
- Thompson, V.; Kennedy-Asser, A.T.; Vosper, E.; Lo, Y.T.E.; Huntingford, C.; Andrews, O.; Collins, M.; Hegerl, G.C.; Mitchell, D. The 2021 western North America heat wave among the most extreme events ever recorded globally. Sci. Adv. 2022, 8, eabm6860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, P.; Mahendran, R.; Huang, W.; Gao, Y.; Yang, Z.; Ye, T.; Wen, B.; Wu, Y.; Li, S.; et al. Global climate change and human health: Pathways and possible solutions. Eco-Environ. Health 2022, 1, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gauer, R.; Meyers, B.K. Heat-Related Illnesses. Am. Fam. Physician 2019, 99, 482–489. [Google Scholar]
- Guo, Y.; Chen, H.; Wang, Q.-J.; Qi, X.; Li, Q.; Fu, W.; Huang, J.; Yao, C.-Y.; Liu, Z.-Y.; Wang, M.-Z.; et al. Prolonged melatonin treatment promote testicular recovery by enhancing RAC1-mediated apoptotic cell clearance and cell junction-dependent spermatogensis after heat stress. Theriogenology 2021, 162, 22–31. [Google Scholar] [CrossRef]
- Gan, M.; Jing, Y.; Xie, Z.; Ma, J. Potential Function of Testicular MicroRNAs in Heat-Stress-Induced Spermatogenesis Disorders. Int. J. Mol. Sci. 2023, 24, 8809. [Google Scholar] [CrossRef]
- Aldahhan, R.A.; Stanton, P.G.; Ludlow, H.; de Kretser, D.M.; Hedger, M.P. Acute heat-treatment disrupts inhibin-related protein production and gene expression in the adult rat testis. Mol. Cell. Endocrinol. 2019, 498, 110546. [Google Scholar] [CrossRef]
- Setchell, B.P. The effects of heat on the testes of mammals. Anim. Reprod. 2018, 3, 81–91. [Google Scholar]
- Widlak, W.; Vydra, N. The Role of Heat Shock Factors in Mammalian Spermatogenesis. Adv. Anat. Embryol. Cell Biol. 2017, 222, 45–65. [Google Scholar] [CrossRef]
- Paul, C.; Melton, D.W.; Saunders, P.T. Do heat stress and deficits in DNA repair pathways have a negative impact on male fertility? Mol. Hum. Reprod. 2008, 14, 1–8. [Google Scholar] [CrossRef]
- van Zelst, S.J.; Zupp, J.L.; Hayman, D.L.; Setchell, B.P. X-Y chromosome dissociation in mice and rats exposed to increased testicular or environmental temperatures. Reprod. Fertil. Dev. 1995, 7, 1117–1121. [Google Scholar] [CrossRef]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K.; Kharwar, R.K. Co-treatment of testosterone and estrogen mitigates heat-induced testicular dysfunctions in a rat model. J. Steroid Biochem. Mol. Biol. 2021, 214, 106011. [Google Scholar] [CrossRef] [PubMed]
- Netherton, J.K.; Robinson, B.R.; Ogle, R.A.; Gunn, A.; Villaverde, A.; Colyvas, K.; Wise, C.; Russo, T.; Dowdell, A.; Baker, M.A. Seasonal variation in bull semen quality demonstrates there are heat-sensitive and heat-tolerant bulls. Sci. Rep. 2022, 12, 15322. [Google Scholar] [CrossRef]
- Patton, G.C.; Sawyer, S.M.; Santelli, J.S.; Ross, D.A.; Afifi, R.; Allen, N.B.; Arora, M.; Azzopardi, P.; Baldwin, W.; Bonell, C.; et al. Our future: A Lancet commission on adolescent health and wellbeing. Lancet 2016, 387, 2423–2478. [Google Scholar] [CrossRef] [PubMed]
- Patton, G.C.; Olsson, C.A.; Skirbekk, V.; Saffery, R.; Wlodek, M.E.; Azzopardi, P.S.; Stonawski, M.; Rasmussen, B.; Spry, E.; Francis, K.; et al. Adolescence and the next generation. Nature 2018, 554, 458–466. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet. Child Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef]
- Global, regional, and national mortality among young people aged 10-24 years, 1950-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 398, 1593–1618. [CrossRef]
- Yüzen, D.; Graf, I.; Diemert, A.; Arck, P.C. Climate change and pregnancy complications: From hormones to the immune response. Front. Endocrinol. 2023, 14, 1149284. [Google Scholar] [CrossRef]
- Abreu, A.P.; Kaiser, U.B. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016, 4, 254–264. [Google Scholar] [CrossRef]
- Schneider, J.S.; Rissman, E.F. Gonadotropin-releasing hormone II: A multi-purpose neuropeptide. Integr. Comp. Biol. 2008, 48, 588–595. [Google Scholar] [CrossRef]
- Plant, T.M.; Marshall, G.R. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001, 22, 764–786. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Keenan, D.M.; Liu, P.Y.; Iranmanesh, A.; Takahashi, P.Y.; Nehra, A.X. The aging male hypothalamic-pituitary-gonadal axis: Pulsatility and feedback. Mol. Cell. Endocrinol. 2009, 299, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.J.; Wang, C. LH and non-SHBG testosterone and estradiol levels during testosterone replacement of hypogonadal men: Further evidence that steroid negative feedback increases as men grow older. J. Androl. 2010, 31, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Ketchem, J.M.; Bowman, E.J.; Isales, C.M. Male sex hormones, aging, and inflammation. Biogerontology 2023, 24, 1–25. [Google Scholar] [CrossRef]
- Aggarwal, A.; Upadhyay, R. Heat Stress and Hormones. In Heat Stress and Animal Productivity; Aggarwal, A., Upadhyay, R., Eds.; Springer: Chennai, India, 2013; pp. 27–51. [Google Scholar] [CrossRef]
- Lu, B.B.; Liang, W.; Liang, C.; Yu, Z.Q.; Xie, X.Z.; Chen, Z. Effect of Heat Stress on Expression of Main Reproductive Hormone in Hypothalamic-Pituitary-Gonadal Axis of Wenchang Chicks. Braz. J. Poult. Sci. 2021, 23, eRBCA-2019-1056. [Google Scholar] [CrossRef]
- Khan, I.; Mesalam, A.; Heo, Y.S.; Lee, S.-H.; Nabi, G.; Kong, I.-K. Heat Stress as a Barrier to Successful Reproduction and Potential Alleviation Strategies in Cattle. Animals 2023, 13, 2359. [Google Scholar] [CrossRef]
- Kim, H.-D.; Kim, Y.-J.; Jang, M.; Bae, S.-G.; Yun, S.-H.; Lee, M.-R.; Seo, Y.-R.; Cho, J.-K.; Kim, S.-J.; Lee, W.-J. Heat Stress during Summer Attenuates Expression of the Hypothalamic Kisspeptin, an Upstream Regulator of the Hypothalamic–Pituitary–Gonadal Axis, in Domestic Sows. Animals 2022, 12, 2967. [Google Scholar] [CrossRef]
- Navarro, V.M.; Tena-Sempere, M. Neuroendocrine control by kisspeptins: Role in metabolic regulation of fertility. Nat. Rev. Endocrinol. 2012, 8, 40–53. [Google Scholar] [CrossRef]
- Aggarwal, A.; Upadhyay, R. Heat Stress and Reproduction. In Heat Stress and Animal Productivity; Springer: Chennai, India, 2013; pp. 79–111. [Google Scholar] [CrossRef]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.P.; Gao, H.B.; Dong, Q.; Ge, R.; Wang, Q.; Chai, W.R.; Feng, X.; Sottas, C. Stress hormone and male reproductive function. Cell Tissue Res. 2005, 322, 147–153. [Google Scholar] [CrossRef]
- Rockett, J.C.; Mapp, F.L.; Garges, J.B.; Luft, J.C.; Mori, C.; Dix, D.J. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol. Reprod. 2001, 65, 229–239. [Google Scholar] [CrossRef]
- Hamilton, T.R.; Mendes, C.M.; de Castro, L.S.; de Assis, P.M.; Siqueira, A.F.; Delgado Jde, C.; Goissis, M.D.; Muiño-Blanco, T.; Cebrián-Pérez, J.; Nichi, M.; et al. Evaluation of Lasting Effects of Heat Stress on Sperm Profile and Oxidative Status of Ram Semen and Epididymal Sperm. Oxidative Med. Cell. Longev. 2016, 2016, 1687657. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.G.; Farias, J.G.; Henríquez-Olavarrieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The hypoxic testicle: Physiology and pathophysiology. Oxidative Med. Cell. Longev. 2012, 2012, 929285. [Google Scholar] [CrossRef]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxidative Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef]
- Hou, W.; Dong, Y.; Zhang, J.; Yin, Z.; Wen, H.; Xiong, L.; Li, W. Hypoxia-induced deacetylation is required for tetraploid differentiation in response to testicular ischemia-reperfusion (IR) injury. J. Androl. 2012, 33, 1379–1386. [Google Scholar] [CrossRef]
- Monageng, E.; Offor, U.; Takalani, N.B.; Mohlala, K.; Opuwari, C.S. A Review on the Impact of Oxidative Stress and Medicinal Plants on Leydig Cells. Antioxidants 2023, 12, 1559. [Google Scholar] [CrossRef]
- Cai, H.; Qin, D.; Peng, S. Responses and coping methods of different testicular cell types to heat stress: Overview and perspectives. Biosci. Rep. 2021, 41, BSR20210443. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D.; Sharma, R.K.; du Plessis, S.S.; Agarwal, A. Testicular Heat Stress and Sperm Quality. In Male Infertility: A Complete Guide to Lifestyle and Environmental Factors; du Plessis, S.S., Agarwal, A., Sabanegh, J.E.S., Eds.; Springer: New York, NY, USA, 2014; pp. 105–125. [Google Scholar] [CrossRef]
- Fleming, J.S.; Yu, F.; McDonald, R.M.; Meyers, S.A.; Montgomery, G.W.; Smith, J.F.; Nicholson, H.D. Effects of scrotal heating on sperm surface protein PH-20 expression in sheep. Mol. Reprod. Dev. 2004, 68, 103–114. [Google Scholar] [CrossRef]
- Nichi, M.; Bols, P.E.; Züge, R.M.; Barnabe, V.H.; Goovaerts, I.G.; Barnabe, R.C.; Cortada, C.N. Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology 2006, 66, 822–828. [Google Scholar] [CrossRef]
- Atta, M.S.; Farrag, F.A.; Almadaly, E.A.; Ghoneim, H.A.; Hafez, A.S.; Al Jaouni, S.K.; Mousa, S.A.; El-Far, A.H. Transcriptomic and biochemical effects of pycnogenol in ameliorating heat stress-related oxidative alterations in rats. J. Therm. Biol. 2020, 93, 102683. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, Y.; Zheng, C.; Xu, S.; He, Y.; Yu, G.; Huang, D.; Huang, Y.; Li, M.; Xu, C. Tanshinone IIA protects mouse testes from heat stress injury by inhibiting apoptosis and TGFβ1/Smad2/Smad3 signaling pathway. Cell Stress Chaperones 2023, 28, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Blumer, C.G.; Fariello, R.M.; Restelli, A.E.; Spaine, D.M.; Bertolla, R.P.; Cedenho, A.P. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil. Steril. 2008, 90, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Habibi, P.; Ostad, S.; Heydari, A.; Monazzam, M.; Foroushani, A.; Ghazi-Khansari, M.; Golbabaei, F. Diagnostic Biomarkers of Heat Stress Induced-DNA in Occupational Exposure: A Systematic Review. J. Health Saf. Work. 2023, 12, 800–819. [Google Scholar]
- Robinson, B.R.; Netherton, J.K.; Ogle, R.A.; Baker, M.A. Testicular heat stress, a historical perspective and two postulates for why male germ cells are heat sensitive. Biol. Rev. 2023, 98, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Meng, T.; Wu, L.; Duan, Y.; Li, G.; Shi, C.; Zhang, H.; Peng, Z.; Fan, C.; Ma, J.; et al. Association between ambient temperature and semen quality: A longitudinal study of 10,802 men in China. Environ. Int. 2020, 135, 105364. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Q.; Ni, H.; Xu, T.; Cai, X.; Dai, T.; Wang, L.; Song, C.; Li, Y.; Li, F.; et al. Effects of temperature anomaly on sperm quality: A multi-center study of 33,234 men. Heliyon 2024, 10, e26765. [Google Scholar] [CrossRef]
- Durdiakova, J.; Ostatnikova, D.; Celec, P. Testosterone and its metabolites—Modulators of brain functions. Acta Neurobiol. Exp. 2011, 71, 434–454. [Google Scholar] [CrossRef]
- Oka, S.; Matsukuma, H.; Horiguchi, N.; Kobayashi, T.; Shiraishi, K. Heat stress upregulates aromatases expression through nuclear DAX-1 deficiency in R2C Leydig tumor cells. Mol. Cell. Endocrinol. 2022, 558, 111766. [Google Scholar] [CrossRef]
- Shiraishi, K.; Oka, S.; Matsuyama, H. Testicular Testosterone and Estradiol Concentrations and Aromatase Expression in Men with Nonobstructive Azoospermia. J. Clin. Endocrinol. Metab. 2021, 106, e1803–e1815. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Kaplan-Zverling, M.; Shen-Orr, Z.; Nave, R.; Herer, P. Seminal plasma androgen/oestrogen balance in infertile men. Int. J. Androl. 2002, 25, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Hamerezaee, M.; Dehghan, S.F.; Golbabaei, F.; Fathi, A.; Barzegar, L.; Heidarnejad, N. Assessment of Semen Quality among Workers Exposed to Heat Stress: A Cross-Sectional Study in a Steel Industry. Saf. Health Work 2018, 9, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat Stress: A Serious Disruptor of the Reproductive Physiology of Dairy Cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.M.; Szelényi, Z.; Szenci, O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals 2024, 14, 1473. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Zhao, W.; Jing, J.; Shao, Y.; Zeng, R.; Wang, C.; Yao, B.; Hang, D. Circulating sex hormone levels in relation to male sperm quality. BMC Urol. 2020, 20, 101. [Google Scholar] [CrossRef]
- Nacusi, L.P.; Tindall, D.J. Targeting 5α-reductase for prostate cancer prevention and treatment. Nat. Rev. Urol. 2011, 8, 378–384. [Google Scholar] [CrossRef]
- Traish, A.M. Negative Impact of Testosterone Deficiency and 5α-Reductase Inhibitors Therapy on Metabolic and Sexual Function in Men. Adv. Exp. Med. Biol. 2017, 1043, 473–526. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Wistuba, J.; Neuhaus, N.; Nieschlag, E. Physiology of Testicular Function. In Andrology: Male Reproductive Health and Dysfunction; Nieschlag, E., Behre, H.M., Kliesch, S., Nieschlag, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 15–54. [Google Scholar] [CrossRef]
- Grumbach, M.M. The neuroendocrinology of human puberty revisited. Horm. Res. 2002, 57 (Suppl. S2), 2–14. [Google Scholar] [CrossRef]
- Preston, B.T.; Stevenson, I.R.; Lincoln, G.A.; Monfort, S.L.; Pilkington, J.G.; Wilson, K. Testes size, testosterone production and reproductive behaviour in a natural mammalian mating system. J. Anim. Ecol. 2012, 81, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, W.; Wang, Z.; Huang, R.; Xia, H.; Li, Z.; Deng, J.; Ye, T. Hormone Regulation in Testicular Development and Function. Int. J. Mol. Sci. 2024, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Rastrelli, G.; Hackett, G.; Seminara, S.B.; Huhtaniemi, I.T.; Rey, R.A.; Hellstrom, W.J.G.; Palmert, M.R.; Corona, G.; Dohle, G.R.; et al. Paediatric and adult-onset male hypogonadism. Nat. Rev. Dis. Primers 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Hoai, B.; Hoang, L. Ultrasonic testicular size of 24,440 adult Vietnamese men and the correlation with age and hormonal profiles. Andrologia 2022, 54, e14333. [Google Scholar] [CrossRef]
- Baxter-Jones, A.D.; Faulkner, R.A.; Forwood, M.R.; Mirwald, R.L.; Bailey, D.A. Bone mineral accrual from 8 to 30 years of age: An estimation of peak bone mass. J. Bone Miner. Res. 2011, 26, 1729–1739. [Google Scholar] [CrossRef]
- Whiting, S.J.; Vatanparast, H.; Baxter-Jones, A.; Faulkner, R.A.; Mirwald, R.; Bailey, D.A. Factors that affect bone mineral accrual in the adolescent growth spurt. J. Nutr. 2004, 134, 696s–700s. [Google Scholar] [CrossRef]
- Ağırdil, Y. The growth plate: A physiologic overview. EFORT Open Rev. 2020, 5, 498–507. [Google Scholar] [CrossRef]
- Juul, A. The effects of oestrogens on linear bone growth. Hum. Reprod. Update 2001, 7, 303–313. [Google Scholar] [CrossRef]
- Nilsson, O.; Marino, R.; De Luca, F.; Phillip, M.; Baron, J. Endocrine regulation of the growth plate. Horm. Res. 2005, 64, 157–165. [Google Scholar] [CrossRef]
- Aldahhan, R.A.; Stanton, P.G. Heat stress response of somatic cells in the testis. Mol. Cell. Endocrinol. 2021, 527, 111216. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd; Khosla, S.; Atkinson, E.J.; Oconnor, M.K.; Ofallon, W.M.; Riggs, B.L. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos. Int. 2000, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Warming, L.; Hassager, C.; Christiansen, C. Changes in bone mineral density with age in men and women: A longitudinal study. Osteoporosis international: A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. Osteoporos. Int. 2002, 13, 105–112. [Google Scholar] [CrossRef]
- Amory, J.K.; Watts, N.B.; Easley, K.A.; Sutton, P.R.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004, 89, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Chen, T.C.; Barber, T.W.; Malabanan, A.O.; Holick, M.F.; Tangpricha, V. Testosterone increases bone mineral density in female-to-male transsexuals: A case series of 15 subjects. Clin. Endocrinol. 2004, 61, 560–566. [Google Scholar] [CrossRef]
- Kirchengast, S.; Angelika, G. Body composition characteristics during puberty in girls and boys from Eastern Austria. Int. J. Anthropol. 2003, 18, 139–151. [Google Scholar] [CrossRef]
- Ethun, K. Chapter 9—Sex and Gender Differences in Body Composition, Lipid Metabolism, and Glucose Regulation. In Sex Differences in Physiology; Neigh, G.N., Mitzelfelt, M.M., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 145–165. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Santosa, S.; Bush, N.C.; Jensen, M.D. Acute Testosterone Deficiency Alters Adipose Tissue Fatty Acid Storage. J. Clin. Endocrinol. Metab. 2017, 102, 3056–3064. [Google Scholar] [CrossRef]
- Weimar, J.D.; DiRusso, C.C.; Delio, R.; Black, P.N. Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids. Amino acid residues within the ATP/AMP signature motif of Escherichia coli FadD are required for enzyme activity and fatty acid transport. J. Biol. Chem. 2002, 277, 29369–29376. [Google Scholar] [CrossRef]
- Bhasin, S.; Woodhouse, L.; Storer, T.W. Proof of the effect of testosterone on skeletal muscle. J. Endocrinol. 2001, 170, 27–38. [Google Scholar] [CrossRef]
- Conti, V.; Russomanno, G.; Corbi, G.; Izzo, V.; Vecchione, C.; Filippelli, A. Adrenoreceptors and nitric oxide in the cardiovascular system. Front. Physiol. 2013, 4, 321. [Google Scholar] [CrossRef]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of testosterone on muscle mass and muscle protein synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Phillips, S.M. Anabolic processes in human skeletal muscle: Restoring the identities of growth hormone and testosterone. Physician Sportsmed. 2010, 38, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Urban, R.J.; Wolf, S.E.; Jiang, J.; Catlin, D.H.; Herndon, D.N.; Wolfe, R.R.; Ferrando, A.A. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J. Clin. Endocrinol. Metab. 1999, 84, 2705–2711. [Google Scholar] [CrossRef]
- Singh, R.; Artaza, J.N.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Bhasin, S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003, 144, 5081–5088. [Google Scholar] [CrossRef] [PubMed]
- Gucenmez, S.; Yildiz, P.; Donderici, O.; Serter, R. The effect of testosterone level on metabolic syndrome: A cross-sectional study. Hormones 2024, 23, 163–169. [Google Scholar] [CrossRef]
- Katznelson, L.; Finkelstein, J.S.; Schoenfeld, D.A.; Rosenthal, D.I.; Anderson, E.J.; Klibanski, A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J. Clin. Endocrinol. Metab. 1996, 81, 4358–4365. [Google Scholar] [CrossRef]
- Mauras, N.; Hayes, V.; Welch, S.; Rini, A.; Helgeson, K.; Dokler, M.; Veldhuis, J.D.; Urban, R.J. Testosterone deficiency in young men: Marked alterations in whole body protein kinetics, strength, and adiposity. J. Clin. Endocrinol. Metab. 1998, 83, 1886–1892. [Google Scholar] [CrossRef]
- Wang, C.; Eyre, D.R.; Clark, R.; Kleinberg, D.; Newman, C.; Iranmanesh, A.; Veldhuis, J.; Dudley, R.E.; Berman, N.; Davidson, T.; et al. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—A clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3654–3662. [Google Scholar] [CrossRef]
- Cohen, J.; Nassau, D.E.; Patel, P.; Ramasamy, R. Low Testosterone in Adolescents & Young Adults. Front. Endocrinol. 2019, 10, 916. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Thornburg, K.L.; Prentice, A.M.; Campisi, S.; Lassi, Z.S.; Koletzko, B.; Bhutta, Z.A. Nutrition in adolescents: Physiology, metabolism, and nutritional needs. Ann. N. Y. Acad. Sci. 2017, 1393, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Jung, Y. Energy Metabolism Changes and Dysregulated Lipid Metabolism in Postmenopausal Women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef]
- Holliday, M.A. Metabolic rate and organ size during growth from infancy to maturity and during late gastation and early infancy. Pediatrics 1971, 47 (Suppl. S2), 169. [Google Scholar]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. S1), 5–41. [Google Scholar] [PubMed]
- Reiter, R.J.; Tan, D.X.; Fuentes-Broto, L. Melatonin: A multitasking molecule. Prog. Brain Res. 2010, 181, 127–151. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar]
- Deng, C.C.; Zhang, J.P. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Zhao, L.; Yao, C.; Xing, X.; Jing, T.; Li, P.; Zhu, Z.; Yang, C.; Zhai, J.; Tian, R.; Chen, H.; et al. Single-cell analysis of developing and azoospermia human testicles reveals central role of Sertoli cells. Nat. Commun. 2020, 11, 5683. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Casao, A.; Perez-Patiño, C.; Parrilla, I.; Muiño-Blanco, T.; Martinez, E.A.; Cebrian-Perez, J.A.; Roca, J. Profile and reproductive roles of seminal plasma melatonin of boar ejaculates used in artificial insemination programs. J. Anim. Sci. 2017, 95, 1660–1668. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Lv, Y.; Li, F.; Su, L.; Qin, Y.; Zeng, W. Melatonin protects the mouse testis against heat-induced damage. Mol. Hum. Reprod. 2020, 26, 65–79. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, H.; Guo, X.; Aierken, A.; Hua, J.; Ma, B.; Peng, S. Melatonin alleviates heat stress-induced testicular damage in dairy goats by inhibiting the PI3K/AKT signaling pathway. Stress Biol. 2022, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Whiting, S.; Lambourne, S.; Aitken, R.J.; Sun, Y.P. Melatonin alleviates heat stress-induced oxidative stress and apoptosis in human spermatozoa. Free Radic. Biol. Med. 2021, 164, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, F.B.; Safdari, H.A.; Almatroudi, A.; Alzohairy, M.A.; Safdari, M.; Amirizadeh, M.; Rehman, S.; Equbal, M.J.; Hoque, M. Prospective therapeutic potential of Tanshinone IIA: An updated overview. Pharmacol. Res. 2021, 164, 105364. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Zhao, Y.; Li, J. Sodium tanshinone IIA sulfonate suppresses heat stress-induced endothelial cell apoptosis by promoting NO production through upregulating the PI3K/AKT/eNOS pathway. Mol. Med. Rep. 2017, 16, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, I.; Monllor, F.; Marchena, A.M.; Ortiz, A.; Lozano, G.; Jiménez, M.I.; Gaspar, P.; García, J.F.; Pariente, J.A.; Rodríguez, A.B.; et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J. Pineal Res. 2014, 57, 333–339. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef]
- Hu, S.; Liu, D.; Liu, S.; Li, C.; Guo, J. Lycium barbarum Polysaccharide Ameliorates Heat-Stress-Induced Impairment of Primary Sertoli Cells and the Blood-Testis Barrier in Rat via Androgen Receptor and Akt Phosphorylation. Evidence-Based Complement. Altern. Med. 2021, 2021, 5574202. [Google Scholar] [CrossRef]
- Fadl, A.M.; Abdelnaby, E.A. Supplemental dietary zinc sulphate and folic acid combination improves testicular volume and haemodynamics, testosterone levels and semen quality in rams under heat stress conditions. Reprod. Domest. Anim. 2022, 57, 567–576. [Google Scholar] [CrossRef]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-H. Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods. Nutrients 2024, 16, 3032. https://doi.org/10.3390/nu16173032
Ko S-H. Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods. Nutrients. 2024; 16(17):3032. https://doi.org/10.3390/nu16173032
Chicago/Turabian StyleKo, Seong-Hee. 2024. "Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods" Nutrients 16, no. 17: 3032. https://doi.org/10.3390/nu16173032
APA StyleKo, S.-H. (2024). Effects of Heat Stress-Induced Sex Hormone Dysregulation on Reproduction and Growth in Male Adolescents and Beneficial Foods. Nutrients, 16(17), 3032. https://doi.org/10.3390/nu16173032






