Investigating the Impact of Nutrition and Oxidative Stress on Attention Deficit Hyperactivity Disorder
Abstract
:1. Introduction
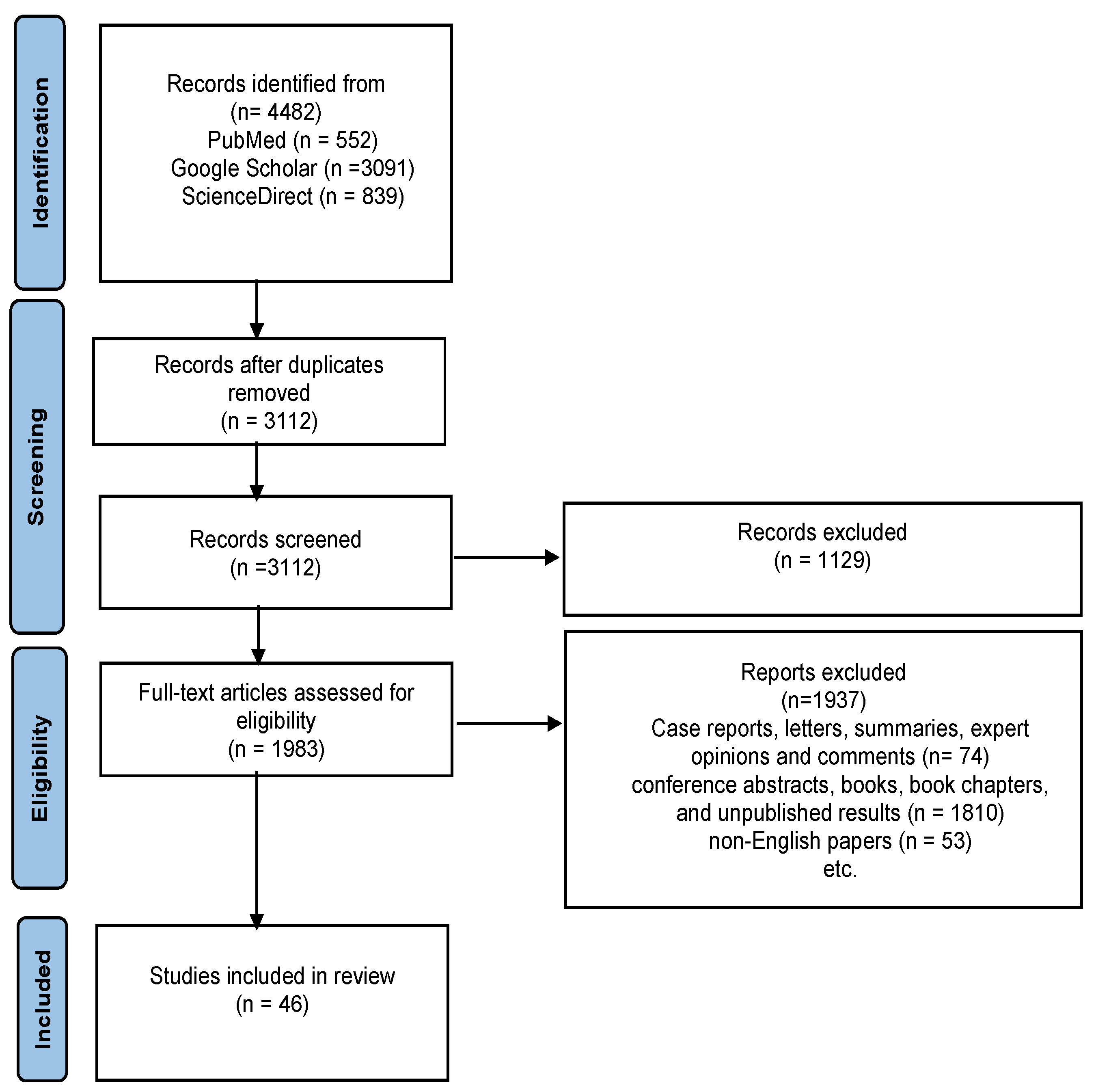
2. Methodology
2.1. Search Strategy
2.2. Excluding Criteria
2.3. Data Extraction
2.4. Data Synthesis
3. ADHD
3.1. Factors Influencing ADHD Symptoms
3.2. Factors Influencing the Pathogenesis of ADHD
4. Oxidative Stress in ADHD
5. Food Diet in ADHD
6. ADHD vs. Gut−Brain Axis
7. Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weibel, S.; Menard, O.; Ionita, A.; Boumendjel, M.; Cabelguen, C.; Kraemer, C.; Micoulaud-Franchi, J.-A.; Bioulac, S.; Perroud, N.; Sauvaget, A.; et al. Practical Considerations for the Evaluation and Management of Attention Deficit Hyperactivity Disorder (ADHD) in Adults. Encephale 2020, 46, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-Deficit/Hyperactivity Disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Kooij, J.; Bijlenga, D.; Salerno, L.; Jaeschke, R.; Bitter, I.; Balázs, J.; Thome, J.; Dom, G.; Kasper, S.; Filipe, C.N.; et al. Updated European Consensus Statement on Diagnosis and Treatment of Adult ADHD. Eur. Psychiatry 2019, 56, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Glatt, S.J. A Comparison of the Efficacy of Medications for Adult Attention-Deficit/Hyperactivity Disorder Using Meta-Analysis of Effect Sizes. J. Clin. Psychiatry 2010, 71, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef]
- Lopresti, A.L. Oxidative and Nitrosative Stress in ADHD: Possible Causes and the Potential of Antioxidant-Targeted Therapies. ADHD Atten. Deficit Hyperact. Disord. 2015, 7, 237–247. [Google Scholar] [CrossRef]
- Guney, E.; Cetin, F.H.; Alisik, M.; Tunca, H.; Torun, Y.T.; Iseri, E.; Taner, Y.I.; Cayci, B.; Erel, O. Attention Deficit Hyperactivity Disorder and Oxidative Stress: A Short Term Follow up Study. Psychiatry Res. 2015, 229, 310–317. [Google Scholar] [CrossRef]
- Verlaet, A.A.J.; Breynaert, A.; Ceulemans, B.; De Bruyne, T.; Fransen, E.; Pieters, L.; Savelkoul, H.F.J.; Hermans, N. Oxidative Stress and Immune Aberrancies in Attention-Deficit/Hyperactivity Disorder (ADHD): A Case–Control Comparison. Eur. Child. Adolesc. Psychiatry 2019, 28, 719–729. [Google Scholar] [CrossRef]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative Stress in Psychiatric Disorders: Evidence Base and Therapeutic Implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Verlaet, A.A.J.; Maasakkers, C.M.; Hermans, N.; Savelkoul, H.F.J. Rationale for Dietary Antioxidant Treatment of ADHD. Nutrients 2018, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W. Omega-3 Fatty Acids and Mental Health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Pinto, S.; Correia-de-Sá, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating Patterns and Dietary Interventions in ADHD: A Narrative Review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef]
- Morandini, H.A.; Watson, P.A.; Barbaro, P.; Rao, P. Brain Iron Concentration in Childhood ADHD: A Systematic Review of Neuroimaging Studies. J. Psychiatr. Res. 2024, 173, 200–209. [Google Scholar] [CrossRef]
- Shirvani-Rad, S.; Ejtahed, H.-S.; Marvasti, F.E.; Taghavi, M.; Sharifi, F.; Arzaghi, S.M.; Larijani, B. The Role of Gut Microbiota-Brain Axis in Pathophysiology of ADHD: A Systematic Review. J. Atten. Disord. 2022, 26, 1698–1710. [Google Scholar] [CrossRef] [PubMed]
- Mathee, K.; Cickovski, T.; Deoraj, A.; Stollstorff, M.; Narasimhan, G. The Gut Microbiome and Neuropsychiatric Disorders: Implications for Attention Deficit Hyperactivity Disorder (ADHD). J. Med. Microbiol. 2020, 69, 14–24. [Google Scholar] [CrossRef]
- Altomare, A.; Di Rosa, C.; Imperia, E.; Emerenziani, S.; Cicala, M.; Guarino, M.P.L. Diarrhea Predominant-Irritable Bowel Syndrome (Ibs-d): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2021, 13, 1506. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health—Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Larcombe, S.; Hutton, M.L.; Lyras, D. Involvement of Bacteria Other Than Clostridium Difficile in Antibiotic-Associated Diarrhoea. Trends Microbiol. 2016, 24, 463–476. [Google Scholar] [CrossRef]
- Dobrosavljevic, M.; Larsson, H.; Cortese, S. The Diagnosis and Treatment of Attention-Deficit Hyperactivity Disorder (ADHD) in Older Adults. Expert. Rev. Neurother. 2023, 23, 883–893. [Google Scholar] [CrossRef]
- Regan, S.L.; Williams, M.T.; Vorhees, C.V. Review of Rodent Models of Attention Deficit Hyperactivity Disorder. Neurosci. Biobehav. Rev. 2022, 132, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Esperón, C.A.S.; Babatope, T.T. Attention Deficit Hyperactivity Disorder and Eating Disorders: An Overlooked Comorbidity? An Sist Sanit Navar. 2022, 45, e0994. [Google Scholar]
- Wüstner, A.; Otto, C.; Schlack, R.; Hölling, H.; Klasen, F.; Ravens-Sieberer, U. Risk and Protective Factors for the Development of ADHD Symptoms in Children and Adolescents: Results of the Longitudinal BELLA Study. PLoS ONE 2019, 14, e0214412. [Google Scholar] [CrossRef]
- Wylock, J.-F.; Borghini, A.; Slama, H.; Delvenne, V. Child Attachment and ADHD: A Systematic Review. Eur. Child. Adolesc. Psychiatry 2023, 32, 5–16. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M.; Jefferies, R.; Stergiakouli, E. What Causes Attention Deficit Hyperactivity Disorder? Arch. Dis. Child. 2012, 97, 260–265. [Google Scholar] [CrossRef]
- Agnew--Blais, J.C.; Wertz, J.; Arseneault, L.; Belsky, D.W.; Danese, A.; Pingault, J.; Polanczyk, G.V.; Sugden, K.; Williams, B.; Moffitt, T.E. Mother’s and Children’s ADHD Genetic Risk, Household Chaos and Children’s ADHD Symptoms: A Gene–Environment Correlation Study. J. Child. Psychol. Psychiatry 2022, 63, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Blunden, S.L.; Milte, C.M.; Sinn, N. Diet and Sleep in Children with Attention Deficit Hyperactivity Disorder: Preliminary Data in Australian Children. J. Child. Health Care 2011, 15, 14–24. [Google Scholar] [CrossRef]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a Risk Factor for Attention Deficit Hyperactivity Disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Carvalho, G.F.d.S.; Costa, T.V.M.M.; Nascimento, A.M.; Wolff, B.M.; Damasceno, J.G.; Vieira, L.L.; Almeida, V.T.; de Oliveira, Y.G.; de Mello, C.B.; Muszkat, M.; et al. DNA Methylation Epi-Signature and Biological Age in Attention Deficit Hyperactivity Disorder Patients. Clin. Neurol. Neurosurg. 2023, 228, 107714. [Google Scholar] [CrossRef]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef]
- Matthews, M.; Nigg, J.T.; Fair, D.A. Attention Deficit Hyperactivity Disorder. Curr. Top. Behav. Neurosci. 2013, 16, 235–266. [Google Scholar] [CrossRef]
- Rajaprakash, M.M.; Leppert, M.M.L. Attention-Deficit/Hyperactivity Disorder. Pediatr. Rev. 2022, 43, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Gavin, B.; McNicholas, F. ADHD: Science, Stigma and Service Implications. Ir. J. Psychol. Med. 2018, 35, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Wolraich, M.L.; Chan, E.; Froehlich, T.; Lynch, R.L.; Bax, A.; Redwine, S.T.; Ihyembe, D.; Hagan, J.F. ADHD Diagnosis and Treatment Guidelines: A Historical Perspective. Pediatrics 2019, 144, e20191682. [Google Scholar] [CrossRef]
- Fraticelli, S.; Caratelli, G.; de Berardis, D.; Ducci, G.; Pettorruso, M.; Martinotti, G.; Di Cesare, G.; Di Giannantonio, M. Gender Differences in Attention Deficit Hyperactivity Disorder: An Update of the Current Evidence. Riv. Psichiatr. 2022, 57, 159–164. [Google Scholar] [CrossRef]
- Breda, V.; Cerqueira, R.O.; Ceolin, G.; Koning, E.; Fabe, J.; McDonald, A.; Gomes, F.A.; Brietzke, E. Is There a Place for Dietetic Interventions in Adult ADHD? Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 119, 110613. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Smit, D.; Pimenta, M.G.; Arns, M. Neurofeedback as a Treatment Intervention in ADHD: Current Evidence and Practice. Curr. Psychiatry Rep. 2019, 21, 1–7. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Halldner, L.; Zetterqvist, J.; Sjölander, A.; Serlachius, E.; Fazel, S.; Långström, N.; Larsson, H. Medication for Attention Deficit–Hyperactivity Disorder and Criminality. N. Engl. J. Med. 2012, 367, 2006–2014. [Google Scholar] [CrossRef]
- Chang, C.-H.; Yu, C.-J.; Du, J.-C.; Chiou, H.-C.; Hou, J.-W.; Yang, W.; Chen, C.-F.; Chen, H.-C.; Chen, Y.-S.; Hwang, B.; et al. The Associations among Organophosphate Pesticide Exposure, Oxidative Stress, and Genetic Polymorphisms of Paraoxonases in Children with Attention Deficit/Hyperactivity Disorder. Sci. Total. Environ. 2021, 773, 145604. [Google Scholar] [CrossRef]
- da Silva, B.S.; Grevet, E.H.; Silva, L.C.F.; Ramos, J.K.N.; Rovaris, D.L.; Bau, C.H.D. An Overview on Neurobiology and Therapeutics of Attention-Deficit/Hyperactivity Disorder. Discov. Ment. Health 2023, 3, 1–21. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Swanson, J.M. Dopamine in Drug Abuse and Addiction: Results from Imaging Studies and Treatment Implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar] [CrossRef]
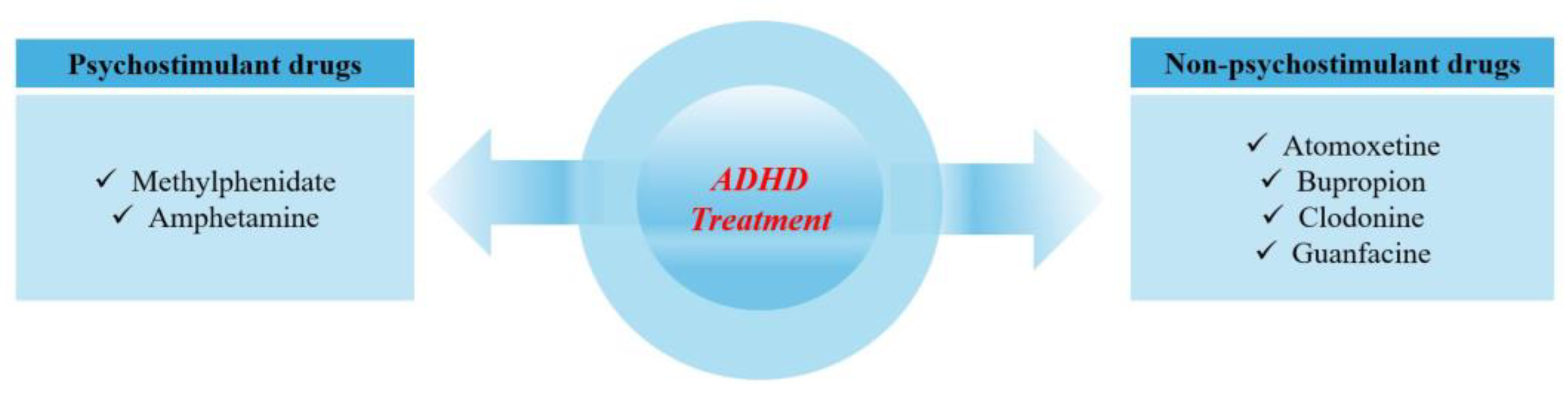
- Engert, V.; Pruessner, J.C. Dopaminergic and Noradrenergic Contributions to Functionality in ADHD: The Role of Methylphenidate. Curr. Neuropharmacol. 2009, 6, 322–328. [Google Scholar] [CrossRef] [PubMed]
- A Staller, J.; Faraone, S.V. Targeting the Dopamine System in the Treatment of Attention-Deficit/Hyperactivity Disorder. Expert. Rev. Neurother. 2007, 7, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.; McCarthy, K.; Mancuso, E.; Gendron, C. Atomoxetine for the Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: A Review. Neuropsychiatr. Dis. Treat. 2009, 5, 215–226. [Google Scholar] [CrossRef]
- Hazell, P.L.; Stuart, J.E. A Randomized Controlled Trial of Clonidine Added to Psychostimulant Medication for Hyperactive and Aggressive Children. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 886–894. [Google Scholar] [CrossRef]
- Pelsser, L.M.; Frankena, K.; Toorman, J.; Pereira, R.R. Diet and ADHD, Reviewing the Evidence: A Systematic Review of Meta-Analyses of Double-Blind Placebo-Controlled Trials Evaluating the Efficacy of Diet Interventions on the Behavior of Children with ADHD. PLoS ONE 2017, 12, e0169277. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.; Koerting, J.; Smith, E.; McCann, D.C.; Thompson, M. Early Detection and Intervention for Attention-Deficit/Hyperactivity Disorder. Expert. Rev. Neurother. 2011, 11, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.T.; Walick, K.S.; Rivera, J.C. Preliminary Evidence of an Association Between ADHD Medications and Diminished Bone Health in Children and Adolescents. J. Pediatr. Orthop. 2017, 37, 348–354. [Google Scholar] [CrossRef]
- Honarvar, N.M.; Samadi, M.; Chimeh, M.S.; Gholami, F.; Bahrampour, N.; Jalali, M.; Effatpanah, M.; Yekaninejad, M.S.; Abdolahi, M.; Chamari, M. Effect of Vitamin D on Paraxonase-1, Total Antioxidant Capacity, and 8-Isoprostan in Children with Attention Deficit Hyperactivity Disorder. Int. J. Clin. Pract. 2022, 2022, 4836731. [Google Scholar] [CrossRef]
- Schmitz, F.; Scherer, E.B.S.; Machado, F.R.; da Cunha, A.A.; Tagliari, B.; Netto, C.A.; Wyse, A.T.S. Methylphenidate Induces Lipid and Protein Damage in Prefrontal Cortex, but Not in Cerebellum, Striatum and Hippocampus of Juvenile Rats. Metab. Brain Dis. 2012, 27, 605–612. [Google Scholar] [CrossRef]
- Foschiera, L.N.; Schmitz, F.; Wyse, A.T. Evidence of Methylphenidate Effect on Mitochondria, Redox Homeostasis, and Inflammatory Aspects: Insights from Animal Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110518. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, A.C.; Frey, B.N.; Valvassori, S.S.; Zanotto, C.; Gomes, K.M.; Comim, C.M.; Cassini, C.; Stertz, L.; Ribeiro, L.C.; Quevedo, J.; et al. DNA Damage in Rats after Treatment with Methylphenidate. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Barrio, C.; Arias-Sánchez, S.; Martín-Monzón, I. The Gut Microbiota-Brain Axis, Psychobiotics and Its Influence on Brain and Behaviour: A Systematic Review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef] [PubMed]
- Kul, M.; Unal, F.; Kandemir, H.; Sarkarati, B.; Kilinc, K.; Kandemir, S.B. Evaluation of Oxidative Metabolism in Child and Adolescent Patients with Attention Deficit Hyperactivity Disorder. Psychiatry Investig. 2015, 12, 361–366. [Google Scholar] [CrossRef]
- Koç, S.; Güler, E.M.; Derin, S.; Gültekin, F.; Aktaş, S. Oxidative and Inflammatory Parameters in Children and Adolescents with ADHD. J. Atten. Disord. 2023, 27, 880–886. [Google Scholar] [CrossRef]
- Chen, J.-R.; Hsu, S.-F.; Hsu, C.-D.; Hwang, L.-H.; Yang, S.-C. Dietary Patterns and Blood Fatty Acid Composition in Children with Attention-Deficit Hyperactivity Disorder in Taiwan. J. Nutr. Biochem. 2004, 15, 467–472. [Google Scholar] [CrossRef]
- Naeini, A.A.; Nasim, S.; Najafi, M.; Ghazvini, M.; Hassanzadeh, A. Relationship between Antioxidant Status and Attention Deficit Hyperactivity Disorder among Children. Int. J. Prev. Med. 2019, 10, 41. [Google Scholar] [CrossRef]
- Avcil, S.; Uysal, P.; Yenisey, Ç.; Abas, B.I. Elevated Melatonin Levels in Children with Attention Deficit Hyperactivity Disorder: Relationship to Oxidative and Nitrosative Stress. J. Atten. Disord. 2021, 25, 693–703. [Google Scholar] [CrossRef]
- Ceylan, M.; Sener, S.; Bayraktar, A.C.; Kavutcu, M. Oxidative Imbalance in Child and Adolescent Patients with Attention-Deficit/Hyperactivity Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1491–1494. [Google Scholar] [CrossRef]
- Garre-Morata, L.; de Haro, T.; Villén, R.G.; Fernández-López, M.L.; Escames, G.; Molina-Carballo, A.; Acuña-Castroviejo, D. Changes in Cortisol and in Oxidative/Nitrosative Stress Indicators after ADHD Treatment. Antioxidants 2024, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Selek, S.; Savas, H.A.; Gergerlioglu, H.S.; Bulut, M.; Yilmaz, H.R. Oxidative Imbalance in Adult Attention Deficit/Hyperactivity Disorder. Biol. Psychol. 2008, 79, 256–259. [Google Scholar] [CrossRef]
- Hassan, A.; Karim, E.; Adham, E.; Hassan, A.I.; El, A.A.; El-Mahdy, A. ORIGINAL ARTICLES Nutiritional and Metabolic Disturbances in Attention Deficit Hyperactivity Disease. Res. J. Med. Med. Sci. 2011, 6, 10–16. [Google Scholar]
- Elhady, M.; Youness, E.R.; Mostafa, R.S.I.; Aziz, A.A.; Hussein, R. Oxidative Stress Contribution to Attention Deficit Hyperactivity Disorder in Children with Epilepsy. Appl. Neuropsychol. Child. 2019, 8, 347–354. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Ruchi, K.; Kumar, A.S.; Sunil, G.; Bashir, A.; Prabhat, S. Antioxidant Activity in Children with ADHD—A Comparison in Untreated and Treated Subjects with Normal Children. IIUM Med. J. Malays. 2011, 10. [Google Scholar] [CrossRef]
- Chovanová, Z.; Muchová, J.; Sivoňová, M.; Dvořáková, M.; Žitňanová, I.; Waczulíková, I.; Trebatická, J.; Škodáček, I.; Ďuračková, Z. Effect of Polyphenolic Extract, Pycnogenol®, on the Level of 8-Oxoguanine in Children Suffering from Attention Deficit/Hyperactivity Disorder. Free. Radic. Res. 2006, 40, 1003–1010. [Google Scholar] [CrossRef]
- Dvořáková, M.; Sivoňová, M.; Trebatická, J.; Škodáček, I.; Waczuliková, I.; Muchová, J.; Ďuračková, Z. The Effect of Polyphenolic Extract from Pine Bark, Pycnogenol®on the Level of Glutathione in Children Suffering from Attention Deficit Hyperactivity Disorder (ADHD). Redox Rep. 2006, 11, 163–172. [Google Scholar] [CrossRef]
- Oztop, D.; Altun, H.; Baskol, G.; Ozsoy, S. Oxidative Stress in Children with Attention Deficit Hyperactivity Disorder. Clin. Biochem. 2012, 45, 745–748. [Google Scholar] [CrossRef]
- Comim, C.M.; Gomes, K.M.; Réus, G.Z.; Petronilho, F.; Ferreira, G.K.; Streck, E.L.; Dal-Pizzol, F.; Quevedo, J. Methylphenidate Treatment Causes Oxidative Stress and Alters Energetic Metabolism in an Animal Model of Attention-Deficit Hyperactivity Disorder. Acta Neuropsychiatr. 2014, 26, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Faraji, F.; Mozaffari, S. The Neuroprotective Effect of Curcumin Against Nicotine-Induced Neurotoxicity Is Mediated by CREB–BDNF Signaling Pathway. Neurochem. Res. 2017, 42, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Leffa, D.T.; Bellaver, B.; de Oliveira, C.; de Macedo, I.C.; de Freitas, J.S.; Grevet, E.H.; Caumo, W.; Rohde, L.A.; Quincozes-Santos, A.; Torres, I.L.S. Increased Oxidative Parameters and Decreased Cytokine Levels in an Animal Model of Attention-Deficit/Hyperactivity Disorder. Neurochem. Res. 2017, 42, 3084–3092. [Google Scholar] [CrossRef] [PubMed]
- Russo, A. Decreased Serum Cu/Zn SOD Associated with High Copper in Children with Attention Deficit Hyperactivity Disorder (ADHD). J. Central Nerv. Syst. Dis. 2010, 2, 9–14. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Motevalian, M.; Shabab, B.; Fatima, S. Effects of Acute Doses of Methylphenidate on Inflammation and Oxidative Stress in Isolated Hippocampus and Cerebral Cortex of Adult Rats. J. Neural Transm. 2017, 124, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Dam, S.A.; Mostert, J.C.; Szopinska-Tokov, J.W.; Bloemendaal, M.; Amato, M.; Arias-Vasquez, A. The Role of the Gut-Brain Axis in Attention-Deficit/Hyperactivity Disorder. Gastroenterol. Clin. N. Am. 2019, 48, 407–431. [Google Scholar] [CrossRef]
- Spreckley, E.; Murphy, K.G. The L-Cell in Nutritional Sensing and the Regulation of Appetite. Front. Nutr. 2015, 2, 23. [Google Scholar] [CrossRef]
- Arora, T.; Akrami, R.; Pais, R.; Bergqvist, L.; Johansson, B.R.; Schwartz, T.W.; Reimann, F.; Gribble, F.M.; Bäckhed, F. Microbial Regulation of the L Cell Transcriptome. Sci. Rep. 2018, 8, 1207. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Scuto, M.; Modafferi, S.; Rampulla, F.; Zimbone, V.; Tomasello, M.; Spano’, S.; Ontario, M.; Palmeri, A.; Salinaro, A.T.; Siracusa, R.; et al. Redox Modulation of Stress Resilience by Crocus sativus L. for Potential Neuroprotective and Anti-Neuroinflammatory Applications in Brain Disorders: From Molecular Basis to Therapy. Mech. Ageing Dev. 2022, 205, 111686. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Salinaro, A.T.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut–Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical Composition and Antimicrobial Activity of the Essential Oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, K.K.S.; Wallace, K.B. Disruption of the Keap1/Nrf2-Antioxidant Response System after Chronic Doxorubicin Exposure In Vivo. Cardiovasc. Toxicol. 2020, 20, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lei, Y.; Lei, S.; Gong, X. Cardioprotective Effects of Corilagin on Doxorubicin induced Cardiotoxicity via P13K/Akt and NF-ΚB Signaling Pathways in a Rat Model. Toxicol. Mech. Methods 2022, 32, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2022, 27, 167. [Google Scholar] [CrossRef]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium Erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef]
- Mattson, M.P.; Leak, R.K. The Hormesis Principle of Neuroplasticity and Neuroprotection. Cell Metab. 2024, 36, 315–337. [Google Scholar] [CrossRef]
- Modafferi, S.; Lupo, G.; Tomasello, M.; Rampulla, F.; Ontario, M.; Scuto, M.; Salinaro, A.T.; Arcidiacono, A.; Anfuso, C.D.; Legmouz, M.; et al. Antioxidants, Hormetic Nutrition, and Autism. Curr. Neuropharmacol. 2023, 22, 1156–1168. [Google Scholar] [CrossRef]
- Cordaro, M.; Scuto, M.; Siracusa, R.; D’Amico, R.; Filippo Peritore, A.; Gugliandolo, E.; Fusco, R.; Crupi, R.; Impellizzeri, D.; Pozzebon, M.; et al. Effect of N-Palmitoylethanolamine-Oxazoline on Comorbid Neuropsychiatric Disturbance Associated with Inflammatory Bowel Disease. FASEB J. 2020, 34, 4085–4106. [Google Scholar] [CrossRef]
- Hontelez, S.; Stobernack, T.; Pelsser, L.M.; van Baarlen, P.; Frankena, K.; Groefsema, M.M.; Kleerebezem, M.; Pereira, R.R.; Postma, E.M.; Smeets, P.A.M.; et al. Correlation between Brain Function and ADHD Symptom Changes in Children with ADHD Following a Few-Foods Diet: An Open-Label Intervention Trial. Sci. Rep. 2021, 11, 22205. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chernova, L.N.; Tinkov, A.A. Magnesium Status in Children with Attention-Deficit/Hyperactivity Disorder and/or Autism Spectrum Disorder. J. Korean Acad. Child Adolesc. Psychiatry 2020, 31, 41–45. [Google Scholar] [CrossRef]
- Wankerl, B.; Hauser, J.; Makulska-Gertruda, E.; Reißmann, A.; Sontag, T.A.; Tucha, O.; Lange, K.W. Neurobiologische Grundlagen Der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung. TT—[Neurobiology of Attention Deficit Hyperactivity Disorder]. Fortschritte Neurol. Psychiatr. 2014, 82, 9–29. [Google Scholar] [CrossRef]
- Kelly, B.D. Attention-Deficit Hyperactivity Disorder: A Clinical Review of the Concept, Diagnosis and Management. Ir. J. Psychol. Med. 2018, 35, 157–161. [Google Scholar] [CrossRef]
- San Mauro Martin, I.; Sanz Rojo, S.; González Cosano, L.; Conty de la Campa, R.; Garicano Vilar, E.; Blumenfeld Olivares, J.A. Impulsiveness in Children with Attention-Deficit/Hyperactivity Disorder after an 8-Week Intervention with the Mediterranean Diet and/or Omega-3 Fatty Acids: A Randomised Clinical Trial. Neurologia 2022, 37, 513–523. [Google Scholar] [CrossRef]
- Ly, V.; Bottelier, M.; Hoekstra, P.J.; Vasquez, A.A.; Buitelaar, J.K.; Rommelse, N.N. Elimination Diets’ Efficacy and Mechanisms in Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder. Eur. Child. Adolesc. Psychiatry 2017, 26, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- González, H.F.; Visentin, S. Micronutrients and Neurodevelopment: An Update. Arch. Argent. Pediatr. 2016, 114, 570–575. [Google Scholar] [CrossRef]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in Adolescent Growth and Development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chao, J.C.-J.; Chernova, L.N.; Shakieva, R.A.; Kopylov, P.Y.; Skalny, A.A.; et al. Serum Zinc, Copper, Zinc-to-Copper Ratio, and Other Essential Elements and Minerals in Children with Attention Deficit/Hyperactivity Disorder (ADHD). J. Trace Elem. Med. Biol. 2020, 58, 126445. [Google Scholar] [CrossRef]
- Martins, A.C.; Krum, B.N.; Queirós, L.S.S.; Tinkov, A.A.; Skalny, A.V.; Bowman, A.B.; Aschner, M. Manganese in the Diet: Bioaccessibility, Adequate Intake, and Neurotoxicological Effects. J. Agric. Food Chem. 2020, 68, 12893–12903. [Google Scholar] [CrossRef]
- Saper, R.B.; Rash, R. Zinc: An Essential Micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc Requirements and the Risks and Benefits of Zinc Supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional Iron Deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, J.; Cheng, Y.; Li, R.; Wang, Y.; Chen, Y.; Scott, T.; Tucker, K.L. Magnesium and Cognitive Health in Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2024, 15, 100272. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. Int. Rev. J. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in Food and the Human Body: A Review. Sci. Total. Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Sager, M. Selenium in Agriculture, Food, and Nutrition. Pure Appl. Chem. 2006, 78, 111–133. [Google Scholar] [CrossRef]
- Noah, L.; Dye, L.; De Fer, B.B.; Mazur, A.; Pickering, G.; Pouteau, E. Effect of Magnesium and Vitamin B6 Supplementation on Mental Health and Quality of Life in Stressed Healthy Adults: Post—Hoc Analysis of a Randomised Controlled Trial. Stress. Health 2021, 37, 1000–1009. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, Vitamin B6 and Related Pathways. Mol. Asp. Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Roškar, R.; Hickey, N.; Geremia, S. Vitamin B12 in Foods, Food Supplements, and Medicines—A Review of Its Role and Properties with a Focus on Its Stability. Molecules 2023, 28, 240. [Google Scholar] [CrossRef]
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12—Multifaceted In Vivo Functions and In Vitro Applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef] [PubMed]
- Benedik, E. Sources of Vitamin D for Humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Giordano, E.; Visioli, F. Long-Chain Omega 3 Fatty Acids: Molecular Bases of Potential Antioxidant Actions. Prostagland. Leukot. Essent. Fat. Acids 2014, 90, 1–4. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Manganese: Its Role in Disease and Health. Met. Ions Life Sci. 2019, 19, 253–266. [Google Scholar] [CrossRef]
- Lucchini, R.; Placidi, D.; Cagna, G.; Fedrighi, C.; Oppini, M.; Peli, M.; Zoni, S. Manganese and Developmental Neurotoxicity. Adv. Neurobiol. 2017, 18, 13–34. [Google Scholar]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Aschner, M.; Martins, A.C.; Oliveira-Paula, G.H.; Skalny, A.V.; Zaitseva, I.P.; Bowman, A.B.; Kirichuk, A.A.; Santamaria, A.; Tizabi, Y.; Tinkov, A.A. Manganese in Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder: The State of the Art. Curr. Res. Toxicol. 2024, 6, 100170. [Google Scholar] [CrossRef]
- Farias, A.C.; Cunha, A.; Benko, C.R.; McCracken, J.T.; Costa, M.T.; Farias, L.G.; Cordeiro, M.L. Manganese in Children with Attention-Deficit/Hyperactivity Disorder: Relationship with Methylphenidate Exposure. J. Child. Adolesc. Psychopharmacol. 2010, 20, 113–118. [Google Scholar] [CrossRef]
- Robberecht, H.; Verlaet, A.A.J.; Breynaert, A.; de Bruyne, T.; Hermans, N. Magnesium, Iron, Zinc, Copper and Selenium Status in Attention-Deficit/Hyperactivity Disorder (ADHD). Molecules 2020, 25, 4440. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; Allen, K.L.; Jacoby, P.; Trapp, G.S.; Gallagher, C.M.; Byrne, S.M.; Oddy, W.H. Low Dietary Intake of Magnesium is Associated with Increased Externalising Behaviours in Adolescents. Public. Health Nutr. 2015, 18, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Huss, M.; Völp, A.; Stauss-Grabo, M. Supplementation of Polyunsaturated Fatty Acids, Magnesium and Zinc in Children Seeking Medical Advice for Attention-Deficit/Hyperactivity Problems—An Observational Cohort Study. Lipids Health Dis. 2010, 9, 105. [Google Scholar] [CrossRef]
- Granero, R.; Pardo-Garrido, A.; Carpio-Toro, I.L.; Ramírez-Coronel, A.A.; Martínez-Suárez, P.C.; Reivan-Ortiz, G.G. The Role of Iron and Zinc in the Treatment of ADHD among Children and Adolescents: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 4059. [Google Scholar] [CrossRef]
- Ghoreishy, S.M.; Mousavi, S.E.; Asoudeh, F.; Mohammadi, H. Zinc Status in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Observational Studies. Sci. Rep. 2021, 11, 14612. [Google Scholar] [CrossRef]
- Sinn, N. Nutritional and Dietary Influences on Attention Deficit Hyperactivity Disorder. Nutr. Rev. 2008, 66, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain. Res. Manag. 2018, 2018, 1–44. [Google Scholar] [CrossRef]
- Noorazar, S.G.; Malek, A.; Aghaei, S.M.; Yasamineh, N.; Kalejahi, P. The Efficacy of Zinc Augmentation in Children with Attention Deficit Hyperactivity Disorder under Treatment with Methylphenidate: A Randomized Controlled Trial. Asian J. Psychiatry 2020, 48, 101868. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Mulqueen, J. Nutritional Supplements for the Treatment of ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Unal, D.; Çelebi, F.; Bildik, H.N.; Koyuncu, A.; Karahan, S. Vitamin B12 and Haemoglobin Levels May be Related with ADHD Symptoms: A Study in Turkish Children with ADHD. Psychiatry Clin. Psychopharmacol. 2019, 29, 515–519. [Google Scholar] [CrossRef]
- Landaas, E.T.; Aarsland, T.I.M.; Ulvik, A.; Halmøy, A.; Ueland, P.M.; Haavik, J. Vitamin Levels in Adults with ADHD. BJPsych Open 2016, 2, 377–384. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, Q.; Ren, P.; Xiang, W.; Xiao, L. The Synaptic and Circuit Functions of Vitamin D in Neurodevelopment Disorders. Neuropsychiatr. Dis. Treat. 2023, 19, 1515–1530. [Google Scholar] [CrossRef]
- Joshi, K.; Lad, S.; Kale, M.; Patwardhan, B.; Mahadik, S.P.; Patni, B.; Chaudhary, A.; Bhave, S.; Pandit, A. Supplementation with Flax Oil and Vitamin C Improves the Outcome of Attention Deficit Hyperactivity Disorder (ADHD). Prostagland. Leukot. Essent. Fat. Acids 2006, 74, 17–21. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Su, K.-P.; Mondelli, V.; Pariante, C.M. Omega-3 Polyunsaturated Fatty Acids in Youths with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Clinical Trials and Biological Studies. Neuropsychopharmacology 2018, 43, 534–545. [Google Scholar] [CrossRef]
- Gillies, D.; Leach, M.J.; Algorta, G.P. Polyunsaturated Fatty Acids (PUFA) for Attention Deficit Hyperactivity Disorder (ADHD) in Children and Adolescents. Cochrane Database Syst. Rev. 2023, 2023, CD007986. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Haddad, B. The Effect of Dietary Education on ADHD, a Randomized Controlled Clinical Trial. Ann. Gen. Psychiatry 2015, 14, 12. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, D.W.; Hong, Y.-S.; Kim, Y.-M.; Seo, J.-H.; Choe, B.M.; Park, J.H.; Kang, J.-W.; Yoo, J.-H.; Chueh, H.W.; et al. Dietary Patterns in Children with Attention Deficit/Hyperactivity Disorder (ADHD). Nutrients 2014, 6, 1539–1553. [Google Scholar] [CrossRef]
- Bilici, M.; Yıldirim, F.; Kandil, S.; Bekaroğlu, M.; Yildirmiş, S.; Değer, O.; Ülgen, M.; Yildiran, A.; Aksu, H. Double-Blind, Placebo-Controlled Study of Zinc Sulfate in the Treatment of Attention Deficit Hyperactivity Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; DiSilvestro, R.A.; Bozzolo, D.; Bozzolo, H.; Crowl, L.; Fernandez, S.; Ramadan, Y.; Thompson, S.; Mo, X.; Abdel-Rasoul, M.; et al. Zinc for Attention-Deficit/Hyperactivity Disorder: Placebo-Controlled Double-Blind Pilot Trial Alone and Combined with Amphetamine. J. Child Adolesc. Psychopharmacol. 2011, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E.; Lecendreux, M.; Deron, J.; Marchand, M.; Cortese, S.; Zaïm, M.; Mouren, M.C.; Arnulf, I. Effects of Iron Supplementation on Attention Deficit Hyperactivity Disorder in Children. Pediatr. Neurol. 2008, 38, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Starobrat-Hermelin, B.; Kozielec, T. The Effects of Magnesium Physiological Supplementation on Hyperactivity in Children with Attention Deficit Hyperactivity Disorder (ADHD). Posit. Response Magnes. Oral Load. Test 1997, 10, 149–156. [Google Scholar]
- Dehbokri, N.; Noorazar, G.; Ghaffari, A.; Mehdizadeh, G.; Sarbakhsh, P.; Ghaffary, S. Effect of Vitamin D Treatment in Children with Attention-Deficit Hyperactivity Disorder. World J. Pediatr. 2019, 15, 78–84. [Google Scholar] [CrossRef]
- Elshorbagy, H.H.; Barseem, N.F.; Abdelghani, W.E.; Suliman, H.A.I.; Al-Shokary, A.H.; Abdulsamea, S.E.; Elsadek, A.E.; Maksoud, Y.H.A.; El Din, D.M.A.E.-H.N. Impact of Vitamin D Supplementation on Attention-Deficit Hyperactivity Disorder in Children. Ann. Pharmacother. 2018, 52, 623–631. [Google Scholar] [CrossRef]
- Hemamy, M.; Pahlavani, N.; Amanollahi, A.; Islam, S.M.S.; McVicar, J.; Askari, G.; Malekahmadi, M. The Effect of Vitamin D and Magnesium Supplementation on the Mental Health Status of Attention-Deficit Hyperactive Children: A Randomized Controlled Trial. BMC Pediatr. 2021, 21, 178. [Google Scholar] [CrossRef]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.; Demmelmair, H.; Koletzko, B.; van der Velden, M.G.D.S.; Eilander, A.; Hoeksma, M.; et al. Reduced Symptoms of Inattention after Dietary Omega-3 Fatty Acid Supplementation in Boys with and without Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef]
- Checa-Ros, A.; Jeréz-Calero, A.; Molina-Carballo, A.; Campoy, C.; Muñoz-Hoyos, A. Current Evidence on the Role of the Gut Microbiome in ADHD Pathophysiology and Therapeutic Implications. Nutrients 2021, 13, 249. [Google Scholar] [CrossRef]
- Healy-Stoffel, M.; Levant, B. N-3 (Omega-3) Fatty Acids: Effects on Brain Dopamine Systems and Potential Role in the Etiology and Treatment of Neuropsychiatric Disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 216–232. [Google Scholar] [CrossRef]
- Sukmajaya, A.C.; Lusida, M.I.; Soetjipto; Setiawati, Y. Systematic Review of Gut Microbiota and Attention-Deficit Hyperactivity Disorder (ADHD). Ann. Gen. Psychiatry 2021, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Guo, P.; Li, Y.; Liu, L.; Yan, R.; Liu, S.; Wang, S.; Xue, F.; Zhou, X.; Yuan, Z. Role of the Gut-Brain Axis in the Shared Genetic Etiology between Gastrointestinal Tract Diseases and Psychiatric Disorders: A Genome-Wide Pleiotropic Analysis. JAMA Psychiatry 2023, 80, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. 2015, 28, 203–209.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Suez, J.; Elinav, E. The Interplay between the Innate Immune System and the microbiota. Curr. Opin. Immunol. 2014, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzki, J.C.; Glass, C.K.; Mazmanian, S.K. Microbiome–Microglia Connections via the Gut–Brain Axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut Microbiome in ADHD and Its Relation to Neural Reward Anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Hurn, D.; Hermanus, D. Gut Bacteria and Neuropsychiatric Disorders. Microorganisms 2021, 9, 2583. [Google Scholar] [CrossRef]
- Taş, E.; Ülgen, K.O. Understanding the ADHD-Gut Axis by Metabolic Network Analysis. Metabolites 2023, 13, 592. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Chattipakorn, N.; Chattipakorn, S.C. Possible Links between Gut-Microbiota and Attention-Deficit/Hyperactivity Disorders in Children and Adolescents. Eur. J. Nutr. 2020, 59, 3391–3403. [Google Scholar] [CrossRef]
- Lange, K.W.; Lange, K.M.; Nakamura, Y.; Reissmann, A. Nutrition in the Management of ADHD: A Review of Recent Research. Curr. Nutr. Rep. 2023, 12, 383–394. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between Drugs and the Gut Microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Bloemendaal, M.; Aarts, E.; et al. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms 2020, 8, 406. [Google Scholar] [CrossRef]
- Stiernborg, M.; Debelius, J.; Yang, L.L.; Skott, E.; Millischer, V.; Giacobini, M.; Melas, P.A.; Boulund, F.; Lavebratt, C. Bacterial Gut Microbiome Differences in Adults with ADHD and in Children with ADHD on Psychostimulant Medication. Brain, Behav. Immun. 2023, 110, 310–321. [Google Scholar] [CrossRef]
| Sample | Types of Biomarkers | Biomarkers | Level Compared to Control/Treatment | Reference |
|---|---|---|---|---|
| Plasma | Enzymes | GPx | Significantly lower | [60] |
| [63] | ||||
| Activity did not change * | [48] | |||
| CAT | Higher than the controls, but this difference was not statistically significant | [60] | ||
| Increased * | [61] | |||
| Significantly lower | [63] | |||
| SOD | Not significantly | [60] | ||
| Significantly lower | [62,63] | |||
| Antioxidants | TAS | Significantly lower | [55] | |
| Decreased | [68,69] | |||
| No statistical differences | [8] | |||
| GSH | Higher | [9] | ||
| GLA | Significantly higher | [57] | ||
| Oxidants | LPO | Lower * | [61] | |
| AOPP | ||||
| NOx | ||||
| Enzyme | GRd | Increased * | ||
| Antioxidant | total thiols | Significantly lower | [8] | |
| Enzyme | GST | Significantly lower | [63] | |
| Serum | Enzyme | SOD1 | Significantly lower | [74] |
| Antioxidant | Melatonin | Higher | [59] | |
| TAC | Significantly lower | [58] | ||
| Enzyme | CAT | |||
| Antioxidant | GSH | |||
| Oxidant | MDA | No significant difference between the two groups | ||
| Increased | [64] | |||
| Statistically significantly Lower | [70] | |||
| Saliva | Enzyme | CAT | Reduced | [67] |
| Rat brain homogenates-Tx MPH | Enzyme | SOD | Decreased * | [71] |
| Significantly decreased activity * | [75] | |||
| CAT | Decreased * | [71] | ||
| Antioxidant | GSH | Significantly decreased activity * | [75] | |
| Enzyme | GPx | |||
| Rat brain homogenates | Antioxidant | GSH | Lower | [73] |
| Enzymes | SOD | No difference | ||
| CAT | ||||
| GPx | ||||
| not specified | Oxidant | TOS | Significantly higher | [56] |
| Antioxidant | TAS | Lower than in the control group |
| Microutrients | Types | Effects | Food | References |
|---|---|---|---|---|
| Minerals | Mn | Protects against OS | Grains, rice, nuts, seafood, chocolate, fruits, seeds, leafy green vegetables, teas, juice and water | [100] |
| Zn | It helps regulate gene expression, has antioxidant properties and can protect against macular degeneration caused by OS | Meat, seafood, fruits and vegetables, cereals, dairy, legumes, nuts | [101,102] | |
| Fe | An insufficient iron level can be a risk factor for death | It is found in two forms: heme iron from meat and non-heme iron present in vegetables, legumes, cereals, nuts | [103] | |
| Mg | It crosses the blood-brain barrier and plays a key role in neuronal maturation and central nervous system function | Vegetables (spinach), pulses, cereals, fruits, nuts | [104,105] | |
| Se | It acts as a cofactor for GPx enzymes, which play a role in protecting against OS. It reduces lipid oxidation by catalyzing the reduction of peroxides | Vegetables, nuts, fish, grains, meat | [106,107] | |
| Vitamins | B6 | Role in neurotransmitter synthesis (gamma-aminobutyric acid, serotonin and dopamine) and stress reduction | Meat, dairy products, beans, nuts, potatoes and more fruits and vegetables | [108,109] |
| B12 | Essential for DNA function and metabolism. Vitamin B12 deficiency leads to an increase in the proinflammatory cytokine IL-6. Proinflammatory cytokines produce inflammation that increases ROS levels and can lead to OS | Meat, milk, eggs and fish | [110,111] | |
| D | Role in bone metabolism, brain function and regulates Ca. It can stimulate the activity and expression of GGT, which participates in the glutathione cycle between neurons and astrocytes. It increases the level of glutathione to protect neurons, so it can lead to a decrease in ROS | Sun exposure, fatty fish, dairy products, cereals, orange juice, eggs | [48,112] | |
| Polyunsaturated fatty acids | ω-3 PUFAs | They can prevent chronic diseases, reduce lipoperoxidation levels, and reduce the ratio of SOD/CAT enzymes.High doses of omega-3 fatty acids can trigger OS | Fish (mackerel, salmon), microalgae and some microorganisms | [113,114,115] |
| Supplements | Number of Participants (n =) | Age (Years) | Dose | Time | Result | References |
|---|---|---|---|---|---|---|
| Zn | 400 | 9.61 ± 1.7 | 150 mg zinc sulfate | 12 weeks | Positive effects on the symptoms of ADHD | [141] |
| 52 | 6–14 | 15 mg every morning or two times per day + amphetamine | 13 weeks | it had no effect | [142] | |
| 60 | 9.6 ± 1.70 | 0.5–1 mg/kg/day methylphenidate + 10 mg Zn | 6 weeks | Significantly improved attention | [129] | |
| Fe | 23 | 5–8 | 80 mg/day ferrous sulfate | 12 weeks | Improves ADHD symptoms in children with low serum ferritin levels | [143] |
| Mg | 50 | 7–12 | 200 mg/day | 6 months | Positive response to Mg supplementation | [144] |
| Vitamin D | 96 | 9.76 ± 2.38 | 50,000 UI/week 25-hydroxy-vitamin D3 | 6 weeks | A positive effect on ADHD symptoms | [145] |
| 35 | 7–14 | 3000 IU/day 25-hydroxy-vitamin D3 | 12 weeks | may improve cognitive functions related to ADHD | [146] | |
| Mg + Vitamin D | 74 | 6–12 | vitamin D (50.000 UI/week) + Mg supplements (6 mg/kg/day) | 8 weeks | Reduced social problems and anxiety in children with ADHD | [147] |
| ω-3 PUFAs | 40 | 8–14 | 10 g of full fat (80%) margarine daily enriched with either 650 mg of EPA/DHA | 16 weeks | ω-3 PUFA supplementation may increase the performance of pharmacological treatments for ADHD | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visternicu, M.; Rarinca, V.; Burlui, V.; Halitchi, G.; Ciobică, A.; Singeap, A.-M.; Dobrin, R.; Mavroudis, I.; Trifan, A. Investigating the Impact of Nutrition and Oxidative Stress on Attention Deficit Hyperactivity Disorder. Nutrients 2024, 16, 3113. https://doi.org/10.3390/nu16183113
Visternicu M, Rarinca V, Burlui V, Halitchi G, Ciobică A, Singeap A-M, Dobrin R, Mavroudis I, Trifan A. Investigating the Impact of Nutrition and Oxidative Stress on Attention Deficit Hyperactivity Disorder. Nutrients. 2024; 16(18):3113. https://doi.org/10.3390/nu16183113
Chicago/Turabian StyleVisternicu, Malina, Viorica Rarinca, Vasile Burlui, Gabriela Halitchi, Alin Ciobică, Ana-Maria Singeap, Romeo Dobrin, Ioannis Mavroudis, and Anca Trifan. 2024. "Investigating the Impact of Nutrition and Oxidative Stress on Attention Deficit Hyperactivity Disorder" Nutrients 16, no. 18: 3113. https://doi.org/10.3390/nu16183113










