Synergistic Enhancement of 5-Fluorouracil Chemotherapeutic Efficacy by Taurine in Colon Cancer Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.2. Colon Analysis and Processing
2.3. Immunohistochemical Assay
2.4. Statistical Analysis
3. Results
Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACS Releases Colorectal Cancer Estimates for 2024|Colorectal Cancer Alliance. Available online: https://colorectalcancer.org/article/acs-releases-colorectal-cancer-estimates-2024 (accessed on 26 June 2024).
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 26 June 2024).
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of Oncogenic KRAS in the Prognosis, Diagnosis and Treatment of Colorectal Cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Zhou, J.; Chen, Z.-R.; Chng, W.-J. P53 Mutations in Colorectal Cancer- Molecular Pathogenesis and Pharmacological Reactivation. World J. Gastroenterol. 2015, 21, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Serebriiskii, I.G.; Pavlov, V.A.; Andrianov, G.V.; Litwin, S.; Basickes, S.; Newberg, J.Y.; Frampton, G.M.; Meyer, J.E.; Golemis, E.A. Source, Co-Occurrence, and Prognostic Value of PTEN Mutations or Loss in Colorectal Cancer. npj Genom. Med. 2023, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Khaniki, S.H.; Shokoohi, F.; Esmaily, H.; Kerachian, M.A. Analyzing Aberrant DNA Methylation in Colorectal Cancer Uncovered Intangible Heterogeneity of Gene Effects in the Survival Time of Patients. Sci. Rep. 2023, 13, 22104. [Google Scholar]
- Li, Z.; Shen, J.; Chan, M.T.V.; Wu, W.K.K. TUG1: A Pivotal Oncogenic Long Non-coding RNA of Human Cancers. Cell Prolif. 2016, 49, 471–475. [Google Scholar] [CrossRef]
- Rattray, N.J.W.; Charkoftaki, G.; Rattray, Z.; Hansen, J.E.; Vasiliou, V.; Johnson, C.H. Environmental Influences in the Etiology of Colorectal Cancer: The Premise of Metabolomics. Curr. Pharmacol. Rep. 2017, 3, 114–125. [Google Scholar] [CrossRef]
- Albrecht, J.; Schousboe, A. Taurine Interaction with Neurotransmitter Receptors in the CNS: An Update. Neurochem. Res. 2005, 30, 1615–1621. [Google Scholar] [CrossRef]
- Santulli, G.; Kansakar, U.; Varzideh, F.; Mone, P.; Jankauskas, S.S.; Lombardi, A. Functional Role of Taurine in Aging and Cardiovascular Health: An Updated Overview. Nutrients 2023, 15, 4236. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “Very Essential” Amino Acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Huxtable, R.J. Physiological Actions of Taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological Role of Taurine—From Organism to Organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G. Idiosyncratic Nutrient Requirements of Cats Appear to Be Diet-Induced Evolutionary Adaptations. Nutr. Res. Rev. 2002, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The Appeal of a Safe Amino Acid for Skeletal Muscle Disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine Deficiency as a Driver of Aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- El-Hakim, H.A.; El-Agouza, M.; El-Shimy, R.A.; Mohammed, H.G.; El-Gendy, H.A.A. The Prospect of Using Serum Taurine Level as a Potential Biomarker for Early Detection of Colorectal Carcinoma and Its Correlation with Other Prognostic Markers. Case Med. Res. 2019, 5, 1. [Google Scholar] [CrossRef]
- Wang, G.; Ma, N.; He, F.; Kawanishi, S.; Kobayashi, H.; Oikawa, S.; Murata, M. Taurine Attenuates Carcinogenicity in Ulcerative Colitis-Colorectal Cancer Mouse Model. Oxidative Med. Cell. Longev. 2020, 2020, 7935917. [Google Scholar] [CrossRef]
- Man Chin, C.; Hartmann Jornada, D.; Chiba, D.E.; Scarim, C.B.; De Andrade, C.R.; Gabriel, E.A. The Preventive Effect of Taurine in Early Stage of Colon Chemical Carcinogenesis. Int. J. Complement. Altern. Med. 2021, 14, 242–244. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, S.; Wang, Y.; Xu, B.; Wan, F. Mechanism of Taurine-Induced Apoptosis in Human Colon Cancer Cells. Acta Biochim. Biophys. Sin. 2014, 46, 261–272. [Google Scholar] [CrossRef]
- Liu, J.-X.; Li, W.; Li, J.-T.; Liu, F.; Zhou, L. Screening Key Long Non-Coding RNAs in Early-Stage Colon Adenocarcinoma by RNA-Sequencing. Epigenomics 2018, 10, 1215–1228. [Google Scholar] [CrossRef]
- Ibrahim, B.A.; Gobran, M.A.; Metwalli, A.-E.M.; Abd Elhady, W.A.; Tolba, A.M.; Omar, W.E. Interplay of LncRNA TUG1 and TGF-β/P53 Expression in Colorectal Cancer. Asian Pac. J. Cancer Prev. 2023, 24, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Detre, S.; Saclani Jotti, G.; Dowsett, M. A “Quickscore” Method for Immunohistochemical Semiquantitation: Validation for Oestrogen Receptor in Breast Carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Zhang, X.; Liu, L.; Tu, S.; Zhu, W.; Yu, L.; Wan, H.; Yu, B.; Wan, F. Roles of the MST1-JNK Signaling Pathway in Apoptosis of Colorectal Cancer Cells Induced by Taurine. Libyan J. Med. 2018, 13, 1500346. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, M.K.; Anandakumar, P.; Sakthisekaran, D. Taurine Abrogates Mammary Carcinogenesis through Induction of Apoptosis in Sprague-Dawley Rats. J. Biochem. Mol. Toxicol. 2018, 32, e22204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, H.; Wang, Y.; Liu, C.; Zhu, W.; Zheng, S.; Wan, F. Taurine Induces the Apoptosis of Breast Cancer Cells by Regulating Apoptosis-Related Proteins of Mitochondria. Int. J. Mol. Med. 2015, 35, 218–226. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.H.; Kim, B.G.; Lee, K.L.; Kim, J.W.; Koh, S. Tauroursodeoxycholic Acid Attenuates Colitis-associated Colon Cancer by Inhibiting Nuclear Factor kappaB Signaling. J. Gastro Hepatol. 2019, 34, 544–551. [Google Scholar] [CrossRef]
- Bosquesi, P.L.; Scarim, C.B.; De Oliveira, J.R.S.; De Oliveira Vizioli, E.; Santos, J.L.D.; Chin, C.M. Protective Effect of Taurine in the Induction of Genotoxicity by Mutagenic Drugs. J. Pharm. Pharmacol. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Orlando, F.A.; Tan, D.; Baltodano, J.D.; Khoury, T.; Gibbs, J.F.; Hassid, V.J.; Ahmed, B.H.; Alrawi, S.J. Aberrant Crypt Foci as Precursors in Colorectal Cancer Progression. J. Surg. Oncol. 2008, 98, 207–213. [Google Scholar] [CrossRef]
- Han, X.; Patters, A.B.; Jones, D.P.; Zelikovic, I.; Chesney, R.W. The Taurine Transporter: Mechanisms of Regulation. Acta Physiol. 2006, 187, 61–73. [Google Scholar] [CrossRef]
- Anderson, C.M.H.; Howard, A.; Walters, J.R.F.; Ganapathy, V.; Thwaites, D.T. Taurine Uptake across the Human Intestinal Brush-border Membrane Is via Two Transporters: H+-coupled PAT1 (SLC36A1) and Na+- and Cl−-dependent TauT (SLC6A6). J. Physiol. 2009, 587, 731–744. [Google Scholar] [CrossRef]
- Richter, M.; Moroniak, S.J.; Michel, H. Identification of Competitive Inhibitors of the Human Taurine Transporter TauT in a Human Kidney Cell Line. Pharmacol. Rep. 2019, 71, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M.; Matsumura, Y. Role of SLC6A6 in Promoting the Survival and Multidrug Resistance of Colorectal Cancer. Sci. Rep. 2014, 4, 4852. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Kyriakopoulos, A.M.; Goulielmaki, M.; Panayiotidis, M.I.; Spandidos, D.A.; Zoumpourlis, V. Significance of Taurine Transporter (TauT) in Homeostasis and Its Layers of Regulation. Mol. Med. Rep. 2020, 22, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Colorectal Carcinogenesis: Review of Human and Experimental Animal Studies. J. Carcinog. 2009, 8, 5. [Google Scholar] [CrossRef]
- Loevenich, L.P.; Tschurtschenthaler, M.; Rokavec, M.; Silva, M.G.; Jesinghaus, M.; Kirchner, T.; Klauschen, F.; Saur, D.; Neumann, J.; Hermeking, H.; et al. SMAD4 Loss Induces C-MYC–Mediated NLE1 Upregulation to Support Protein Biosynthesis, Colorectal Cancer Growth, and Metastasis. Cancer Res. 2022, 82, 4604–4623. [Google Scholar] [CrossRef]
- Takahashi, M.; Fukuda, K.; Sugimura, T.; Wakabayashi, K. Beta-Catenin Is Frequently Mutated and Demonstrates Altered Cellular Location in Azoxymethane-Induced Rat Colon Tumors. Cancer Res. 1998, 58, 42–46. [Google Scholar]
- Takahashi, M.; Wakabayashi, K. Gene Mutations and Altered Gene Expression in Azoxymethane-Induced Colon Carcinogenesis in Rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- Hao, X.P.; Pretlow, T.G.; Rao, J.S.; Pretlow, T.P. Beta-Catenin Expression Is Altered in Human Colonic Aberrant Crypt Foci. Cancer Res. 2001, 61, 8085–8088. [Google Scholar]
- Takahashi, M.; Mutoh, M.; Kawamori, T.; Sugimura, T.; Wakabayashi, K. Altered Expression of Beta-Catenin, Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in Azoxymethane-Induced Rat Colon Carcinogenesis. Carcinogenesis 2000, 21, 1319–1327. [Google Scholar] [CrossRef]
- Perše, M.; Cerar, A. Morphological and Molecular Alterations in 1,2 Dimethylhydrazine and Azoxymethane Induced Colon Carcinogenesis in Rats. BioMed Res. Int. 2011, 2011, 473964. [Google Scholar] [CrossRef] [PubMed]
- Shivapurkar, N.; Belinsky, S.A.; Wolf, D.C.; Tang, Z.; Alabaster, O. Absence of P53 Gene Mutations in Rat Colon Carcinomas Induced by Azoxymethane. Cancer Lett. 1995, 96, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Darzynkiewicz, Z. Cell Cycle Dependent Expression and Stability of the Nuclear Protein Detected by Ki-67 Antibody in HL-60 Cells. Cell Prolif. 1992, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Min, K.; Lee, S.K. Cell Cycle Dysregulation Is Associated with 5-Fluorouracil Resistance in Gastric Cancer Cells. Anticancer. Res. 2020, 40, 3247–3254. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Vinayagam, R.; Arokia Vijaya Anand, M.; Isa, N.M.; Ponnaiyan, R. Biochemical and Molecular Aspects of 1,2-Dimethylhydrazine (DMH)-Induced Colon Carcinogenesis: A Review. Toxicol. Res. 2020, 9, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA Localization and Function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, J.; Fan, Q.; Li, Z.; Dou, R.; Lin, Z.; Chen, Z.; Xu, Y.; Huang, Z.; Lan, J.; et al. A Prognostic Cuproptosis-Related lncRNA Predictive Signature for Bladder Cancer Patients. Hum. Cell 2023, 36, 798–811. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-Coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef]
- Guo, C.; Qi, Y.; Qu, J.; Gai, L.; Shi, Y.; Yuan, C. Pathophysiological Functions of the lncRNA TUG1. Curr. Pharm. Des. 2020, 26, 688–700. [Google Scholar] [CrossRef]
- Fan, S.; Yang, Z.; Ke, Z.; Huang, K.; Liu, N.; Fang, X.; Wang, K. Downregulation of the Long Non-Coding RNA TUG1 Is Associated with Cell Proliferation, Migration, and Invasion in Breast Cancer. Biomed. Pharmacother. 2017, 95, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, N.; Ghorbian, S. Analysis of Clinical Important of LncRNA-HOTAIR Gene Variations and Ovarian Cancer Susceptibility. Mol. Biol. Rep. 2020, 47, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, X.; Li, X.; Ma, Y.; Goel, A. Long Non-Coding RNAs in Colorectal Cancer: Novel Oncogenic Mechanisms and Promising Clinical Applications. Cancer Lett. 2021, 504, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shen, X. Long Noncoding RNAs: Functions and Mechanisms in Colon Cancer. Mol. Cancer 2020, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, K.; Liu, K.; Huang, J.; Chen, J.; Zhang, J.; Zhang, N. Long Noncoding RNA BC200 Regulates Cell Growth and Invasion in Colon Cancer. Int. J. Biochem. Cell Biol. 2018, 99, 219–225. [Google Scholar] [CrossRef]
- Jiang, H.; Li, T.; Qu, Y.; Wang, X.; Li, B.; Song, J.; Sun, X.; Tang, Y.; Wan, J.; Yu, Y.; et al. Long Non-Coding RNA SNHG15 Interacts with and Stabilizes Transcription Factor Slug and Promotes Colon Cancer Progression. Cancer Lett. 2018, 425, 78–87. [Google Scholar] [CrossRef]
- Cao, T.; Zhang, W.; Wang, Q.; Wang, C.; Ma, W.; Zhang, C.; Ge, M.; Tian, M.; Yu, J.; Jiao, A.; et al. Cancer SLC6A6-Mediated Taurine Uptake Transactivates Immune Checkpoint Genes and Induces Exhaustion in CD8+ T Cells. Cell 2024, 187, 2288–2304.e27. [Google Scholar] [CrossRef]
- Calabresi, P.; Goulette, F.A.; Darnowski, J.W. Taurolidine: Cytotoxic and Mechanistic Evaluation of a Novel Antineoplastic Agent1. Cancer Res. 2001, 61, 6816–6821. [Google Scholar]
- Stendel, R.; Picht, T.; Schilling, A.; Heidenreich, J.; Loddenkemper, C.; Jänisch, W.; Brock, M. Treatment of Glioblastoma with Intravenous Taurolidine. First Clinical Experience. Anticancer. Res. 2004, 24, 1143–1147. [Google Scholar]
- Jacobi, C.A.; Menenakos, C.; Braumann, C. Taurolidine—A New Drug with Anti-Tumor and Anti-Angiogenic Effects. Anti-Cancer Drugs 2005, 16, 917–921. [Google Scholar] [CrossRef]
- Neary, P.M.; Hallihan, P.; Wang, J.H.; Pfirrmann, R.W.; Bouchier-Hayes, D.J.; Redmond, H.P. The Evolving Role of Taurolidine in Cancer Therapy. Ann. Surg. Oncol. 2010, 17, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Taurine MSDS—808616—MilliporeSigma. Available online: https://www.emdmillipore.com/CA/en/product/msds/MDA_CHEM-808616?ReferrerURL=https%3A%2F%2Fwww.google.com%2F&bd=1 (accessed on 21 August 2024).
- Schwarzer, R.; Kivaranovic, D.; Mandorfer, M.; Paternostro, R.; Wolrab, D.; Heinisch, B.; Reiberger, T.; Ferlitsch, M.; Gerner, C.; Trauner, M.; et al. Randomised Clinical Study: The Effects of Oral Taurine 6g/Day vs Placebo on Portal Hypertension. Aliment. Pharmacol. Ther. 2018, 47, 86–94. [Google Scholar] [CrossRef] [PubMed]








| Group A—Induced (DMH) (43) | Group B—Non-Induced (Saline Solution) (40) | ||
|---|---|---|---|
| C (+) | Vehicle * (10) | C (-) | Vehicle * (10) |
| TAU (+) | Taurine ** (11) | TAU (-) | Taurine ** (10) |
| 5-FU (+) | 5-FU *** (11) | 5-FU (-) | 5-FU *** (10) |
| 5-FU+TAU (+) | Taurine ** e 5-FU ***; (11) | 5-FU+TAU (-) | Taurine ** e 5-FU *** (10) |
| C (-) | C (+) | TAU (-) | TAU (+) | 5FU (-) | 5FU (+) | 5FU + TAU (-) | 5FU + TAU (+) | ||
|---|---|---|---|---|---|---|---|---|---|
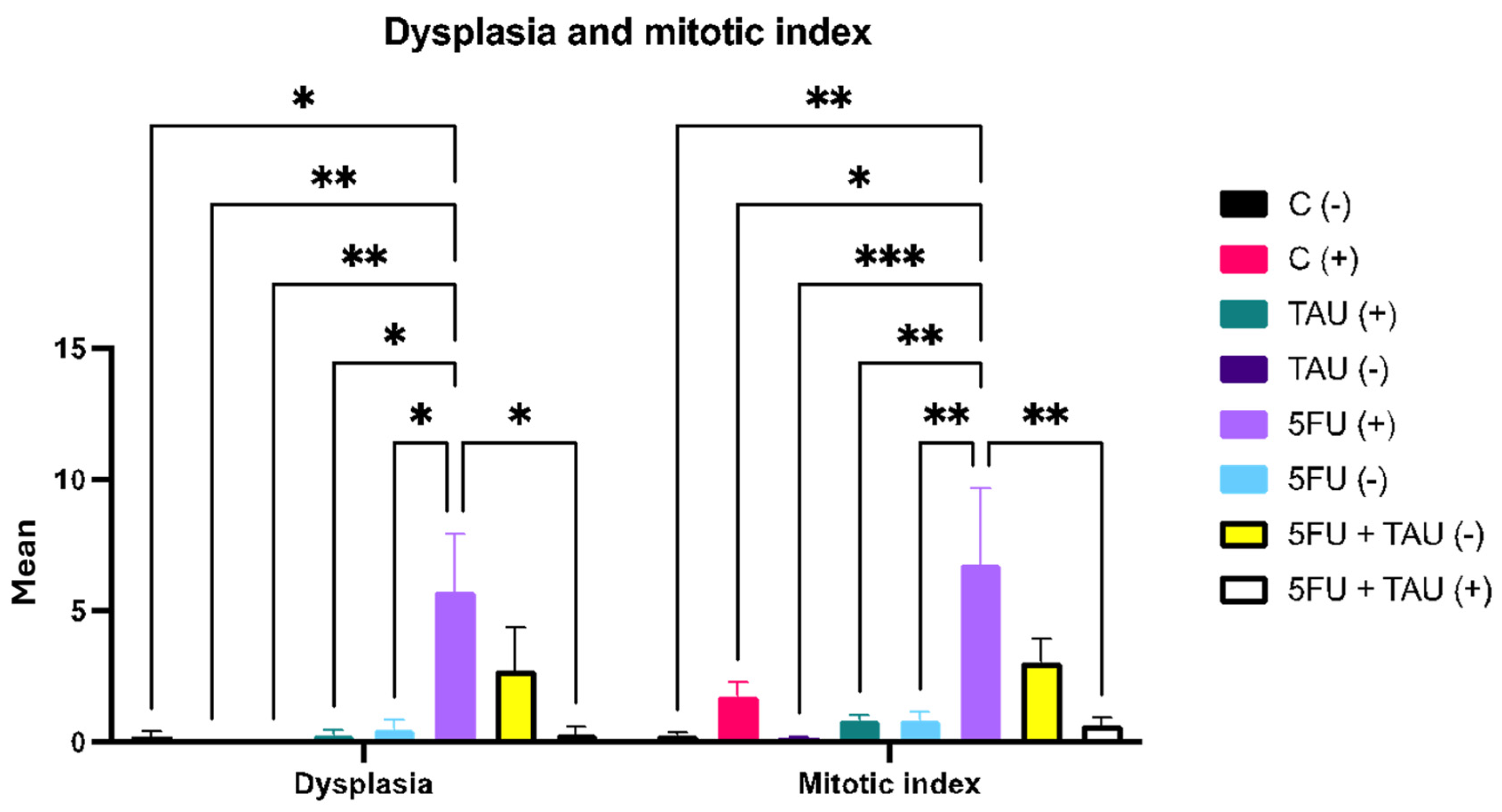
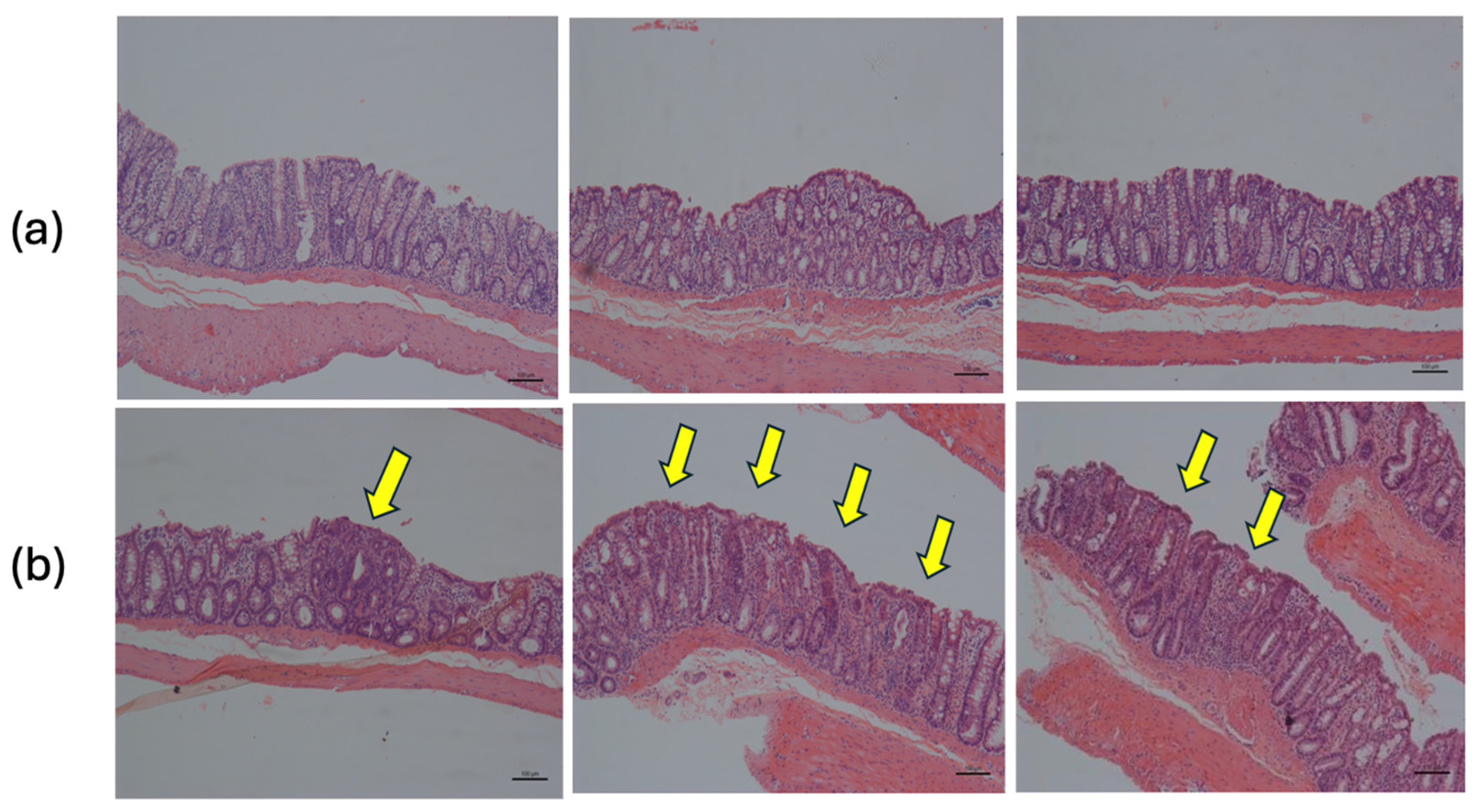
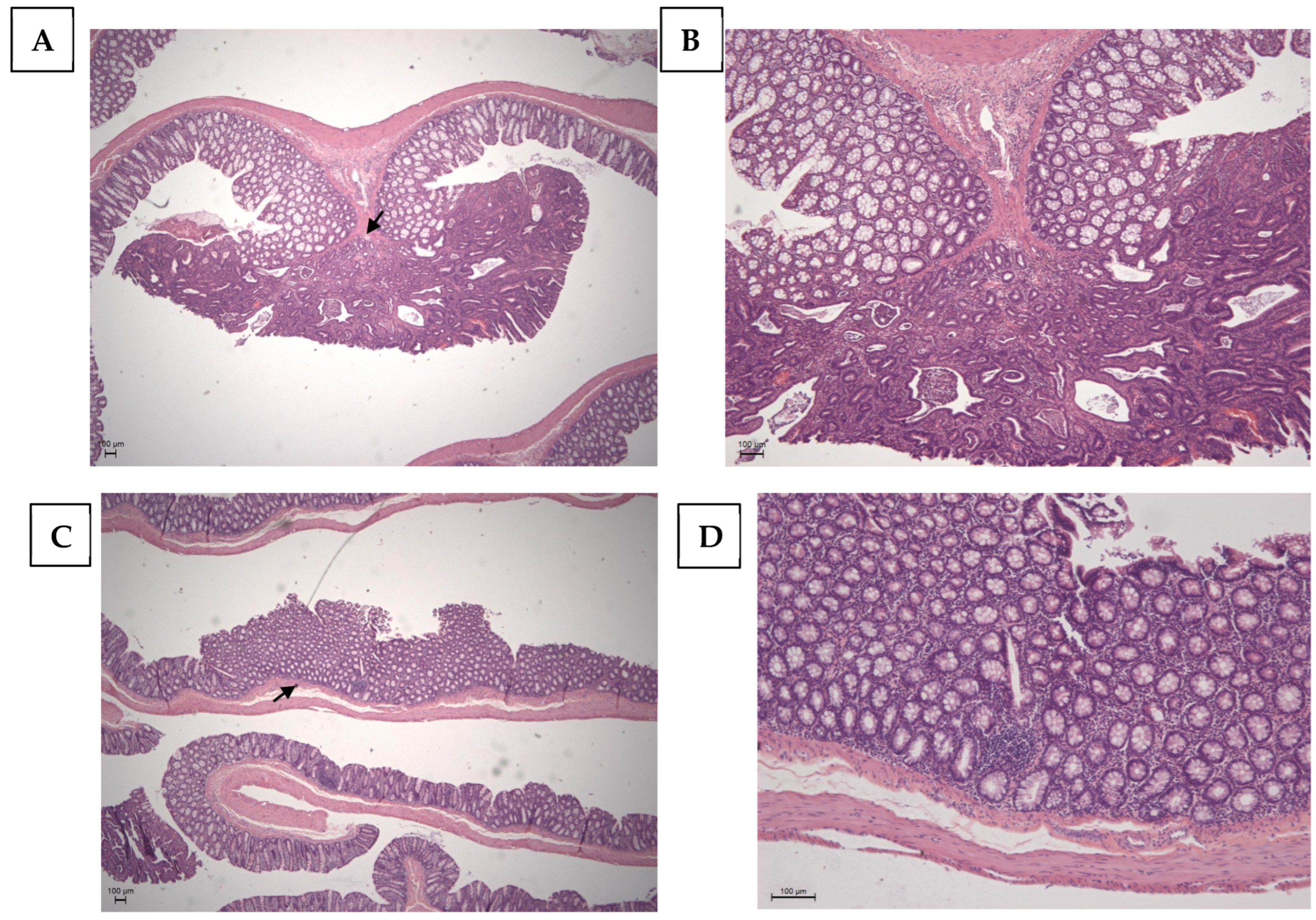
| Adenoma (A) | 0.00 | 0.10 ± 0.10 a | 0.00 | 0.09 ± 0.09 a | 0.00 | 0.18 ± 0.12 a. | 0.00 | 0.09 ± 0.09 a | |
| Adenocarcinoma (AC) | 0.00 | 0.40 ± 0.22 a,d,h | 0.00 | 0.09 ± 0.09 a,b,f,h | 0.00 | 0.45 ± 0.20 a,d,h | 0.00 | 0.00 a,b,d,f | |
| Inflammatory infiltrate | 0.59 ± 0.09 | 0.56 ± 0.06 | 0.65 ± 0.15 | 0.67 ± 0.09 | 0.94 ± 0.13 a,c,d,f,h | 0.67 ± 0.06 | 0.59 ± 0.09 | 0.71 ± 0.08 e | |
| Submucosal inflammation Intensity | 2.25 ± 0.01 | 2.20 ± 0.08 | 2.50 ± 0.11 | 2.82 ± 0.10 g | 2.50 ± 0.13 | 2.27 ± 0.12 | 2.25 ± 0.12 | 2.36 ± 0.12 | |
| Mucosa—loss of continuity | 0.40 ± 0.16 | 0.10 ± 0.10 i | 0.20 ± 0.13 a,b,e,f,g,h | 0.18± 0.12 | 0.0 g | 0.91 ± 0.09 | 0.40 ± 0.16 b,c,d,e | 0.55 ± 0.16 i | |
| Mucosa appearance | 1.80 ± 0.25 | 2.00 ± 0.15 | 1.80 ± 0.13 | 2.00 ± 0.19 | 2.10 ± 0.10 | 1.09 ± 0.09 i | 1.80 ± 0.25 | 1.36 ± 0.15 i | |
| Muscular—loss of continuity | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 ± 0.09 | |
| Muscular tissue appearance | 1.6 ± 0.16 | 1.90 ± 0.10 | 1.80 ± 0.13 | 1.82 ± 0.18 | 2.10 ± 0.10 | 1.18 ± 0.12 b,c,d,e,g | 1.60 ± 0.16 | 1.36 ± 0.15 b,c,d,e,g | |
| Mucus production | 2.20 ± 0.24 h | 2.05 ± 0.09 h | 1.95 ± 0.14 h | 1.95 ± 0.20 h | 2.30 ± 0.13 h | 1.41 ± 0.22 | 2.20 ± 0.24 h | 1.81 ± 0.19 h | |
| Group | Adenomas (%) | Adenocarcinomas (%) |
|---|---|---|
| C(+) | 20% | 80% |
| 5FU(+) | 30% | 70% |
| TAU(+) | 50% | 50% |
| 5FU+TAU(+) | 100% adenomas | 0% adenocarcinomas |
| Group | Control + | Taurine + | 5-FU + | 5-FU/Taurine + |
|---|---|---|---|---|
| n = 20 (σ) | n = 13 (σ) | n = 20 (σ) | n = 12 (σ) | |
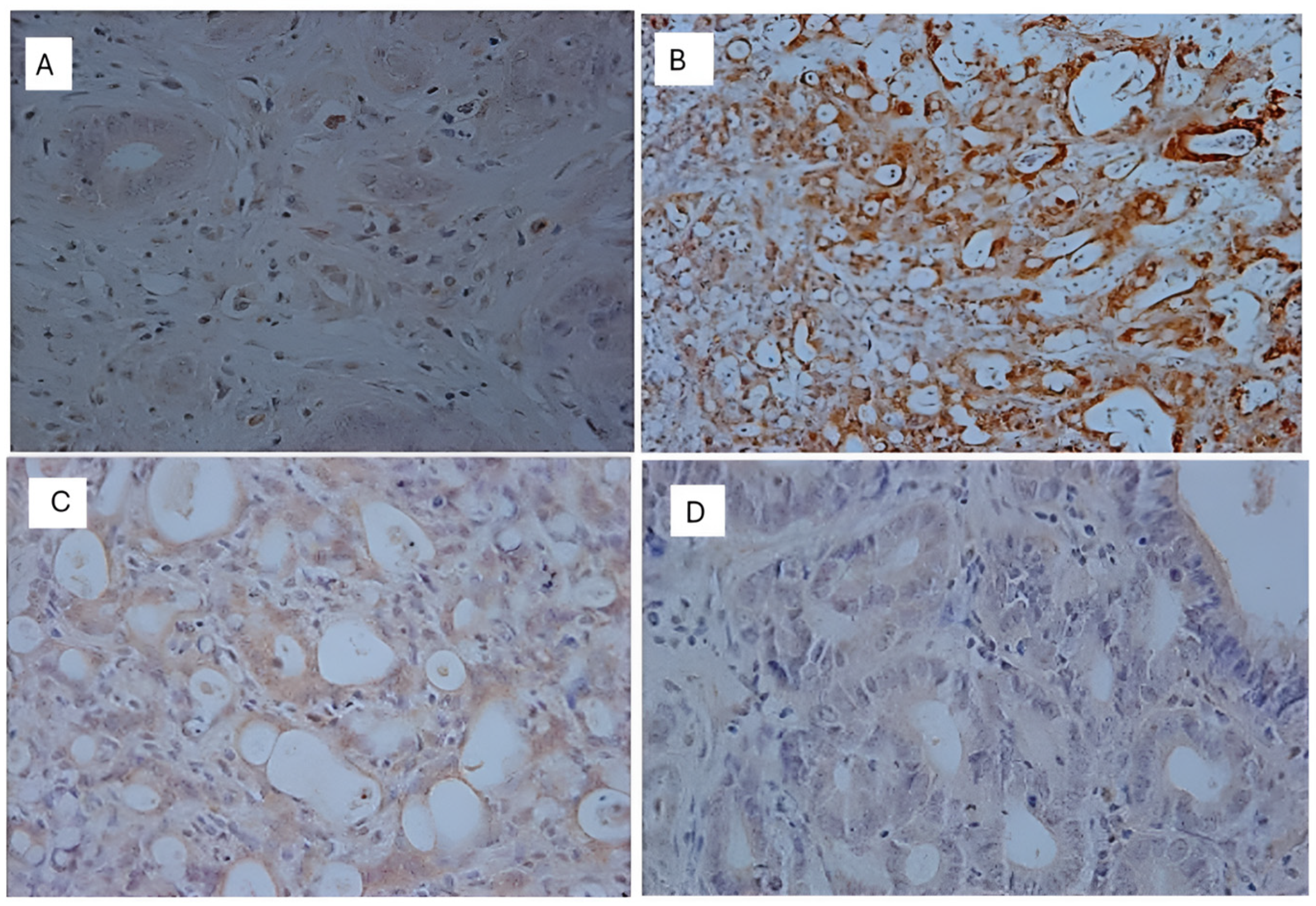
| β-catenin (Q) | 3.65 ± 0.67 | 3.92 ± 0.28 | 3.90 ± 0.31 | 4.00 ± 0.00 |
| β-catenin (I) | 2.40 ± 0.68 | 1.92 ± 0.86 | 3.00 ± 0.00 | 2.58 ± 0.79 |
| β-catenin (S) | 8.70 ± 2.87 | 7.62 ± 3.55 | 11.70 ± 0.92 | 10.33 ± 3.71 a,b ****, c ** |
| p-53 (Q) | 0.30 ± 0.73 | 0.62 ± 1.12 | 0.50 ± 2.01 | 0.33 ± 0.49 |
| p-53 (I) | 0.20 ± 0.52 | 0.54 ± 0.78 | 0.10 ± 0.45 | 0.67 ± 0.98 |
| p-53 (S) | 0.40 ± 1.05 | 1.00 ± 2.20 | 0.10 ± 0.45 | 0.67 ± 0.98 |
| K-ras (Q) | 1.05 ± 1.50 | 1.31 ± 1.49 | 1.10 ± 1.48 | 1.92 ± 1.38 |
| K-ras (I) | 0.60 ± 0.88 | 0.77 ± 0.93 | 0.60 ± 0.75 | 1.42 ± 0.90 |
| K-ras (S) | 1.50 ± 2.33 | 1.69 ± 1.97 | 1.40 ± 2.06 | 2.92 ± 2.11 a,c * |
| Ki-67 (Q) | 0.25 ± 0.44 | 0.46 ± 0.52 | 0.20 ± 0.41) | 0.45 ± 0.52 |
| Ki-67 (I) | 0.80 ± 1.44 | 1.62 ± 1.56 | 0.75 ± 1.33 | 1.09 ± 1.30 |
| Ki-67 (S) | 0.80 ± 1.44 | 1.38 ± 1.56 | 0.60 ± 1.23 | 1.00 ± 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jornada, D.H.; Boreski, D.; Chiba, D.E.; Ligeiro, D.; Luz, M.A.M.; Gabriel, E.A.; Scarim, C.B.; de Andrade, C.R.; Chin, C.M. Synergistic Enhancement of 5-Fluorouracil Chemotherapeutic Efficacy by Taurine in Colon Cancer Rat Model. Nutrients 2024, 16, 3047. https://doi.org/10.3390/nu16183047
Jornada DH, Boreski D, Chiba DE, Ligeiro D, Luz MAM, Gabriel EA, Scarim CB, de Andrade CR, Chin CM. Synergistic Enhancement of 5-Fluorouracil Chemotherapeutic Efficacy by Taurine in Colon Cancer Rat Model. Nutrients. 2024; 16(18):3047. https://doi.org/10.3390/nu16183047
Chicago/Turabian StyleJornada, Daniela Hartmann, Diogo Boreski, Diego Eidy Chiba, Denise Ligeiro, Marcus Alexandre Mendes Luz, Edmo Atique Gabriel, Cauê Benito Scarim, Cleverton Roberto de Andrade, and Chung Man Chin. 2024. "Synergistic Enhancement of 5-Fluorouracil Chemotherapeutic Efficacy by Taurine in Colon Cancer Rat Model" Nutrients 16, no. 18: 3047. https://doi.org/10.3390/nu16183047







