Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics
Abstract
:1. Introduction
2. Identification of Biomarkers Associated with MAFLD
3. MASLD Pathogenesis
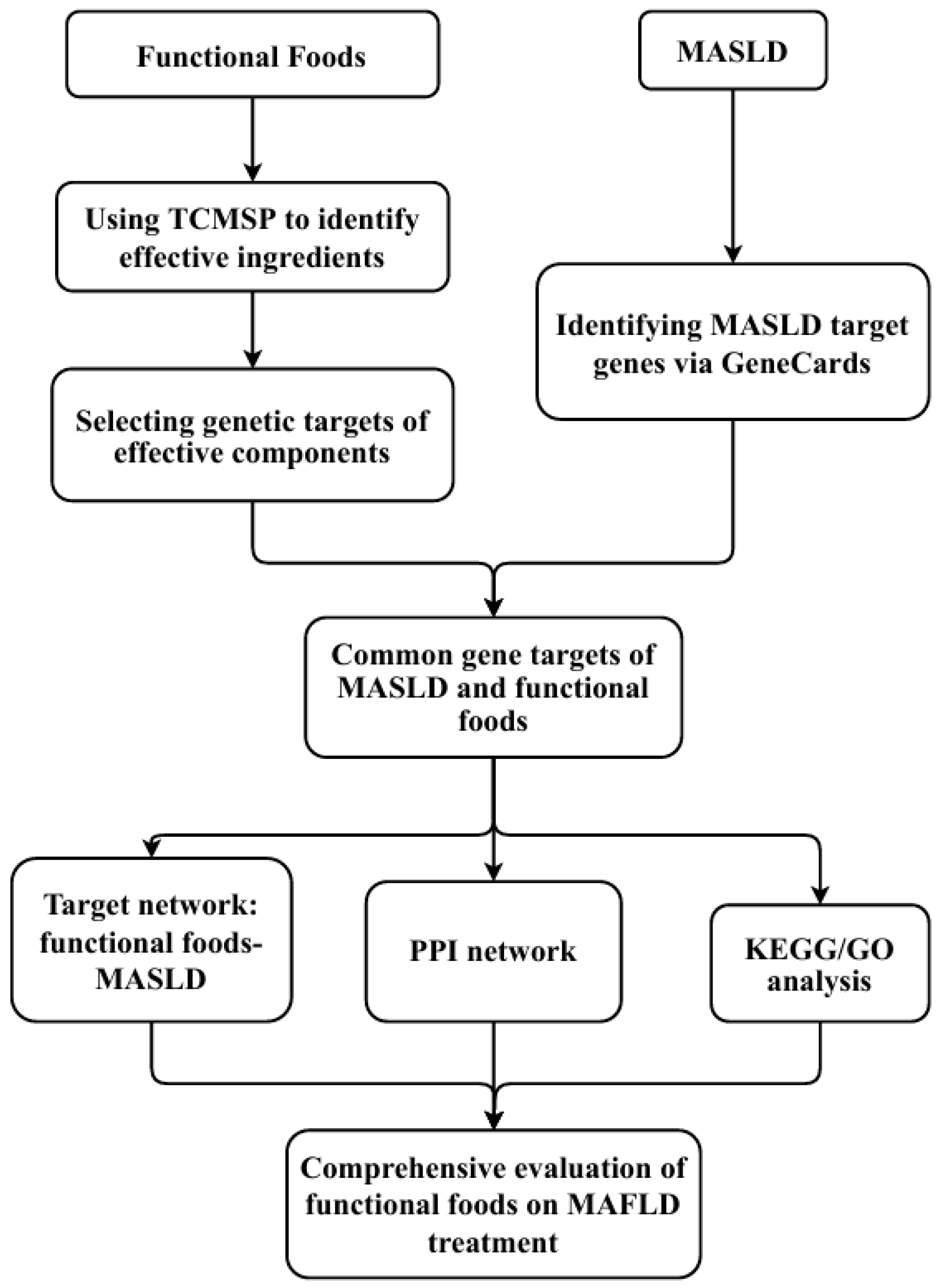
4. Impact of Food Components on MASLD: Application of Network Pharmacology
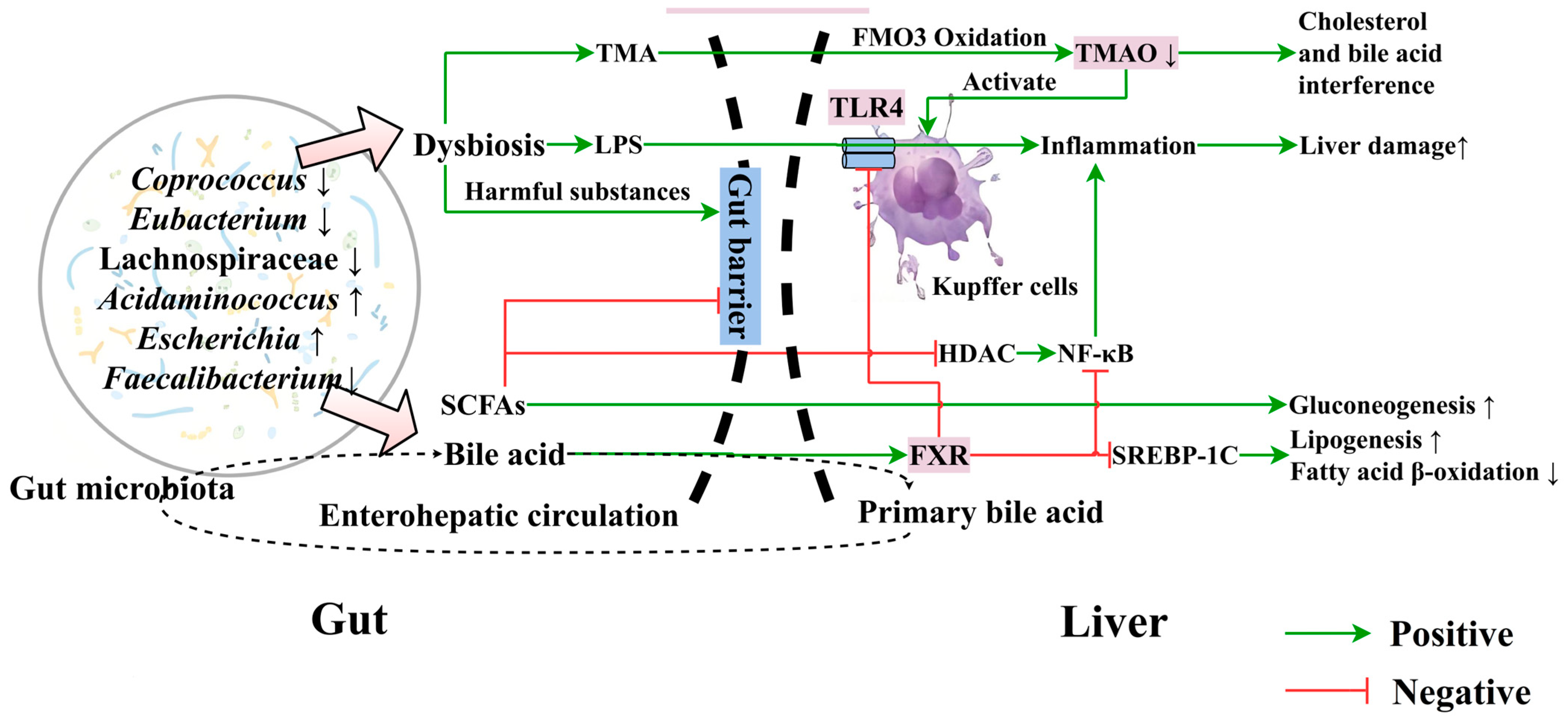
5. Association of Gut Microbiota and Their Metabolites with MASLD
5.1. Association of Gut Microbiota with MASLD
5.2. Relationship between Gut Microbial Metabolites and MASLD
5.2.1. Bile Acids
5.2.2. Short-Chain Fatty Acids (SCFAs)
5.2.3. Trimethylamine (TMA) and Trimethylamine N-Oxide (TMAO)
5.3. Effects of Food on Gut Microbiota Balance and the Gut–Liver Axis
5.4. Potential of Modulating the Gut Microbiota to Improve MASLD
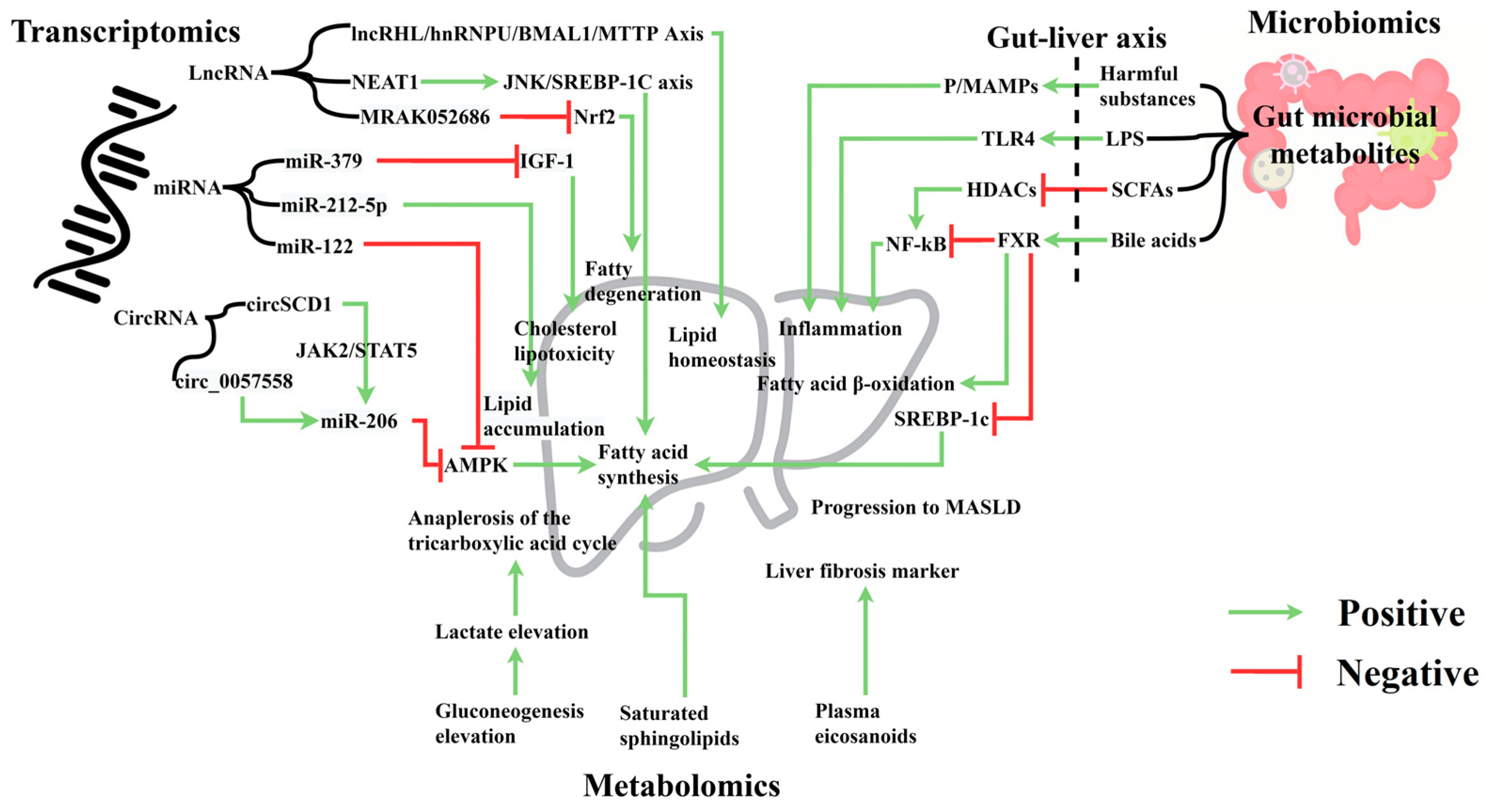
6. Omics Approaches in MASLD Research
6.1. Transcriptomics and MASLD
6.1.1. Micro RNA (miRNA)
6.1.2. lncRNA
6.1.3. circRNA
6.2. Metabolomics and MASLD
6.3. Importance of Integrated Multi-Omics Analysis
7. Impact of the Korean HFF Regulatory Framework and Its Implications for MASLD Research
8. Future Research Pathways and Final Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, J.-M.; Lu, X.-L.; Lin, X.-Y.; Hong, M.-Z.; Weng, S.; Pan, J.-S. Global burden of adult non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has been steadily increasing over the past decades and is expected to persist in the future. Transl. Gastroenterol. Hepatol. 2024, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xiao, W.; Fan, X. A review of MASLD-related hepatocellular carcinoma: Progress in pathogenesis, early detection, and therapeutic interventions. Front. Med. 2024, 11, 1410668. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Iida, S.; Katsuyama, H. Metabolic-Dysfunction-Associated Steatotic Liver Disease-Its Pathophysiology, Association with Atherosclerosis and Cardiovascular Disease, and Treatments. Int. J. Mol. Sci. 2023, 24, 15473. [Google Scholar] [CrossRef]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Liu, M.; Park, S. The Role of PNPLA3_rs738409 Gene Variant, Lifestyle Factors, and Bioactive Compounds in Nonalcoholic Fatty Liver Disease: A Population-Based and Molecular Approach towards Healthy Nutrition. Nutrients 2024, 16, 1239. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, X.; Wang, X.; Zhou, J.Y.; Park, S. Microbial Dysbiosis Linked to Metabolic Dysfunction-Associated Fatty Liver Disease in Asians: Prevotella copri Promotes Lipopolysaccharide Biosynthesis and Network Instability in the Prevotella Enterotype. Int. J. Mol. Sci. 2024, 25, 2183. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, J.; Song, L.; Xu, J.; Lu, H.; Sun, J.; Hou, J.; Chen, J.; Wu, W.; Gong, L. Multi-Omics Integration Analysis Identifies Lipid Disorder of a Non-Alcoholic Fatty Liver Disease (NAFLD) Mouse Model Improved by Zexie–Baizhu Decoction. Front. Pharmacol. 2022, 13, 858795. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Iser, D.; Ryan, M. Fatty liver disease--a practical guide for GPs. Aust. Fam. Physician 2013, 42, 444–447. [Google Scholar] [PubMed]
- Macek, P.; Biskup, M.; Terek-Derszniak, M.; Stachura, M.; Krol, H.; Gozdz, S.; Zak, M. Optimal Body Fat Percentage Cut-Off Values in Predicting the Obesity-Related Cardiovascular Risk Factors: A Cross-Sectional Cohort Study. Diab. Metab. Syndr. Obes. 2020, 13, 1587–1597. [Google Scholar] [CrossRef]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 2008, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.-f.; Wang, Q.-y.; Zhao, X.-y.; Sun, Y.-m.; Wu, X.-n.; Yang, L.-l.; Lu, Z.-z.; Ou, X.-j.; Jia, J.-d.; You, H. Histological assessment based on liver biopsy: The value and challenges in NASH drug development. Acta Pharmacol. Sin. 2022, 43, 1200–1209. [Google Scholar] [CrossRef]
- Jang, W.; Song, J.S. Non-invasive imaging methods to evaluate non-alcoholic fatty liver disease with fat quantification: A review. Diagnostics 2023, 13, 1852. [Google Scholar] [CrossRef]
- Noor, F.; Tahir ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef]
- Wei, X.; Hou, W.; Liang, J.; Fang, P.; Dou, B.; Wang, Z.; Sai, J.; Xu, T.; Ma, C.; Zhang, Q.; et al. Network Pharmacology-Based Analysis on the Potential Biological Mechanisms of Sinisan Against Non-Alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 693701. [Google Scholar] [CrossRef]
- Eguchi, Y.; Wong, G.; Akhtar, O.; Sumida, Y. Non-invasive diagnosis of non-alcoholic steatohepatitis and advanced fibrosis in Japan: A targeted literature review. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2020, 50, 645–655. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Cholongitas, E. Diagnosis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Concepts. Curr. Pharm. Des. 2018, 24, 4574–4586. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.Y.; Ma, Y.D.; Guo, X.Z.; Ye, Y.F.; Xie, C. Bile acid and receptors: Biology and drug discovery for nonalcoholic fatty liver disease. Acta Pharmacol. Sin. 2022, 43, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.; Canbay, A. Why Bile Acids Are So Important in Non-Alcoholic Fatty Liver Disease (NAFLD) Progression. Cells 2019, 8, 1358. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wei, Y.; Peng, J.; Wang, S.; Chen, G.; Sun, J. The Potential Role of C-Reactive Protein in Metabolic-Dysfunction-Associated Fatty Liver Disease and Aging. Biomedicines 2023, 11, 2711. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.; Okeefe, J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019, 6, e000890. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Muzurović, E.; Peng, C.C.-H.; Belanger, M.J.; Sanoudou, D.; Mikhailidis, D.P.; Mantzoros, C.S. Nonalcoholic fatty liver disease and cardiovascular disease: A review of shared cardiometabolic risk factors. Hypertension 2022, 79, 1319–1326. [Google Scholar] [CrossRef]
- Francque, S.; Wong, V.W.-S. NAFLD in lean individuals: Not a benign disease. Gut 2022, 71, 234–236. [Google Scholar] [CrossRef]
- Kwon, Y.; Gottmann, P.; Wang, S.; Tissink, J.; Motzler, K.; Sekar, R.; Albrecht, W.; Cadenas, C.; Hengstler, J.G.; Schürmann, A. Induction of steatosis in primary human hepatocytes recapitulates key pathophysiological aspects of metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 2024, 26. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N.; Japan Study Group of Nafld Jsg-Nafld. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2020, 21, 3863. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.C. 100th anniversary of the discovery of insulin perspective: Insulin and adipose tissue fatty acid metabolism. Am. J. Physiol. Endocrin. Metab. 2021, 320, E653–E670. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kubota, N.; Yamauchi, T.; Kadowaki, T. Role of insulin resistance in MAFLD. Int. J. Mol. Sci. 2021, 22, 4156. [Google Scholar] [CrossRef]
- Lu, Q.; Tian, X.; Wu, H.; Huang, J.; Li, M.; Mei, Z.; Zhou, L.; Xie, H.; Zheng, S. Metabolic changes of hepatocytes in NAFLD. Front. Physiol. 2021, 12, 710420. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Lundsgaard, A.-M.; Kiens, B.; Bojsen-Møller, K.N. The role of hepatic fat accumulation in glucose and insulin homeostasis dysregulation by the liver. J. Clin. Med. 2021, 10, 390. [Google Scholar] [CrossRef]
- Risi, R.; Vidal-Puig, A.; Bidault, G. An adipocentric perspective of pancreatic lipotoxicity in diabetes pathogenesis. J. Endocrinol. 2024, 262, e230313. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Z.; Qin, H. Examining the Pathogenesis of MAFLD and the Medicinal Properties of Natural Products from a Metabolic Perspective. Metabolites 2024, 14, 218. [Google Scholar] [CrossRef]
- Jaswal, K.; Todd, O.A.; Behnsen, J. Neglected gut microbiome: Interactions of the non-bacterial gut microbiota with enteric pathogens. Gut Microbes 2023, 15, 2226916. [Google Scholar] [CrossRef]
- De Filippis, A.; Ullah, H.; Baldi, A.; Dacrema, M.; Esposito, C.; Garzarella, E.U.; Santarcangelo, C.; Tantipongpiradet, A.; Daglia, M. Gastrointestinal disorders and metabolic syndrome: Dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int. J. Mol. Sci. 2020, 21, 4929. [Google Scholar] [CrossRef]
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Ren, S.-M.; Zhang, Q.-Z.; Jiang, M.; Chen, M.-L.; Xu, X.-J.; Wang, D.-M.; Pan, Y.-N.; Liu, X.-Q. Systematic characterization of the metabolites of defatted walnut powder extract in vivo and screening of the mechanisms against NAFLD by UPLC-Q-Exactive Orbitrap MS combined with network pharmacology. J. Ethnopharmacol. 2022, 285, 114870. [Google Scholar] [CrossRef] [PubMed]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the barriers: The role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef] [PubMed]
- Marroncini, G.; Naldi, L.; Martinelli, S.; Amedei, A. Gut–Liver–Pancreas Axis Crosstalk in Health and Disease: From the Role of Microbial Metabolites to Innovative Microbiota Manipulating Strategies. Biomedicines 2024, 12, 1398. [Google Scholar] [CrossRef]
- Yao, N.; Yang, Y.; Li, X.; Wang, Y.; Guo, R.; Wang, X.; Li, J.; Xie, Z.; Li, B.; Cui, W. Effects of dietary nutrients on fatty liver disease associated with metabolic dysfunction (MAFLD): Based on the intestinal-hepatic Axis. Front. Nutr. 2022, 9, 906511. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.D.; Calabrese, F.M.; D’amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal barrier and permeability in health, obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Jayachandran, M.; Qu, S. Non-alcoholic fatty liver disease and gut microbial dysbiosis-underlying mechanisms and gut microbiota mediated treatment strategies. Rev. Endocr. Metab. Disord. 2023, 24, 1189–1204. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, B. The gut-liver axis in health and disease: The role of gut microbiota-derived signals in liver injury and regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef]
- Li, R.; Mao, Z.; Ye, X.; Zuo, T. Human gut microbiome and liver diseases: From correlation to causation. Microorganisms 2021, 9, 1017. [Google Scholar] [CrossRef]
- Kirundi, J.; Moghadamrad, S.; Urbaniak, C. Microbiome-liver crosstalk: A multihit therapeutic target for liver disease. World J. Gastroenterol. 2023, 29, 1651. [Google Scholar] [CrossRef]
- Su, H.; Liu, J.; Wu, G.; Long, Z.; Fan, J.; Xu, Z.; Liu, J.; Yu, Z.; Cao, M.; Liao, N. Homeostasis of gut microbiota protects against polychlorinated biphenyl 126-induced metabolic dysfunction in liver of mice. Sci. Total Environ. 2020, 720, 137597. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile acid–gut microbiota axis in inflammatory bowel disease: From bench to bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of dietary nutrients in the modulation of gut microbiota: A narrative review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, T.; Wu, X.; Qiu, J.Y. A mixture of mulberry and silk amino acids protected against D-galactosamine induced acute liver damage by attenuating oxidative stress and inflammation in HepG2 cells and rats. Exp. Ther. Med. 2020, 19, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef]
- Park, S.; Kim, C.-J.; Ha, K.-C.; Baek, H.-I.; Yang, H.-J.; Kim, M.-J.; Park, S.-J. Efficacy and safety of aronia, red ginseng, shiitake mushroom, and nattokinase mixture on insulin resistance in prediabetic adults: A randomized, double-blinded, placebo-controlled trial. Foods 2021, 10, 1558. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875.e863. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Ha, S.; Wong, V.W.-S.; Zhang, X.; Yu, J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut 2024. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Stefano, J.; Miele, L.; Ponziani, F.R.; Souza-Basqueira, M.; Okada, L.; de Barros Costa, F.; Toda, K.; Mazo, D.; Sabino, E. Gut microbiome composition in lean patients with NASH is associated with liver damage independent of caloric intake: A prospective pilot study. Nutr. Metab. Cardiovas. Dis. 2018, 28, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Abernathy, G.A.; Dichosa, A.E.; Kumar, A. The age of next-generation therapeutic-microbe discovery: Exploiting microbe-microbe and host-microbe interactions for disease prevention. Infect. Immun. 2022, 90, e0058921. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Portincasa, P.; Wang, D.Q. Bile acid physiology. Ann. Hepatol. 2018, 16, 4–14. [Google Scholar] [CrossRef]
- Arab, J.P.; Karpen, S.J.; Dawson, P.A.; Arrese, M.; Trauner, M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017, 65, 350–362. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, L.; Zou, T.; Lian, S.; Luo, J.; Lu, Y.; Hao, H.; Xu, Y.; Xiang, Y.; Zhang, X.; et al. Ileitis promotes MASLD progression via bile acid modulation and enhanced TGR5 signaling in ileal CD8(+) T cells. J. Hepatol. 2024, 80, 764–777. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, C.; Nichols Robert, G.; Chan Siu, H.J.; Jiang, C.; Hao, R.; Smith Philip, B.; Cai, J.; Simons Margaret, N.; Hatzakis, E.; et al. Farnesoid X Receptor Signaling Shapes the Gut Microbiota and Controls Hepatic Lipid Metabolism. mSystems 2016, 1, e00070-16. [Google Scholar] [CrossRef]
- Wieland, A.; Frank, D.; Harnke, B.; Bambha, K. Systematic review: Microbial dysbiosis and nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2015, 42, 1051–1063. [Google Scholar] [CrossRef]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 2016, 45, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Renga, B.; Mencarelli, A.; Cipriani, S.; D’Amore, C.; Carino, A.; Bruno, A.; Francisci, D.; Zampella, A.; Distrutti, E.; Fiorucci, S. The bile acid sensor FXR is required for immune-regulatory activities of TLR-9 in intestinal inflammation. PLoS ONE 2013, 8, e54472. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lin, J.; Ye, C.; Guo, S.; Jiang, C. Microbial transformations of bile acids and their receptors in the regulation of metabolic dysfunction-associated steatotic liver disease. Liver Res. 2023, 7, 165–176. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, S.; Zhao, X.; Li, H.; Lin, W.; Li, B.; Lu, J.; Sun, Y.; Yuan, J. Community-metabolome correlations of gut microbiota from child-turcotte-pugh of A and B patients. Front. Microbiol. 2016, 7, 1856. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Carr, R.M.; Reid, A.E. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr. Atheroscler. Rep. 2015, 17, 500. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef]
- Brüssow, H.; Parkinson, S.J. You are what you eat. Nat. Biotechnol. 2014, 32, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef] [PubMed]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation – Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.; Hanekamp, D.; Veldhoen, M. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef]
- Corrêa, R.O.; Vieira, A.; Sernaglia, E.; Lancellotti, M.; Vieira, A.; Avila-Campos, M.J.; Rodrigues, H.; Vinolo, M. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell Microbiol. 2017, 19, e12720. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, Z.; Zhong, H.; Luo, R.; Liu, W.; Xiong, S.; Liu, Q.; Liu, M. Gut microbial metabolites in MASLD: Implications of mitochondrial dysfunction in the pathogenesis and treatment. Hepatol. Commun. 2024, 8, e0484. [Google Scholar] [CrossRef]
- Shi, C.; Pei, M.; Wang, Y.; Chen, Q.; Cao, P.; Zhang, L.; Guo, J.; Deng, W.; Wang, L.; Li, X.; et al. Changes of flavin-containing monooxygenases and trimethylamine-N-oxide may be involved in the promotion of non-alcoholic fatty liver disease by intestinal microbiota metabolite trimethylamine. Biochem. Biophys. Res. Commun. 2022, 594, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, Y.; Zhou, R.F.; Chen, X.L.; Wang, C.; Tan, X.Y.; Wang, L.J.; Zheng, R.D.; Zhang, H.W.; Ling, W.H.; et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, Z.-M.; Zhang, L.-L.; Li, J.-m.; Lv, W.-l. The role of gut microbiota in some liver diseases: From an immunological perspective. Front. Immunol. 2022, 13, 923599. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef]
- Wei, L.; Singh, R.; Ghoshal, U.C. Enterochromaffin cells–gut microbiota crosstalk: Underpinning the symptoms, pathogenesis, and pharmacotherapy in disorders of gut-brain interaction. J. Neurogastroenterol. Motil. 2022, 28, 357. [Google Scholar] [CrossRef]
- Li, X.; Guo, K.; Li, T.; Ma, S.; An, S.; Wang, S.; Di, J.; He, S.; Fu, J. 5-HT 2 receptor mediates high-fat diet-induced hepatic steatosis and very low density lipoprotein overproduction in rats. Obes. Res. Clin. Pract. 2018, 12, 16–28. [Google Scholar] [CrossRef]
- Gozzi-Silva, S.C.; Teixeira, F.M.E.; Duarte, A.J.d.S.; Sato, M.N.; Oliveira, L.d.M. Immunomodulatory role of nutrients: How can pulmonary dysfunctions improve? Front. Nutr. 2021, 8, 674258. [Google Scholar] [CrossRef]
- Gaspar, B.S.; Profir, M.; Rosu, O.A.; Ionescu, R.F.; Cretoiu, S.M. The Intestinal Microbiome in Humans: Its Role for a Healthy Life and in the Onset of Diseases. Hum. Physiol. Ann. 2024. [Google Scholar] [CrossRef]
- Chi, X.; Sun, X.; Cheng, D.; Liu, S.; Pan, C.Q.; Xing, H. Intestinal microbiome-targeted therapies improve liver function in alcohol-related liver disease by restoring bifidobacteria: A systematic review and meta-analysis. Front. Pharmacol. 2024, 14, 1274261. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- San Yeoh, B. Dysregulated Gut Microbial Fermentation of Dietary Soluble Fiber Induces Cholestatic Hepatocellular Carcinoma in Mice; The Pennsylvania State University: University Park, PA, USA, 2020. [Google Scholar]
- Stojek, M.; Jabłońska, A.; Adrych, K. The role of fecal microbiota transplantation in the treatment of inflammatory bowel disease. J. Clin. Med. 2021, 10, 4055. [Google Scholar] [CrossRef] [PubMed]
- Kaufmanna, B.; Seyfrieda, N.; Hartmanna, D.; Hartmannb, P. Probiotics, prebiotics, and synbiotics in nonalcoholic fatty liver disease and alcohol-associated liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G42–G61. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Thumu, S.C.R.; Halami, P.M. In vivo safety assessment of Lactobacillus fermentum strains, evaluation of their cholesterol-lowering ability and intestinal microbial modulation. J. Sci. Food Agric. 2020, 100, 705–713. [Google Scholar] [CrossRef]
- de Goede, O.M.; Nachun, D.C.; Ferraro, N.M.; Gloudemans, M.J.; Rao, A.S.; Smail, C.; Eulalio, T.Y.; Aguet, F.; Ng, B.; Xu, J. Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 2021, 184, 2633–2648.e2619. [Google Scholar] [CrossRef]
- Kamble, S.C.; Ghosh, P. Defining omic-based biomarker signatures of metabolic dysfunction-associated steatotic liver disease (MASLD): In vitro studies. Curr. Opin. Biomed. Eng. 2024, 30, 100534. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, L.; Zhang, Z.; Xiang, L.; Yuan, Y.; Zheng, P.; Liu, B.; Ren, X.; Bian, H.; Xie, L. The nuclear orphan receptor NR2F6 promotes hepatic steatosis through upregulation of fatty acid transporter CD36. Adv. Sci. 2020, 7, 2002273. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Yi, Z.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L.; Huang, F. Genome-wide analysis of long noncoding RNA expression profiles in patients with non-alcoholic fatty liver disease. IUBMB Life 2015, 67, 847–852. [Google Scholar] [CrossRef]
- Santosh, B.; Varshney, A.; Yadava, P.K. Non-coding RNAs: Biological functions and applications. Cell Biochem. Funct. 2015, 33, 14–22. [Google Scholar] [CrossRef]
- Long, J.-K.; Dai, W.; Zheng, Y.-W.; Zhao, S.-P. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol. Med. 2019, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiong, Y.; Sheng, Q.; Zhao, S.; Wattacheril, J.; Flynn, C.R. A micro-RNA expression signature for human NAFLD progression. J. Gastroenterol. 2016, 51, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-J.; Long, M.; Dai, R.-J. Acetylation of H3K27 activated lncRNA NEAT1 and promoted hepatic lipid accumulation in non-alcoholic fatty liver disease via regulating miR-212-5p/GRIA3. Mol. Cell. Biochem. 2022, 477, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Koda, M.; Okamoto, T.; Onoyama, T.; Miyoshi, K.; Kishina, M.; Matono, T.; Kato, J.; Tokunaga, S.; Sugihara, T. Serum miR-379 expression is related to the development and progression of hypercholesterolemia in non-alcoholic fatty liver disease. PLoS ONE 2020, 15, e0219412. [Google Scholar] [CrossRef]
- Fang, Z.; Dou, G.; Wang, L. MicroRNAs in the pathogenesis of nonalcoholic fatty liver disease. Int. J. Biol. Sci. 2021, 17, 1851. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Ji, X.; Li, B.; Wang, Y.; Huang, Y.; Zhang, X.; Yu, J.; Zou, R.; Qin, D. Long Noncoding RNA lncRHPL Regulates Hepatic VLDL Secretion by Modulating hnRNPU/BMAL1/MTTP Axis. Diabetes 2022, 71, 1915–1928. [Google Scholar] [CrossRef]
- Jin, S.-S.; Lin, C.-J.; Lin, X.-F.; Zheng, J.-Z.; Guan, H.-Q. Silencing lncRNA NEAT1 reduces nonalcoholic fatty liver fat deposition by regulating the miR-139-5p/c-Jun/SREBP-1c pathway. Ann. Hepatol. 2022, 27, 100584. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Tang, X.; Li, Y.; Xia, P.; Gao, X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015, 13, 24. [Google Scholar] [CrossRef]
- Li, P.; Shan, K.; Liu, Y.; Zhang, Y.; Xu, L.; Xu, L. CircScd1 promotes fatty liver disease via the janus kinase 2/signal transducer and activator of transcription 5 pathway. Dig. Dis. Sci. 2019, 64, 113–122. [Google Scholar] [CrossRef]
- Guo, X.-Y.; He, C.-X.; Wang, Y.-Q.; Sun, C.; Li, G.-M.; Su, Q.; Pan, Q.; Fan, J.-G. Circular RNA profiling and bioinformatic modeling identify its regulatory role in hepatic steatosis. BioMed Res. Int. 2017, 2017, 5936171. [Google Scholar] [CrossRef]
- Chen, X.; Tan, Q.-Q.; Tan, X.-R.; Li, S.-J.; Zhang, X.-X. Circ_0057558 promotes nonalcoholic fatty liver disease by regulating ROCK1/AMPK signaling through targeting miR-206. Cell Death Dis. 2021, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Meex, R.C.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, M.K.; Puri, P.; Koo, B.K.; Joo, S.K.; Jang, S.Y.; Lee, D.H.; Jung, Y.J.; Kim, B.G.; Lee, K.L. Circulating lipidomic alterations in obese and non-obese subjects with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2020, 52, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kobayashi, T.; Honda, Y.; Kessoku, T.; Tomeno, W.; Imajo, K.; Nakahara, T.; Oeda, S.; Nagaoki, Y.; Amano, Y. Metabolomic/lipidomic-based analysis of plasma to diagnose hepatocellular ballooning in patients with non-alcoholic fatty liver disease: A multicenter study. Hepatol. Res. 2020, 50, 955–965. [Google Scholar] [CrossRef]
- Caussy, C.; Chuang, J.-C.; Billin, A.; Hu, T.; Wang, Y.; Subramanian, G.M.; Djedjos, C.S.; Myers, R.P.; Dennis, E.A.; Loomba, R. Plasma eicosanoids as noninvasive biomarkers of liver fibrosis in patients with nonalcoholic steatohepatitis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820923904. [Google Scholar] [CrossRef]
- McGlinchey, A.J.; Govaere, O.; Geng, D.; Ratziu, V.; Allison, M.; Bousier, J.; Petta, S.; de Oliviera, C.; Bugianesi, E.; Schattenberg, J.M. Metabolic signatures across the full spectrum of non-alcoholic fatty liver disease. JHEP Rep. 2022, 4, 100477. [Google Scholar] [CrossRef]
- Haam, J.-H.; Lee, Y.K.; Suh, E.; Kim, Y.-S. Characteristics of Urine Organic Acid Metabolites in Nonalcoholic Fatty Liver Disease Assessed Using Magnetic Resonance Imaging with Elastography in Korean Adults. Diagnostics 2022, 12, 1199. [Google Scholar] [CrossRef]
- Dong, S.; Zhan, Z.-Y.; Cao, H.-Y.; Wu, C.; Bian, Y.-Q.; Li, J.-Y.; Cheng, G.-H.; Liu, P.; Sun, M.-Y. Urinary metabolomics analysis identifies key biomarkers of different stages of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 2771. [Google Scholar] [CrossRef]
- Kim, H.Y. Recent advances in nonalcoholic fatty liver disease metabolomics. Clin. Mol. Hepatol. 2021, 27, 553. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Dong, B. Molecular mechanisms in MASLD/MASH related HCC. Hepatology 2024. [Google Scholar] [CrossRef]
| Name | Normal Values | Description |
|---|---|---|
| Percent body fat | ≤25.8% men ≤37.1% women | Body fat percent greater than the reference range increases the risk of cardiovascular and associated diseases. |
| Hemoglobin A1c (HbA1c) | <5.7% | Elevated levels indicate pre-diabetes or diabetes, depending on the level of elevation. It is a major risk factor for MASLD. |
| Alanine transaminase (ALT) | 7–56 IU/L | Elevation usually indicates liver damage but is not always indicative of liver injury alone. |
| Aspartate transaminase (AST) | 0–35 IU/L | Less specific to the liver than ALT and can reflect damage in many tissues, including the liver. |
| AST/ALT ratio | 1 (1:1) | A higher or lower ratio is a better indicator of liver damage than separately. |
| Gamma-glutamyl transferase (GGT) | 9 to 85 IU/L | Frequently elevated in MASLD, but it is not exclusively indicative of liver disease. |
| L-lactate dehydrogenase | 0.4–1.7 µmol/L | Elevated levels are frequently indicative of liver disease. |
| Total bilirubin | 2–21 µmol/L | Elevated concentrations indicate liver damage |
| Prothrombin time (PT) | 25–41 s | Indicator of the status of blood clotting factor, and a longer time suggests a probable liver injury. |
| Albumin | 3.5 to 5.3 g/dL | Albumin is a protein exclusively made by the liver, and low concentrations are indicative of impaired liver function. |
| Uric acid | M 2.1–8.5 mg/dL F 2.07–7.0 mg/dL | Elevated levels are believed to be highly predictive of steatotic liver disease. |
| Total bile acids | 1–2 μg per mL | Moderate elevation and changes in bile acid compositions by changing farnesoid X receptor (FXR) activity in MASLD [22,23]. |
| C-reactive protein (CRP) | <3 mg/dL | Inflammatory marker for MASLD [24]. |
| Trimethylamine N-oxide (TMAO) | <6 μmol/L | TMA is produced by gut bacteria from dietary precursors (choline, L-carnitine, betaine) and quickly converted to TMAO in the liver. TMA is very low in the serum of a healthy person [25]. |
| Fecal and serum butyrate | Not Assigned | Their concentrations are lower in MASLD patients by 20–50% than in healthy persons [26]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Jung, E.-S.; Chae, S.-W.; Jung, S.-J.; Daily, J.W.; Park, S. Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics. Nutrients 2024, 16, 3061. https://doi.org/10.3390/nu16183061
Yuan H, Jung E-S, Chae S-W, Jung S-J, Daily JW, Park S. Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics. Nutrients. 2024; 16(18):3061. https://doi.org/10.3390/nu16183061
Chicago/Turabian StyleYuan, Heng, Eun-Soo Jung, Soo-Wan Chae, Su-Jin Jung, James W. Daily, and Sunmin Park. 2024. "Biomarkers for Health Functional Foods in Metabolic Dysfunction-Associated Steatotic Liver Disorder (MASLD) Prevention: An Integrative Analysis of Network Pharmacology, Gut Microbiota, and Multi-Omics" Nutrients 16, no. 18: 3061. https://doi.org/10.3390/nu16183061







