Neither an Individualised Nor a Standardised Sodium Bicarbonate Strategy Improved Performance in High-Intensity Repeated Swimming, or a Subsequent 200 m Swimming Time Trial in Highly Trained Female Swimmers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Pre-Experimental Procedures
2.3. Supplement Timings
2.4. Experimental Procedures
2.5. Swimming Exercise Tests
2.6. Statistical Analysis
3. Results
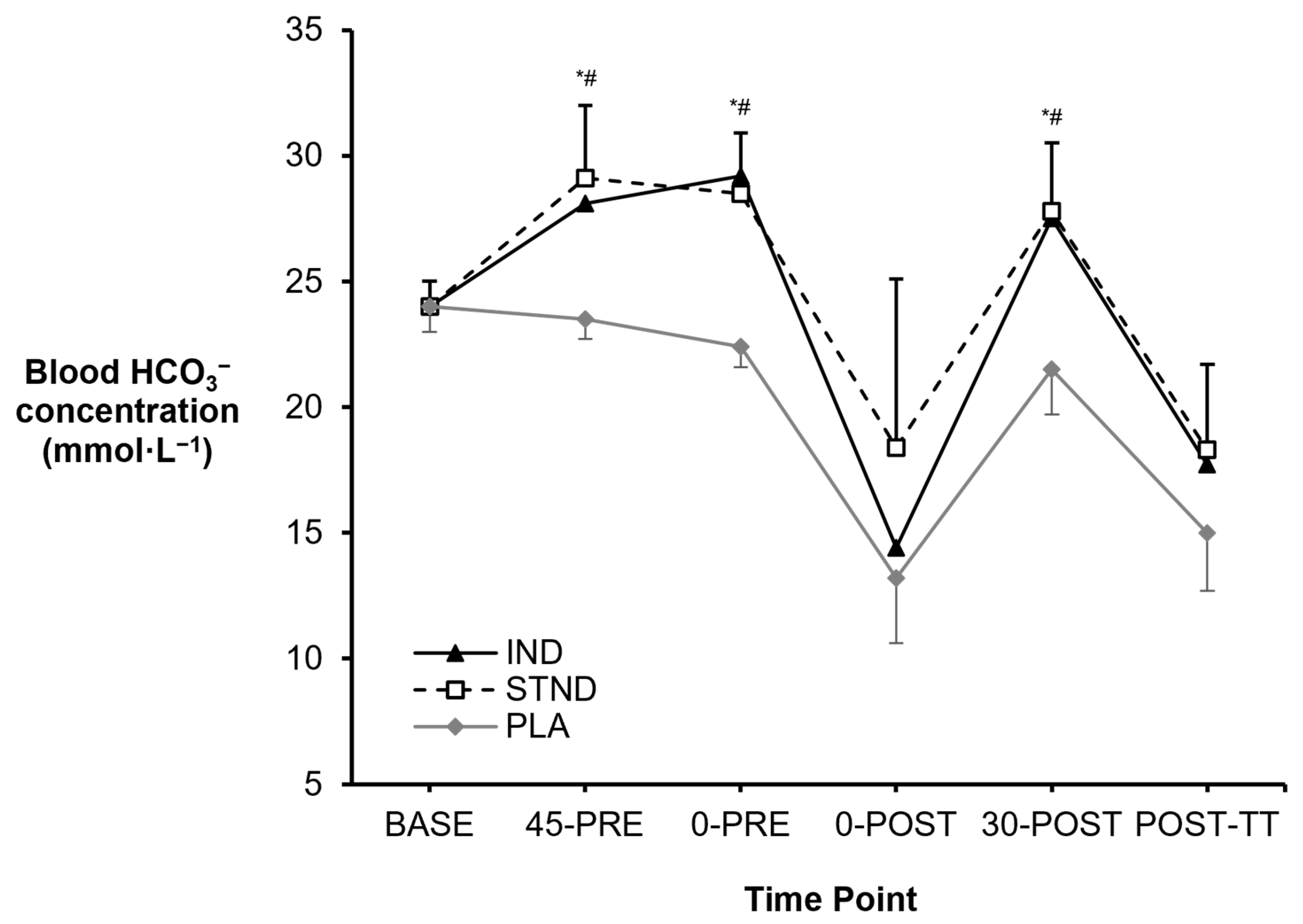
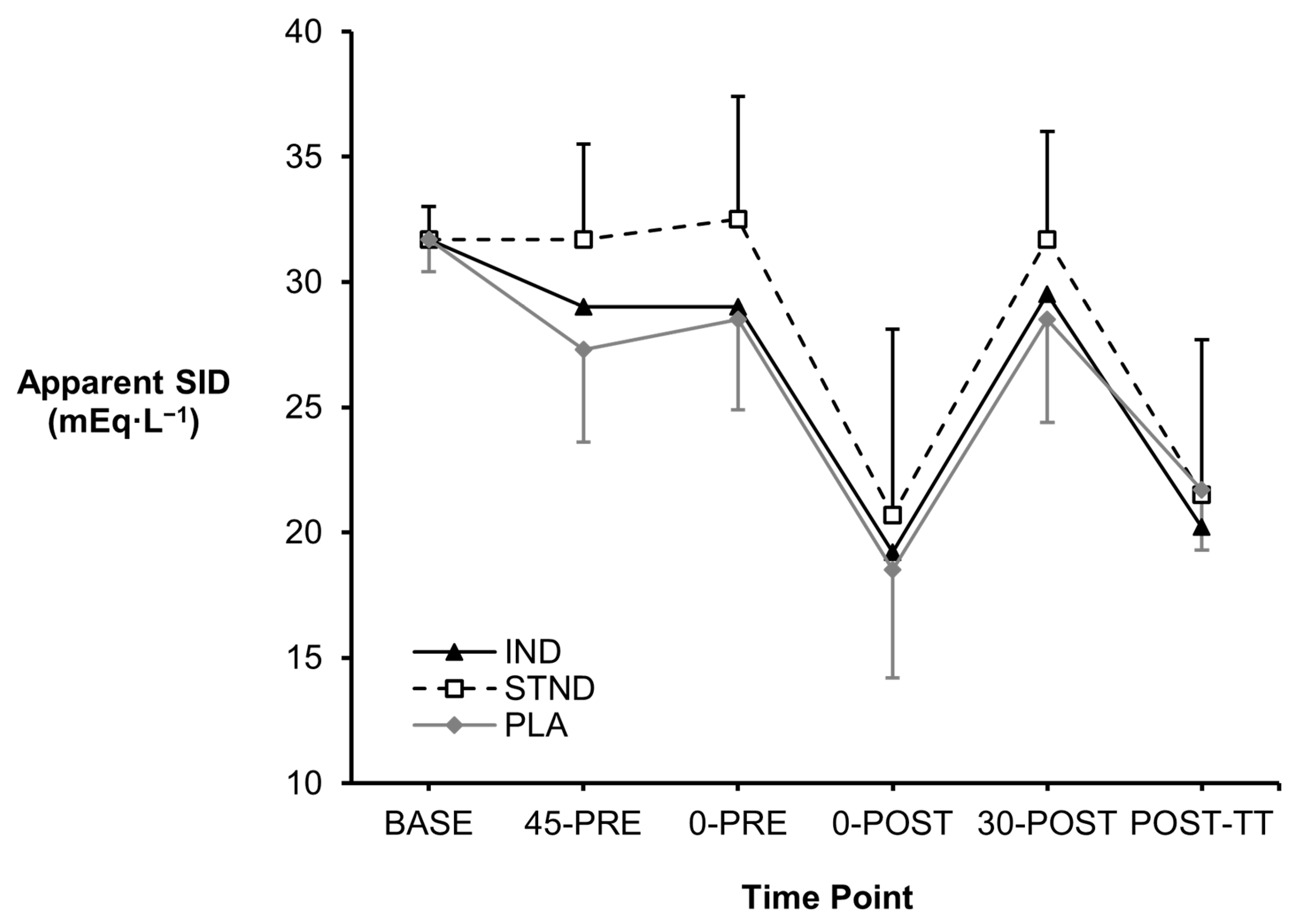
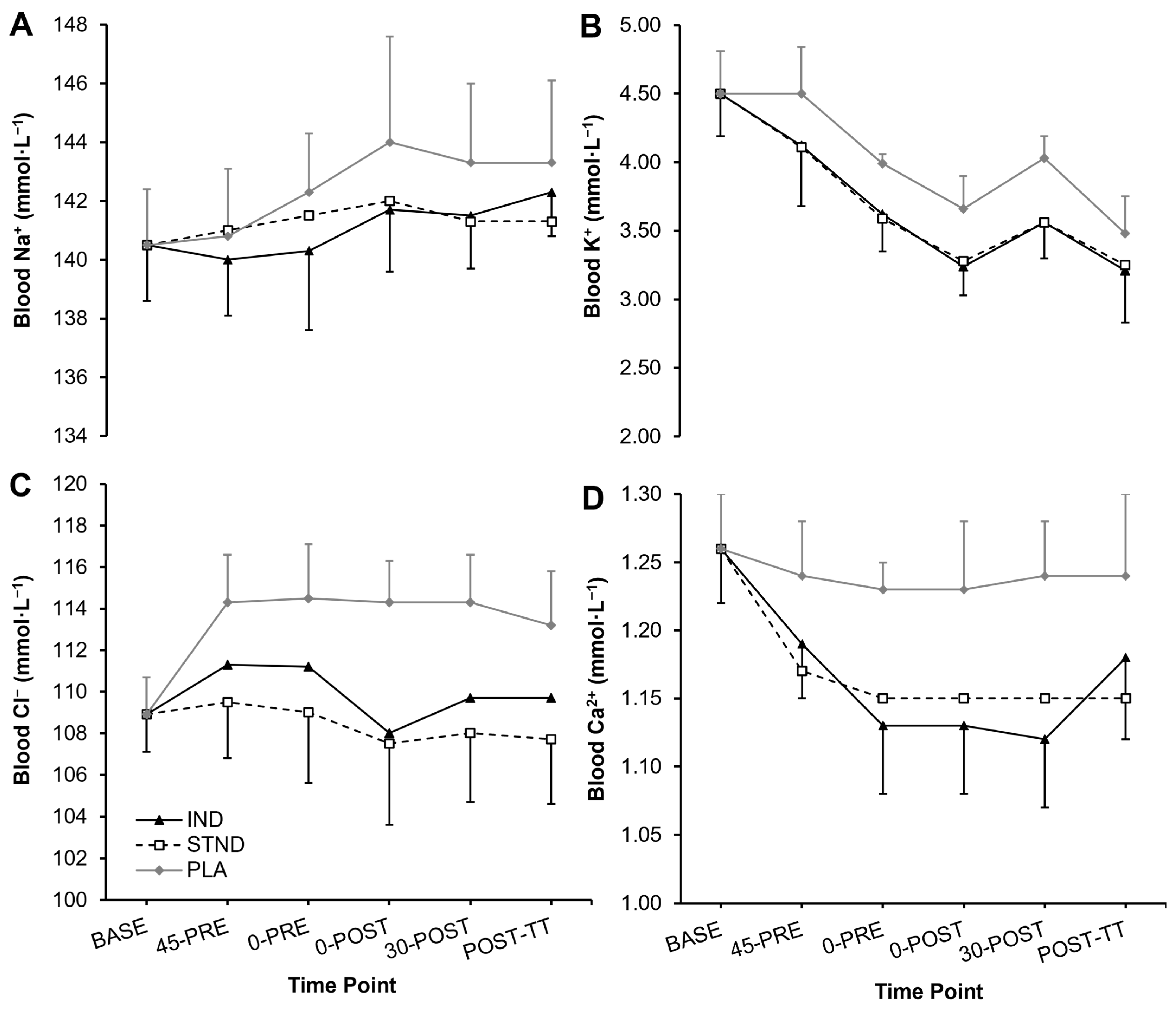
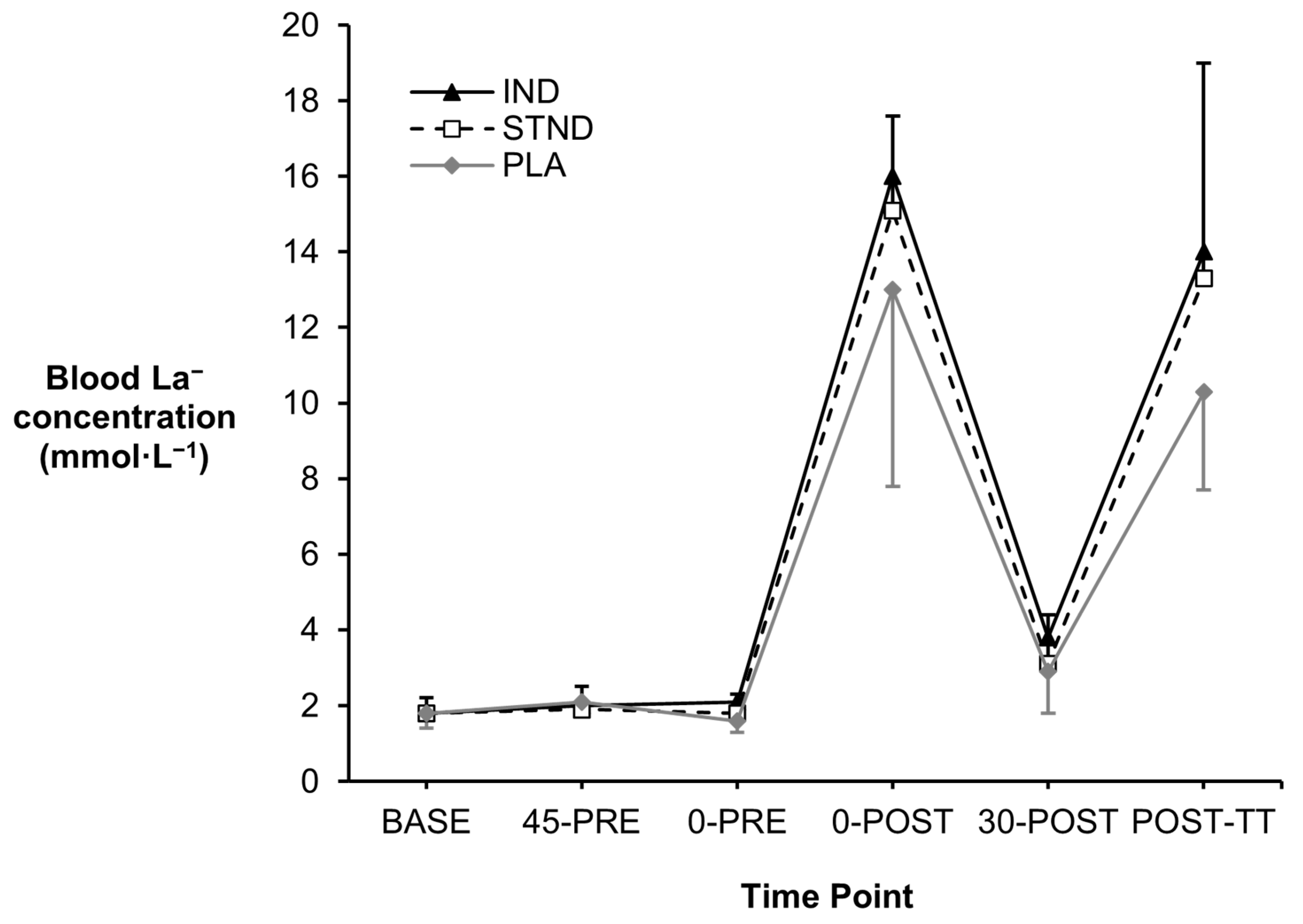
3.1. Blood Metabolites
3.2. Swimming Performance
3.3. Perceptual Measures
3.4. Gastrointestinal Side-Effects
3.5. Order Effects and Supplement Predictions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newbury, J.W.; Foo, W.L.; Cole, M.; Kelly, A.L.; Chessor, R.J.; Sparks, S.A.; Faghy, M.A.; Gough, H.C.; Gough, L.A. Nutritional intakes of highly trained adolescent swimmers before, during, and after a national lockdown in the COVID-19 pandemic. PLoS ONE 2022, 17, e0266238. [Google Scholar] [CrossRef] [PubMed]
- Pollock, S.; Gaoua, N.; Johnston, M.J.; Cooke, K.; Girard, O.; Mileva, K.N. Training regimes and recovery monitoring practices of elite British swimmers. J. Sports Sci. Med. 2019, 18, 577–585. [Google Scholar]
- Vasile, L. Endurance training in performance swimming. Procedia Soc. Behav. Sci. 2014, 117, 232–237. [Google Scholar] [CrossRef]
- Barnett, A. Using recovery modalities between training sessions in elite athletes: Does it help? Sports Med. 2006, 36, 781–796. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Fitts, R.H. The role of acidosis in fatigue: Pro perspective. Med. Sci. Sports Exerc. 2016, 48, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H. Acidosis is not a significant cause of skeletal muscle fatigue. Med. Sci. Sports Exerc. 2016, 48, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Westerblad, H.; Lee, J.A.; Lännergren, J. Role of excitation-contraction coupling in muscle fatigue. Sports Med. 1992, 13, 116–126. [Google Scholar] [CrossRef]
- Hollidge-Horvat, M.G.; Parolin, M.L.; Wong, D.; Jones, N.L.; Heigenhauser, G.J. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E316–E329. [Google Scholar] [CrossRef]
- Sostaric, S.M.; Skinner, S.L.; Brown, M.J.; Sangkabutra, T.; Medved, I.; Medley, T.; Selig, S.E.; Fairweather, I.; Rutar, D.; McKenna, M.J. Alkalosis increases muscle K+ release, but lowers plasma [K+] and delays fatigue during dynamic forearm exercise. J. Physiol. 2006, 570, 185–205. [Google Scholar] [CrossRef]
- Gao, J.P.; Costill, D.L.; Horswill, C.A.; Park, S.H. Sodium bicarbonate ingestion improves performance in interval swimming. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Newbury, J.W.; Price, M. The effects of sodium bicarbonate ingestion on swimming interval performance in trained competitive swimmers. Eur. J. Appl. Physiol. 2023, 123, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Cholewa, J.; Poprzecki, S.; Waskiewicz, Z.; Langfort, J. Effects of sodium bicarbonate ingestion on swim performance in youth athletes. J. Sports Sci. Med. 2009, 8, 45–50. [Google Scholar]
- Campos, E.Z.; Sangali, E.B.; Neto, J.G.; Gobbi, R.B.; Freitas, I.F., Jr.; Papoti, M. Effects of sodium bicarbonate ingestion during an intermittent exercise on blood lactate, stroke parameters, and performance of swimmers. J. Exerc. Physiol. Online 2012, 15, 84–92. [Google Scholar]
- Carr, A.J.; Slater, G.J.; Gore, C.J.; Dawson, B.; Burke, L.M. Effect of sodium bicarbonate on [HCO3−], pH, and gastrointestinal symptoms. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 189–194. [Google Scholar] [CrossRef]
- Boegman, S.; Stellingwerff, T.; Shaw, G.; Clarke, N.; Graham, K.; Cross, R.; Siegler, J.C. The impact of individualizing sodium bicarbonate supplementation strategies on world-class rowing performance. Front. Nutr. 2020, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.F.; Saunders, B.; Yamaguchi, G.; Swinton, P.; Artioli, G.G. Is individualization of sodium bicarbonate ingestion based on time to peak necessary? Med. Sci. Sports Exerc. 2020, 52, 1801–1808. [Google Scholar] [CrossRef]
- Jones, R.L.; Stellingwerff, T.; Artioli, G.G.; Saunders, B.; Cooper, S.; Sale, C. Dose-response of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Newbury, J.W.; Cole, M.; Kelly, A.L.; Chessor, R.J.; Sparks, S.A.; McNaughton, L.R.; Gough, L.A. The time to peak blood bicarbonate (HCO3−), pH, and the strong ion difference (SID) following sodium bicarbonate (NaHCO3) ingestion in highly trained adolescent swimmers. PLoS ONE 2021, 16, e0248456. [Google Scholar] [CrossRef]
- Gough, L.A.; Deb, S.K.; Brown, D.; Sparks, S.A.; McNaughton, L.R. The effects of sodium bicarbonate ingestion on cycling performance and acid base balance recovery in acute normobaric hypoxia. J. Sports Sci. 2019, 37, 1464–1471. [Google Scholar] [CrossRef]
- Pierce, E.F.; Eastman, N.W.; Hammer, W.H.; Lynn, T.D. Effect of induced alkalosis on swimming time trials. J. Sports Sci. 1992, 10, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Pruscino, C.L.; Ross, M.L.; Gregory, J.R.; Savage, B.; Flanagan, T.R. Effects of sodium bicarbonate, caffeine, and their combination on repeated 200-m freestyle performance. Int. J. Sports Nutr. Exerc. Metab. 2008, 18, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Sample size justification. Collabra Psychol. 2022, 8, 33267. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2021, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Boyd, K.T.; Burke, L.M.; Koivisto, A. Nutrition for swimming. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Veiga, S.; Sánchez-Oliver, A.J.; Domínguez, R.; Morencos, E. Analysis of sport supplement consumption by competitive swimmers according to sex and competitive level. Nutrients 2022, 14, 3218. [Google Scholar] [CrossRef]
- Newbury, J.W.; Sparks, S.A.; Cole, M.; Kelly, A.L.; Gough, L.A. Nutritional supplement use in a UK high-performance swimming club. Nutrients 2023, 15, 3306. [Google Scholar] [CrossRef]
- Chung, W.; Shaw, G.; Anderson, M.E.; Pyne, D.B.; Saunders, P.U.; Bishop, D.J.; Burke, L.M. Effect of 10 week beta-alanine supplementation on competition and training performance in elite swimmers. Nutrients 2012, 4, 1441–1453. [Google Scholar] [CrossRef]
- Peyrebrune, M.C.; Stokes, K.; Hall, G.M.; Nevill, M.E. Effect of creatine supplementation on training for competition in elite swimmers. Med. Sci. Sports Exerc. 2005, 37, 2140–2147. [Google Scholar] [CrossRef]
- Lloyd, P. Strong ion calculator—A practical bedside application of modern quantitative acid-base physiology. Crit. Care Resusc. 2004, 6, 285–294. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodriguez, R.; Hamouti, N. Salt and fluid loading: Effects on blood volume and exercise performance. Med. Sport Sci. 2012, 59, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Bernards, J.R.; Sato, K.; Haff, G.G.; Bazyler, C.D. Current research and statistical practices in sport science and a need for change. Sports 2017, 5, 87. [Google Scholar] [CrossRef]
- Heibel, A.B.; Perim, P.H.L.; de Oliveira, L.F.; McNaughton, L.R.; Saunders, B. Time to optimize supplementation: Modifying factors influencing the individual responses to extracellular buffering agents. Front. Nutr. 2018, 5, 35. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Kumstát, M.; Hlinský, T.; Struhár, I.; Thomas, A. Does sodium citrate cause the same ergogenic effect as sodium bicarbonate on swimming performance? J. Hum. Kinet. 2018, 65, 89–98. [Google Scholar] [CrossRef]
- Siegler, J.C.; Marshall, P.W.; Bishop, D.; Shaw, G.; Green, S. Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med. Open 2016, 2, 41. [Google Scholar] [CrossRef]
- Lühker, O.; Berger, M.M.; Pohlmann, A.; Hotz, L.; Gruhlke, T.; Hochreiter, M. Changes in acid-base and ion balance during exercise in normoxia and normobaric hypoxia. Eur. J. Appl. Physiol. 2017, 117, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Brown, D.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. The influence of alkalosis on repeated high-intensity exercise performance and acid-base balance recovery in acute moderate hypoxic conditions. Eur. J. Appl. Physiol. 2018, 118, 2489–2498. [Google Scholar] [CrossRef]
- Gough, L.A.; Rimmer, S.; Sparks, S.A.; McNaughton, L.R.; Higgins, M.F. Post-exercise supplementation of sodium bicarbonate improves acid base balance recovery and subsequent high-intensity boxing specific performance. Front. Nutr. 2019, 6, 155. [Google Scholar] [CrossRef]
- Metheny, N.A.; Krieger, M.M. Salt toxicity: A systematic review and case reports. J. Emerg. Nurs. 2020, 46, 428–439. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Dias, G.F.; da Eira Silva, V.; de Salles Painelli, V.; Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B. (In)consistencies in responses to sodium bicarbonate supplementation: A randomised, repeated measures, counterbalanced and double-blind study. PLoS ONE 2015, 10, e0143086. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.F.; James, R.S.; Price, M.J. The effects of sodium bicarbonate (NaHCO3) ingestion on high intensity cycling capacity. J. Sports Sci. 2013, 31, 972–981. [Google Scholar] [CrossRef]
- McNaughton, L.R. Sodium bicarbonate ingestion and its effects on anaerobic exercise of various durations. J. Sports Sci. 1992, 10, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Mikulic, P. Ergogenic effects of sodium bicarbonate supplementation on middle-, but not short-distance swimming tests: A meta-analysis. J. Diet. Suppl. 2022, 19, 791–802. [Google Scholar] [CrossRef]
- Girard, O.; Mendez-Villanueva, A.; Bishop, D. Repeated-sprint ability—Part I: Factors contributing to fatigue. Sports Med. 2011, 41, 673–694. [Google Scholar] [CrossRef]
- Heubert, R.A.P.; Billat, V.L.; Chassaing, P.; Bocquet, V.; Morton, R.H.; Koralsztein, J.P.; di Prampero, P.E. Effect of a previous sprint on the parameters of the work-time to exhaustion relationship in high intensity cycling. Int. J. Sports Med. 2005, 26, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.A.; Williams, J.J.; Newbury, J.W.; Gurton, W.H. The effects of sodium bicarbonate supplementation at individual time-to-peak blood bicarbonate on 4-km cycling time trial performance in the heat. Eur. J. Sport Sci. 2021, 22, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Batterham, A.M.; Atkinson, G. How big does my sample need to be? A primer on the murky world of sample size estimation. Phys. Ther. Sport 2005, 6, 153–163. [Google Scholar] [CrossRef]
- de Salles Painelli, V.; Roschel, H.; de Jesus, F.; Sale, C.; Harris, R.C.; Solis, M.Y.; Benatti, F.B.; Gualano, B.; Lancha, A.H.; Artioli, G.G. The ergogenic effect of beta-alanine combined with sodium bicarbonate on high-intensity swimming performance. Appl. Physiol. Nutr. Metab. 2013, 38, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.; Minahan, C.; Anderson, M.; Osborne, M. Acute and chronic loading of sodium bicarbonate in highly trained swimmers. Eur. J. Appl. Physiol. 2012, 112, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lindh, A.M.; Peyrebrune, M.C.; Ingham, S.A.; Bailey, D.M.; Folland, J.P. Sodium bicarbonate improves swimming performance. Int. J. Sports Med. 2008, 29, 519–523. [Google Scholar] [CrossRef]
- Colenso-Semple, L.M.; D’Souza, A.C.; Elliott-Sale, K.J.; Phillips, S.M. Current evidence shows no influence of women’s menstrual cycle phase on acute strength performance or adaptations to resistance exercise training. Front. Sports Act. Living 2023, 5, 1054542. [Google Scholar] [CrossRef]
- Saunders, B.; de Oliveira, L.F.; Dolan, E.; Durkalec-Michalski, K.; McNaughton, L.; Artioli, G.G.; Swinton, P.A. Sodium bicarbonate supplementation and the female athlete: A brief commentary with small scale systematic review and meta-analysis. Eur. J. Sport Sci. 2022, 22, 745–754. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Giersch, G.E.; Charkoudian, N.; Stearns, R.L.; Casa, D.J. Fluid balance and hydration considerations for women: Review and future directions. Sports Med. 2020, 50, 253–261. [Google Scholar] [CrossRef]
- Moore, J.; Barlow, D.; Jewell, D.; Kennedy, S. Do gastrointestinal symptoms vary with the menstrual cycle? Br. J. Obstet. Gynaecol. 1998, 105, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
| Performance Variable | Ingestion Strategy | ||
|---|---|---|---|
| IND | STND | PLA | |
| Bout 1 (s) | 48.5 ± 4.7 | 48.9 ± 5.4 | 49.1 ± 4.9 |
| Bout 2 (s) | 48.0 ± 4.6 | 48.7 ± 5.7 | 49.0 ± 5.1 |
| Bout 3 (s) | 48.2 ± 4.9 | 48.8 ± 5.8 | 49.1 ± 5.3 |
| Bout 4 (s) | 47.9 ± 4.8 | 48.6 ± 5.9 | 49.0 ± 5.2 |
| Bout 5 (s) | 48.4 ± 4.6 | 49.0 ± 6.1 | 49.3 ± 5.0 |
| Bout 6 (s) | 48.2 ± 5.1 | 49.2 ± 6.0 | 49.1 ± 5.4 |
| Mean 75 m (s) | 48.2 ± 4.8 | 48.9 ± 5.8 | 49.1 ± 5.1 |
| Mean aggregated (s) | 289.2 ± 28.6 | 293.3 ± 34.8 | 294.5 ± 30.9 |
| Swimmer | Ingestion Strategy | ||
|---|---|---|---|
| IND | STND | PLA | |
| 1 | Stomach Ache 4.9/10 (0-POST) | Stomach Bloating 5/10 (30-POST) | Nausea 3.5/10 (0-PRE) |
| 2 | Stomach Ache 4.1/10 (0-PRE) | Nausea 3.2/10 (45-PRE) | Vomiting 10/10 (45-PRE) |
| 3 | Nausea 6.2/10 (0-POST) | Bowel Urgency 1.7/10 (0-POST) | Bowel Urgency 1.5/10 (POST-TT) |
| 4 | Stomach Ache 0.6/10 (45-PRE) | Stomach Ache 2.4/10 (BASE) | Nausea 8.2/10 (45-PRE) |
| 5 | Nausea 8.9/10 (0-PRE) | Nausea 7/10 (45-PRE) | Nausea 8.7/10 (45-PRE) |
| 6 | Nausea 10/10 (POST-TT) | Nausea 6.6/10 (30-POST) | Nausea 6/10 (0-PRE) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newbury, J.W.; Cole, M.; Kelly, A.L.; Gough, L.A. Neither an Individualised Nor a Standardised Sodium Bicarbonate Strategy Improved Performance in High-Intensity Repeated Swimming, or a Subsequent 200 m Swimming Time Trial in Highly Trained Female Swimmers. Nutrients 2024, 16, 3123. https://doi.org/10.3390/nu16183123
Newbury JW, Cole M, Kelly AL, Gough LA. Neither an Individualised Nor a Standardised Sodium Bicarbonate Strategy Improved Performance in High-Intensity Repeated Swimming, or a Subsequent 200 m Swimming Time Trial in Highly Trained Female Swimmers. Nutrients. 2024; 16(18):3123. https://doi.org/10.3390/nu16183123
Chicago/Turabian StyleNewbury, Josh W., Matthew Cole, Adam L. Kelly, and Lewis A. Gough. 2024. "Neither an Individualised Nor a Standardised Sodium Bicarbonate Strategy Improved Performance in High-Intensity Repeated Swimming, or a Subsequent 200 m Swimming Time Trial in Highly Trained Female Swimmers" Nutrients 16, no. 18: 3123. https://doi.org/10.3390/nu16183123
APA StyleNewbury, J. W., Cole, M., Kelly, A. L., & Gough, L. A. (2024). Neither an Individualised Nor a Standardised Sodium Bicarbonate Strategy Improved Performance in High-Intensity Repeated Swimming, or a Subsequent 200 m Swimming Time Trial in Highly Trained Female Swimmers. Nutrients, 16(18), 3123. https://doi.org/10.3390/nu16183123







