Effects of Maltodextrin–Fructose Supplementation on Inflammatory Biomarkers and Lipidomic Profile Following Endurance Running: A Randomized Placebo-Controlled Cross-Over Trial
Abstract
:1. Introduction
2. Materials and Methods
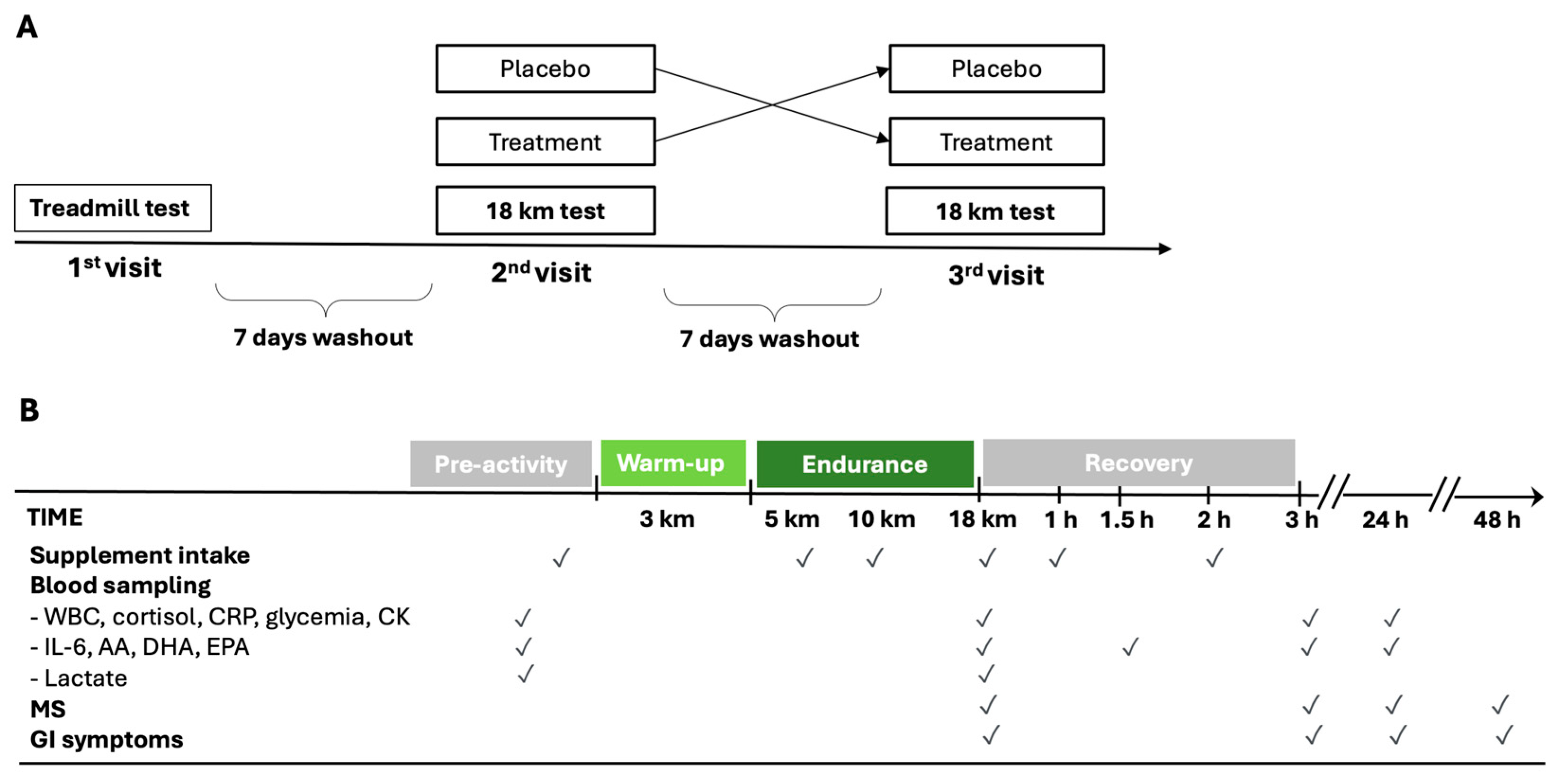
2.1. Study Design and Participants
2.2. Randomization and Procedures
2.3. Supplementation and Dietary Instruction
2.4. Glycemia, Inflammatory, and Damage Biomarkers
2.5. Lipidomic Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Population
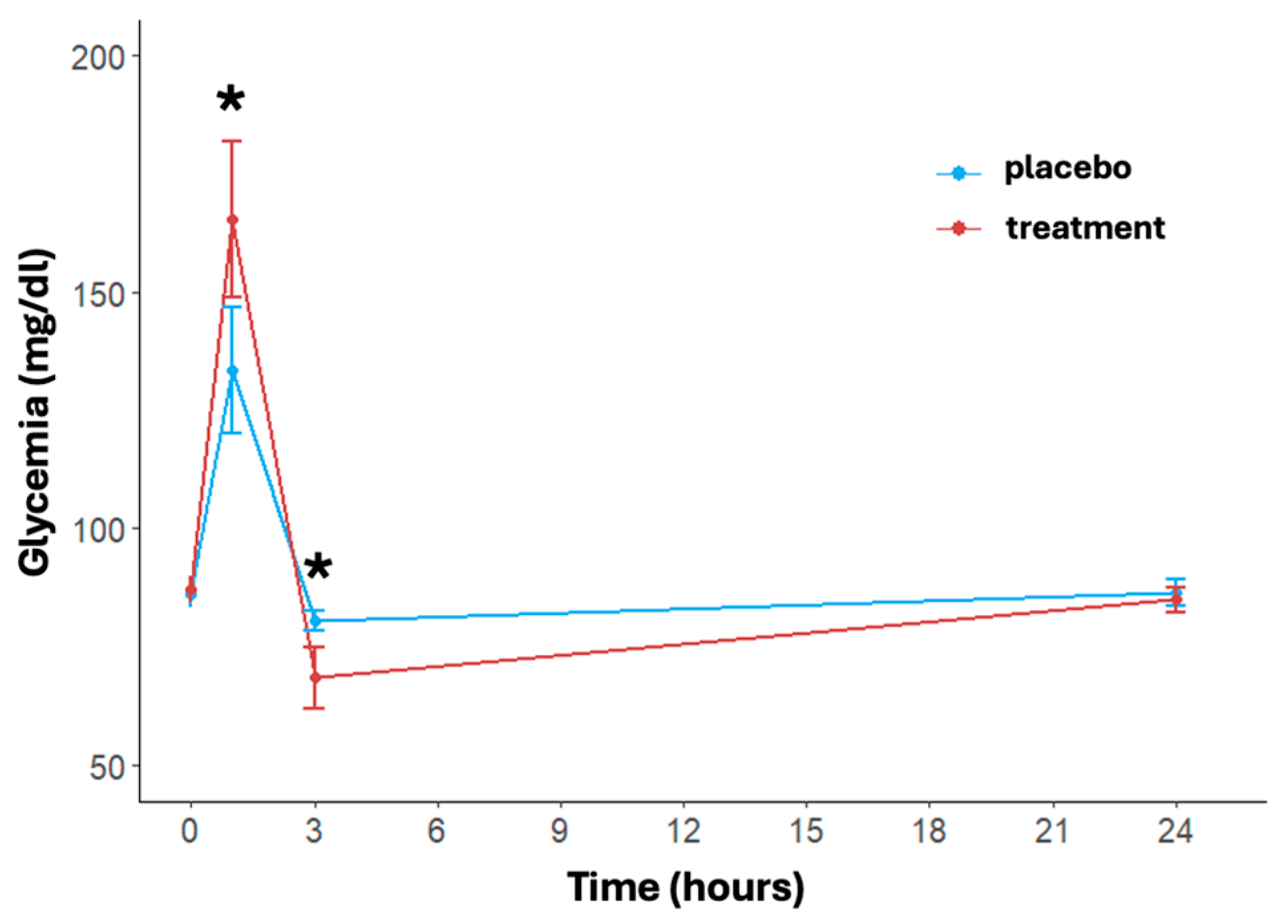
3.2. Glycemia
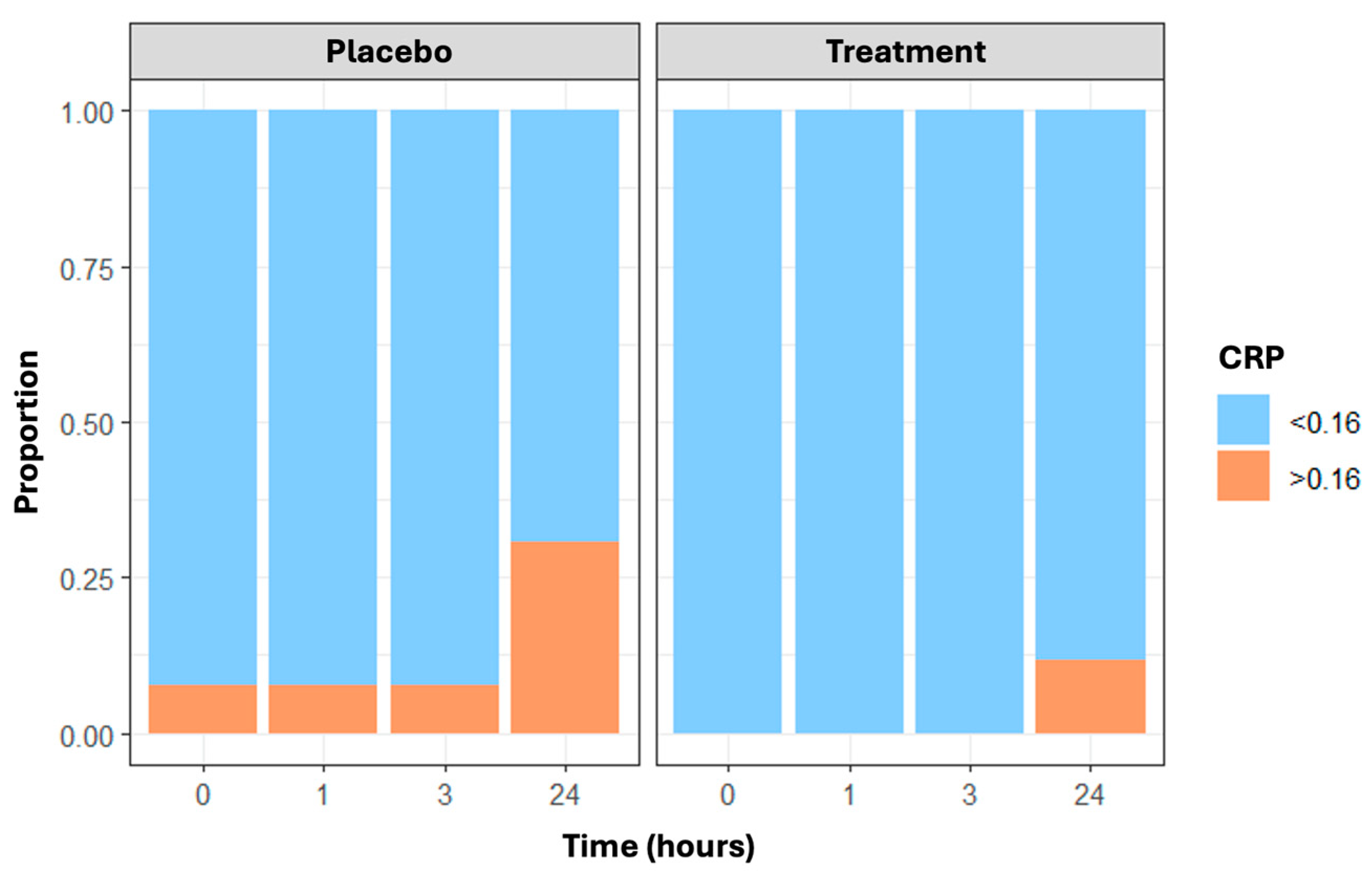
3.3. Inflammatory Biomarkers
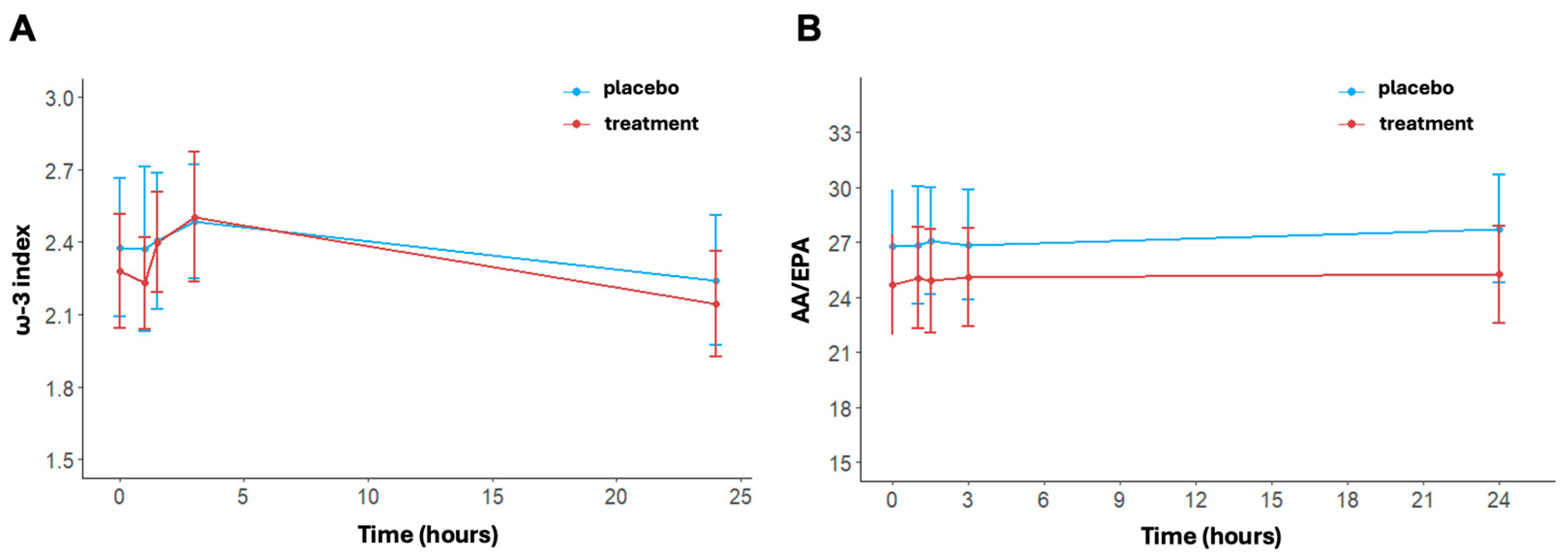
3.4. Lipidomic Profile, ω-3 Index and AA/EPA Ratio
3.5. Creatine Phosphokinase (CK)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Hammad, A.A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Al Saoud, H.; et al. Athletes’ nutritional demands: A narrative review of nutritional requirements. Front. Nutr. 2023, 10, 1331854. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rodríguez, A.; Belinchón-deMiguel, P.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F.; Martínez-Guardado, I.; Villanueva-Tobaldo, C.V.; Clemente-Suárez, V.J. Advances in Understanding the Interplay between Dietary Practices, Body Composition, and Sports Performance in Athletes. Nutrients 2024, 16, 571. [Google Scholar] [CrossRef]
- Jeukendrup, A. A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Med. 2014, 44 (Suppl. S1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Pedersen, P.K.; Rose, P.; Richter, E.A. Carbohydrate supercompensation and muscle glycogen utilization during exhaustive running in highly trained athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.M.; Costill, D.L.; Fink, W.J.; Miller, J.M. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int. J. Sports Med. 1981, 2, 114–118. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J.C. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.; Jeukendrup, A.E. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003, 33, 117–144. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–610. [Google Scholar] [CrossRef]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the Regulation of Inflammatory Responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.S.; de Moura Mello Antunes, B.; Luis Araujo Minari, A.; Vagner Thomatieli Dos Santos, R.; Rosa Neto, J.C.; Santos Lira, F. Macrophage Polarization: Implications on Metabolic Diseases and the Role of Exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef]
- Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Nieman, D.C.; Henson, D.A.; Butterworth, D.E.; Schmitt, R.L.; Bailey, E.M.; Warren, B.J.; Utter, A.; Davis, J.M. Carbohydrate and the cytokine response to 2.5 h of running. J. Appl. Physiol. 1997, 82, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J. Appl. Physiol. 2003, 94, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.; Gleeson, M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 465–479. [Google Scholar] [CrossRef]
- Nieman, D.C.; Mitmesser, S.H. Potential Impact of Nutrition on Immune System Recovery from Heavy Exertion: A Metabolomics Perspective. Nutrients 2017, 9, 513. [Google Scholar] [CrossRef]
- Bermon, S.; Castell, L.; Calder, P.; Bishop, N.; Blomstrand, E.; Mooren, F.; Krüger, K.; Kavazis, A.; Quindry, J.; Senchina, D.; et al. Consensus Statement Immunonutrition and Exercise. Exerc. Immunol. Rev. 2017, 23, 8–50. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand. J. Med. Sci. Sports 2023, 33, 4. [Google Scholar] [CrossRef]
- Steensberg, A.; Febbraio, M.A.; Osada, T.; Schjerling, P.; Van Hall, G.; Saltin, B.; Pedersen, B.K. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J. Physiol. 2001, 537, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Morton, J.P. Ramping up the signal: Promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin. Exp. Pharmacol. Physiol. 2014, 41, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Gobbi, N.; Ali, A.; Berjisian, E.; Hamidvand, A.; Forbes, S.C.; Koozehchian, M.S.; Karayigit, R.; Saunders, B. Carbohydrates and Endurance Exercise: A Narrative Review of a Food First Approach. Nutrients 2023, 15, 1367. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Yang, F.; Jensen, J.; Gao, R.; Yi, L.; Qiu, J. Effects of carbohydrate and protein supplement strategies on endurance capacity and muscle damage of endurance runners: A double blind, controlled crossover trial. J. Int. Soc. Sports Nutr. 2022, 19, 623. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of exercise training on C reactive protein: A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 2017, 51, 670–676. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Medoro, A.; Buonsenso, A.; Centorbi, M.; Calcagno, G.; Scapagnini, G.; Fiorilli, G.; Davinelli, S. Omega-3 Index as a Sport Biomarker: Implications for Cardiovascular Health, Injury Prevention, and Athletic Performance. J. Funct. Morphol. Kinesiol. 2024, 9, 91. [Google Scholar] [CrossRef]
- Davinelli, S.; Intrieri, M.; Corbi, G.; Scapagnini, G. Metabolic indices of polyunsaturated fatty acids: Current evidence, research controversies, and clinical utility. Crit. Rev. Food Sci. Nutr. 2021, 61, 259–274. [Google Scholar] [CrossRef]
- Nieman, D.C. Influence of carbohydrate on the immune response to intensive, prolonged exercise. Exerc. Immunol. Rev. 1998, 4, 64–76. [Google Scholar] [PubMed]
- Robson-Ansley, P.; Walshe, I.; Ward, D. The effect of carbohydrate ingestion on plasma interleukin-6, hepcidin and iron concentrations following prolonged exercise. Cytokine 2011, 53, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Townsend, M.A.; Trinder, D.; Peeling, P. The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur. J. Appl. Physiol. 2012, 112, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Ihalainen, J.K.; Vuorimaa, T.; Puurtinen, R.; Hämäläinen, I.; Mero, A.A. Effects of carbohydrate ingestion on acute leukocyte, cortisol, and interleukin-6 response in high-intensity long-distance running. J. Strength Cond. Res. 2014, 28, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–181. [Google Scholar] [CrossRef]
- Cadario, F.; Pozzi, E.; Rizzollo, S.; Stracuzzi, M.; Beux, S.; Giorgis, A.; Carrera, D.; Fullin, F.; Riso, S.; Rizzo, A.M.; et al. Vitamin D and ω-3 Supplementations in Mediterranean Diet During the 1st Year of Overt Type 1 Diabetes: A Cohort Study. Nutrients 2019, 11, 2158. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Gojanovich, G.; Davis, J.M.; Murphy, E.A.; Mayer, E.P.; Pearce, S.; Dumke, C.L.; Utter, A.C.; McAnulty, S.R.; et al. Influence of carbohydrate on immune function following 2 h cycling. Res. Sports Med. 2006, 14, 225–237. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef]
- Jentjens, R.L.P.G.; Venables, M.C.; Jeukendrup, A.E. Oxidation of exogenous glucose, sucrose, and maltose during prolonged cycling exercise. J. Appl. Physiol. 2004, 96, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.P.G.; Moseley, L.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and fructose during exercise. J. Appl. Physiol. 2004, 96, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.P.G.; Shaw, C.; Birtles, T.; Waring, R.H.; Harding, L.K.; Jeukendrup, A.E. Oxidation of combined ingestion of glucose and sucrose during exercise. Metabolism 2005, 54, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.L.P.G.; Jeukendrup, A.E. High rates of exogenous carbohydrate oxidation from a mixture of glucose and fructose ingested during prolonged cycling exercise. Br. J. Nutr. 2005, 93, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wallis, G.A.; Yeo, S.E.; Blannin, A.K.; Jeukendrup, A.E. Dose-response effects of ingested carbohydrate on exercise metabolism in women. Med. Sci. Sports Exerc. 2007, 39, 131–138. [Google Scholar] [CrossRef]
- Rehrer, N.J.; van Kemenade, M.; Meester, W.; Brouns, F.; Saris, W.H. Gastrointestinal complaints in relation to dietary intake in triathletes. Int. J. Sport Nutr. 1992, 2, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.P.F.; Wiersma, J.W.C.; Koerselman, J.; Akkermans, L.M.A.; Bol, E.; Mosterd, W.L.; De Vries, W.R. The effect of a sports drink on gastroesophageal reflux during a run-bike-run test. Int. J. Sports Med. 2000, 21, 65–70. [Google Scholar] [CrossRef]
- Howlett, K.F.; Watt, M.J.; Hargreaves, M.; Febbraio, M.A. Regulation of glucose kinetics during intense exercise in humans: Effects of α- and β-adrenergic blockade. Metabolism 2003, 52, 1615–1620. [Google Scholar] [CrossRef]
- Davinelli, S.; Intrieri, M.; Ali, S.; Righetti, S.; Mondazzi, L.; Scapagnini, G.; Corbi, G. Omega-3 index and AA/EPA ratio as biomarkers of running-related injuries: An observational study in recreational runners. Eur. J. Sport Sci. 2023, 23, 134–142. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Intrieri, M.; Saso, L.; Scapagnini, G.; Kang, J.X. Targeting NRF2–KEAP1 axis by Omega-3 fatty acids and their derivatives: Emerging opportunities against aging and diseases. Free Radic. Biol. Med. 2022, 193, 736–750. [Google Scholar] [CrossRef]
- Janse De Jonge, X.A.K. Effects of the menstrual cycle on exercise performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef] [PubMed]


| Placebo | Treatment | p-Value * | |
|---|---|---|---|
| 3 km test velocity (km/h) | 13.31 ± 0.75 | 13.44 ± 0.71 | 0.509 |
| 15 km test velocity (km/h) | 15.59 ± 1.46 | 15.59 ± 1.45 | 0.992 |
| Mean heart rate during 3 km (bpm) | 145.96 ± 10.1 | 146.64 ± 11.96 | 0.829 |
| Mean heart rate during 15 km (bpm) | 171.38 ± 8.85 | 171.23 ± 9.18 | 0.951 |
| Maximal heart rate during 15 km (bpm) | 179.58 ± 9.70 | 179.46 ± 8.56 | 0.964 |
| Rating of perceived exertion | 7.15 ± 1.74 | 6.96 ± 1.66 | 0.685 |
| Baseline lactate (mmol/L) | 1.35 ± 0.21 | 1.32 ± 0.24 | 0.629 |
| Post-running lactate (mmol/L) | 5.14 ± 1.95 | 4.64 ± 1.44 | 0.298 |
| Post–pre test delta weight (kg) | −1.33 ± 0.43 | −1.28 ± 0.40 | 0.690 |
| GI symptoms (n) | 6 (23.1) | 4 (15.4) | 0.481 |
| Overall | Placebo | Treatment | p-Value * | |
|---|---|---|---|---|
| Inflammatory and damage markers at baseline | ||||
| White blood cells (n × 109) | 4.96 ± 1.35 | 5.04 ± 1.47 | 4.88 ± 1.25 | 0.671 |
| Neutrophil (n × 109) | 2.58 ± 1.11 | 2.71 ± 1.39 | 2.45 ± 0.74 | 0.409 |
| Cortisol (nmol/L) | 22.36 ± 5.14 | 21.52 ± 5.50 | 23.19 ± 4.71 | 0.247 |
| IL-6 (pg/mL) | 2.49 ± 1.13 | 2.31 ± 0.66 | 2.66 ± 1.45 | 0.269 |
| CRP (mg/dL) | 0.16 ± 0.18 | 0.19 ± 0.26 | 0.14 ± 0.03 | 0.333 |
| CK (IU/L) | 233.77 ± 239.71 | 259.92 ± 321.55 | 207.62 ± 111.37 | 0.437 |
| Lipidomic profile at baseline | ||||
| ω-3 index (%) | 2.33 ± 0.81 | 2.38 ± 0.89 | 2.28 ± 0.73 | 0.669 |
| EPA (%) | 0.36 ± 0.13 | 0.34 ± 0.13 | 0.38 ± 0.14 | 0.329 |
| DHA (%) | 1.97 ± 0.72 | 2.04 ± 0.80 | 1.90 ± 0.64 | 0.509 |
| AA (%) | 8.36 ± 1.64 | 8.32 ± 1.80 | 8.40 ± 1.51 | 0.878 |
| AA/EPA ratio | 25.74 ± 8.96 | 26.80 ± 9.50 | 24.68 ± 8.45 | 0.399 |
| AA (%) | DHA (%) | EPA (%) | ||||
|---|---|---|---|---|---|---|
| Timepoints | Placebo | Treatment | Placebo | Treatment | Placebo | Treatment |
| Baseline | 8.32 ± 1.80 # | 8.40 ± 1.51 # | 2.04 ± 0.80 | 1.90 ± 0.64 # | 0.34 ± 0.13 # | 0.37 ± 0.14 |
| Post-running | 8.55 ± 1.67 # | 8.45 ± 1.07 | 2.02 ± 1.00 | 1.85 ± 0.50 # | 0.34 ± 0.12 | 0.37 ± 0.14 |
| After 1.5 h | 8.62 ± 1.54 | 8.69 ± 1.18 # | 2.06 ± 0.82 | 2.01 ± 0.57 # | 0.34 ± 0.11 * | 0.38 ± 0.14 *,# |
| After 3 h | 9.08 ± 1.54 # | 8.95 ± 1.43 # | 2.12 ± 0.66 | 2.11 ± 0.73 # | 0.36 ± 0.12 # | 0.39 ± 0.15 # |
| After 24 h | 8.45 ± 1.69 * | 8.10 ± 1.22 *,# | 1.91 ± 0.75 | 1.79 ± 0.59 # | 0.32 ± 0.12 # | 0.35 ± 0.12 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righetti, S.; Medoro, A.; Graziano, F.; Mondazzi, L.; Martegani, S.; Chiappero, F.; Casiraghi, E.; Petroni, P.; Corbi, G.; Pina, R.; et al. Effects of Maltodextrin–Fructose Supplementation on Inflammatory Biomarkers and Lipidomic Profile Following Endurance Running: A Randomized Placebo-Controlled Cross-Over Trial. Nutrients 2024, 16, 3078. https://doi.org/10.3390/nu16183078
Righetti S, Medoro A, Graziano F, Mondazzi L, Martegani S, Chiappero F, Casiraghi E, Petroni P, Corbi G, Pina R, et al. Effects of Maltodextrin–Fructose Supplementation on Inflammatory Biomarkers and Lipidomic Profile Following Endurance Running: A Randomized Placebo-Controlled Cross-Over Trial. Nutrients. 2024; 16(18):3078. https://doi.org/10.3390/nu16183078
Chicago/Turabian StyleRighetti, Stefano, Alessandro Medoro, Francesca Graziano, Luca Mondazzi, Serena Martegani, Francesco Chiappero, Elena Casiraghi, Paolo Petroni, Graziamaria Corbi, Riccardo Pina, and et al. 2024. "Effects of Maltodextrin–Fructose Supplementation on Inflammatory Biomarkers and Lipidomic Profile Following Endurance Running: A Randomized Placebo-Controlled Cross-Over Trial" Nutrients 16, no. 18: 3078. https://doi.org/10.3390/nu16183078
APA StyleRighetti, S., Medoro, A., Graziano, F., Mondazzi, L., Martegani, S., Chiappero, F., Casiraghi, E., Petroni, P., Corbi, G., Pina, R., Scapagnini, G., Davinelli, S., & Ricordi, C. (2024). Effects of Maltodextrin–Fructose Supplementation on Inflammatory Biomarkers and Lipidomic Profile Following Endurance Running: A Randomized Placebo-Controlled Cross-Over Trial. Nutrients, 16(18), 3078. https://doi.org/10.3390/nu16183078







