Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Functional Ingredient
2.2.1. Extract Preparation
2.2.2. Total Phenolic Content (TPC)
2.2.3. Determination of Antioxidant Activity
2.2.4. Phytochemical Analysis by LC–MS
2.3. Flour Contamination and OTA Production
2.3.1. OTA Extraction and Bread Analysis
2.3.2. LC-FLD Quantitative Analysis
2.4. Bread Preparation and Baking
2.5. In Vitro Static Digestion Model
2.6. Gastrointestinal Extracts Analysis and Bioaccessibility
2.7. Cell Cultures
2.8. Cell Viability Assay
2.9. Flow Cytometry Analysis
2.9.1. Cell Cycle Analysis
2.9.2. Apoptosis/Necrosis Analysis
2.9.3. ROS Analysis
2.9.4. Mitochondrial Mass and Mitochondrial ROS Analysis
2.10. Statistical Analyses of the Data
3. Results
3.1. Analysis of the Functional Ingredient
3.2. Analysis of Sample Breads and Contaminated Barley Flour
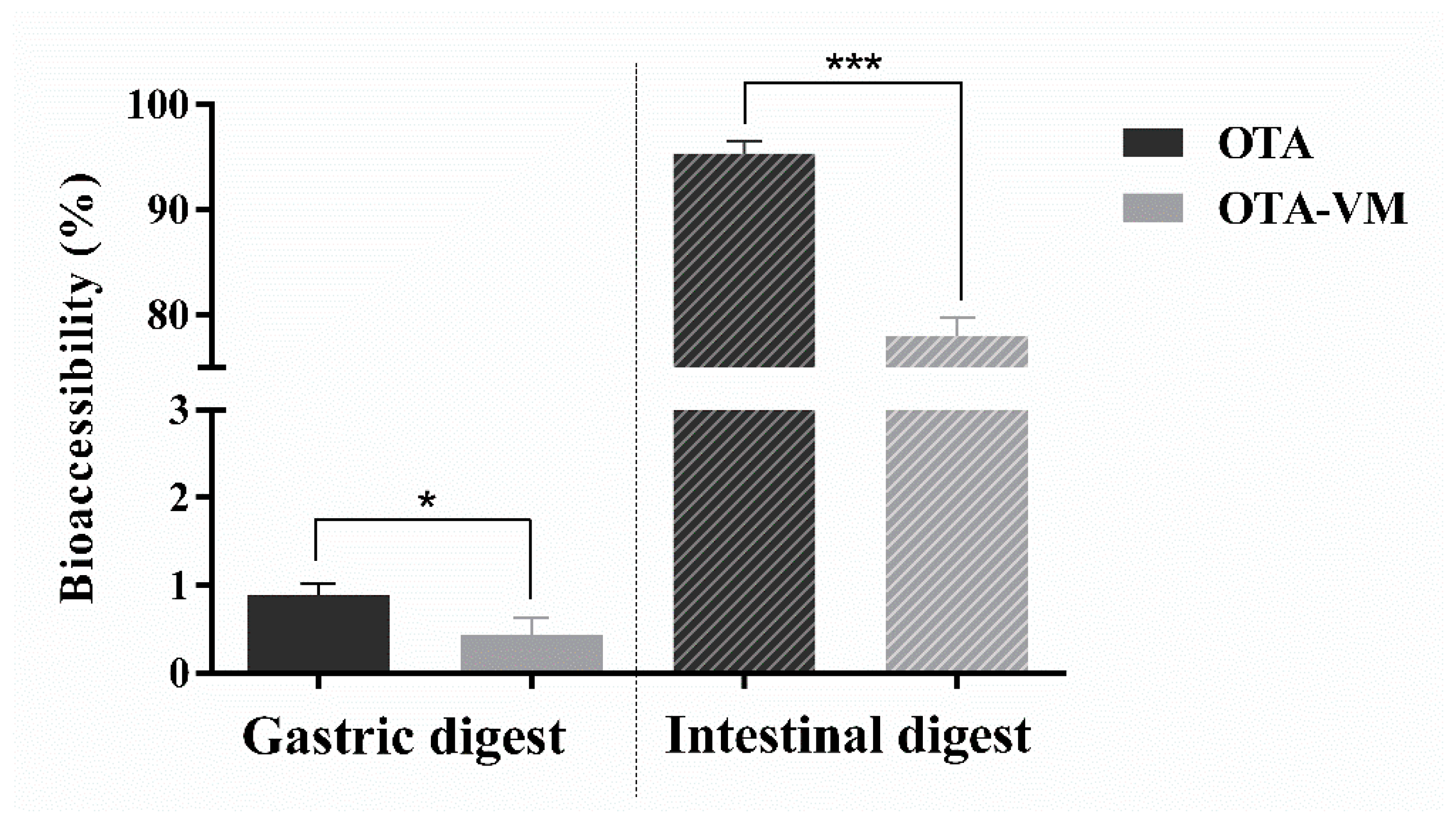
3.3. OTA Bioaccessibility in Gastric and Intestinal Digests
3.4. Cell Viability Results
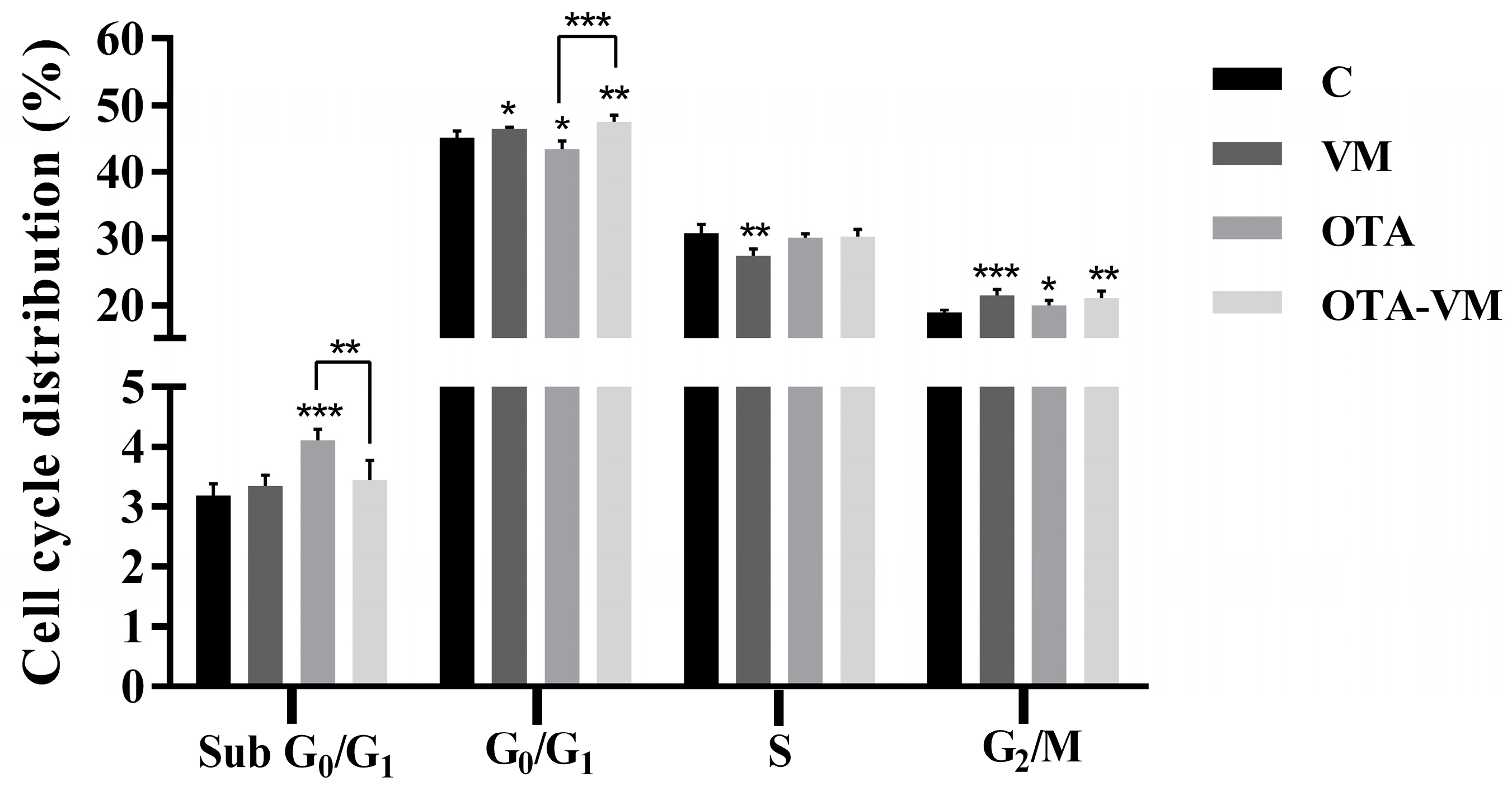
3.5. Cell Cycle Analysis
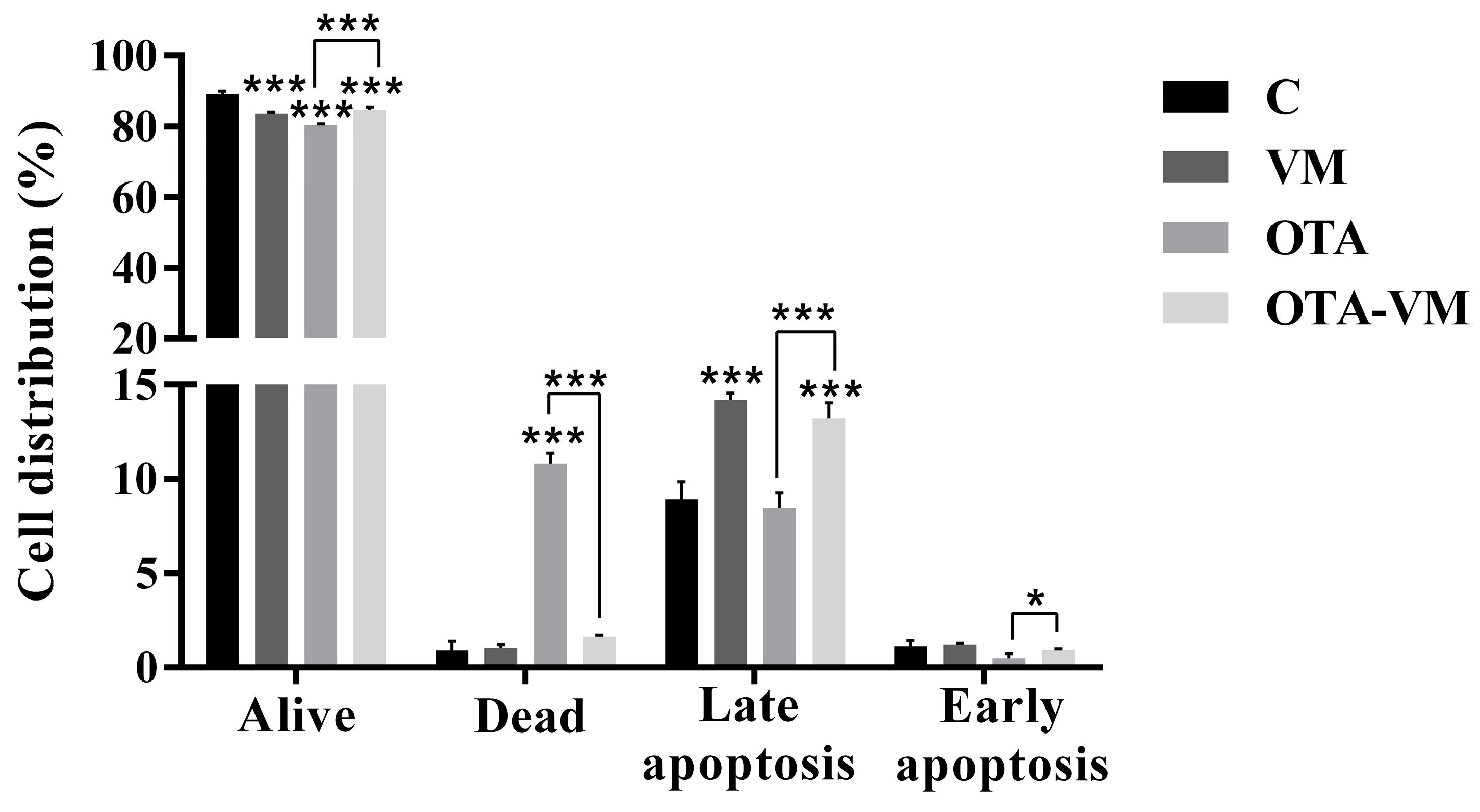
3.6. Apoptosis/Necrosis Analysis
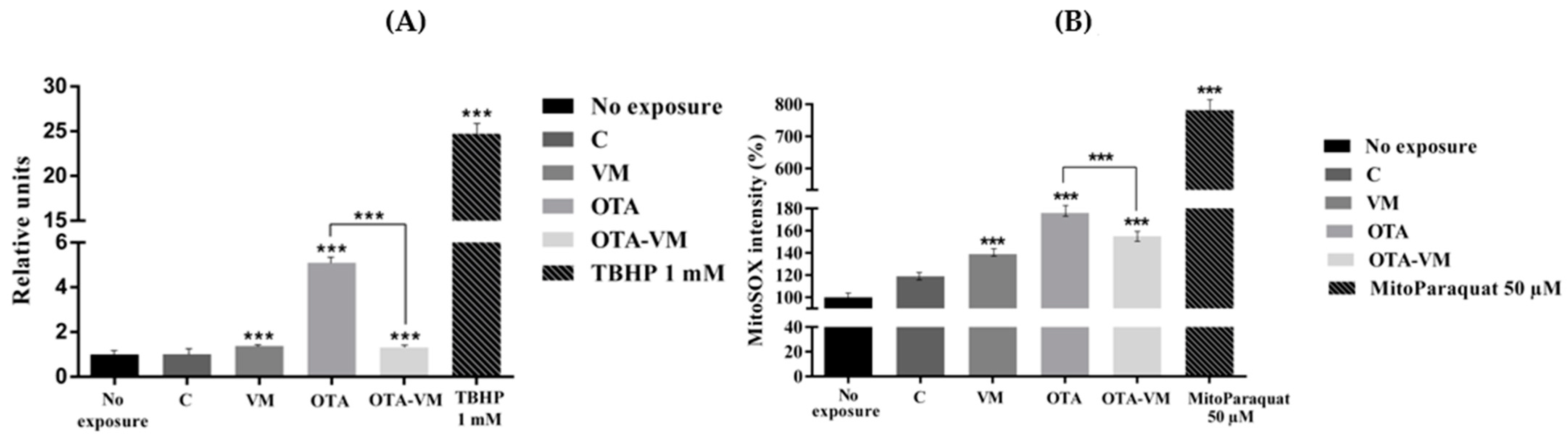
3.7. ROS and Mitochondrial ROS Analysis
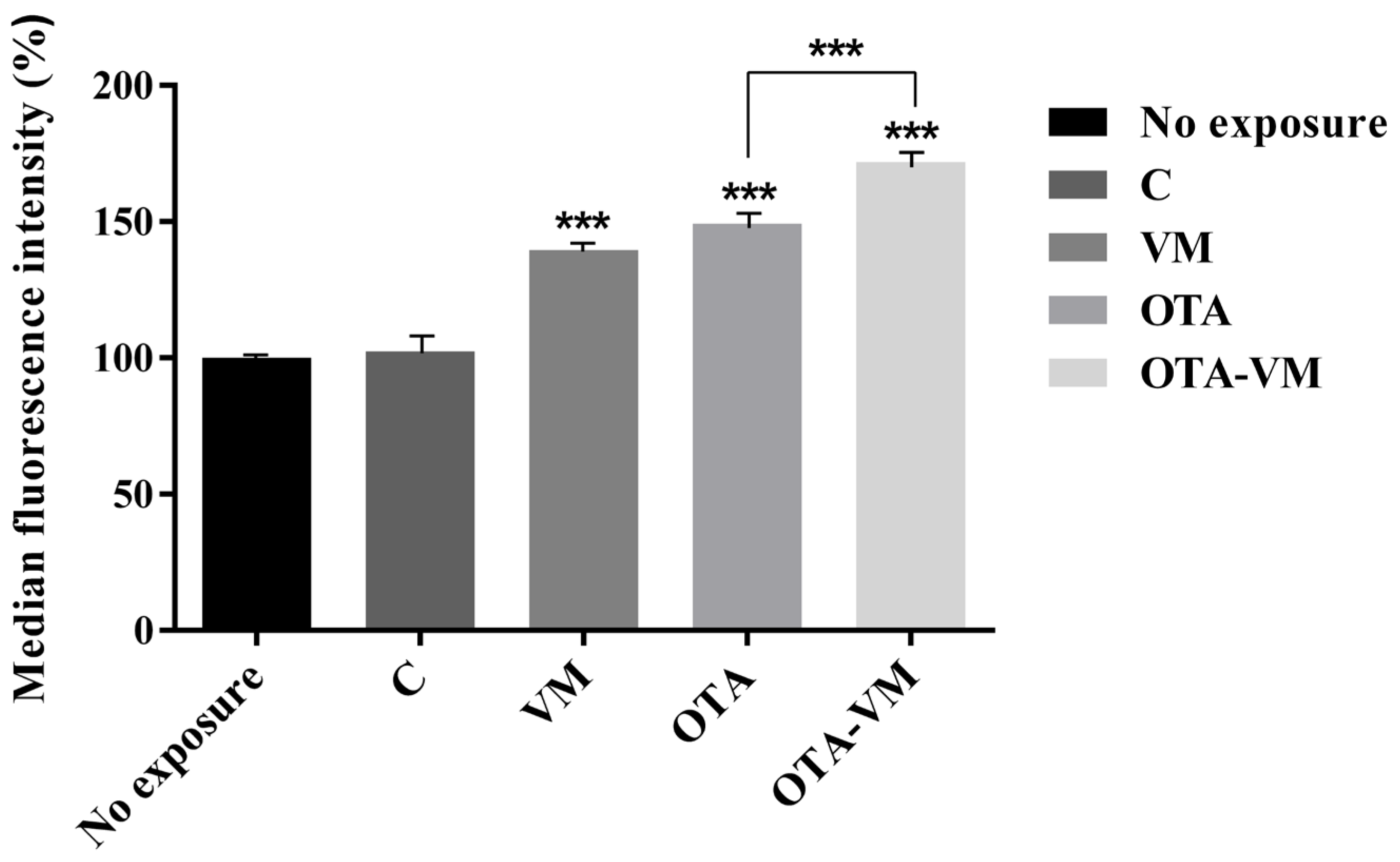
3.8. Mitochondrial Mass Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karsauliya, K.; Yahavi, C.; Pandey, A.; Bhateria, M.; Sonker, A.K.; Pandey, H.; Sharma, M.; Singh, S.P. Co-occurrence of mycotoxins: A review on bioanalytical methods for simultaneous analysis in human biological samples, mixture toxicity and risk assessment strategies. Toxicon 2022, 218, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Sultan Mayar, J.H.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, A.; Atoui, A. Ochratoxin A: General Overview and Actual Molecular Status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Ben Miri, Y.; Benabdallah, A.; Chentir, I.; Djenane, D.; Luvisi, A.; De Bellis, L. Comprehensive Insights into Ochratoxin A: Occurrence, Analysis, and Control Strategies. Foods 2024, 13, 1184. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin biomarkers of exposure: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef]
- Liu, W.-C.; Pushparaj, K.; Meyyazhagan, A.; Arumugam, V.A.; Pappuswamy, M.; Bhotla, H.K.; Baskaran, R.; Issara, U.; Balasubramanian, B.; Khaneghah, A.M. Ochratoxin A as an alarming health threat for livestock and human: A review on molecular interactions, mechanism of toxicity, detection, detoxification, and dietary prophylaxis. Toxicon 2022, 213, 59–75. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2023/915 of 25 April 2023 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2023, 119, 103–157. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 16 September 2024).
- Furlong, E.B.; Buffon, J.G.; Cerqueira, M.B.; Kupski, L. Mitigation of Mycotoxins in Food—Is It Possible? Foods 2024, 13, 1112. [Google Scholar] [CrossRef]
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, 28361. [Google Scholar] [CrossRef]
- Bilawal, A.; Ishfaq, M.; Gantumur, M.A.; Qayum, A.; Shi, R.; Fazilani, S.A.; Anwar, A.; Jiang, Z.; Hou, J. A Review of the Bioactive Ingredients of Berries and Their Applications in Curing Diseases. Food Biosci. 2021, 44, 101407. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Tufail, T.; Javed, M.; Tufail, T.; Arshad, M.U.; Hussain, M.; Gull Khan, S.; Bashir, S.; Al Jbawi, E.; Abdulaali Saewan, S. Phytochemical Profile and Pro-Healthy Properties of Berries. Int. J. Food Prop. 2022, 25, 1714–1735. [Google Scholar] [CrossRef]
- Bunea, A.; Rugină, D.O.; Pintea, A.M.; Sconţa, Z.; Bunea, C.I.; Socaciu, C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not. Bot. Horti Agrobot. 2011, 39, 70–76. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.-J. Anti-Inflammatory Activity of Bilberry (Vaccinium myrtillus L.). Curr. Issues Mol. Biol. 2022, 44, 4570–4583. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.D.; Ryu, D. Practical Strategies to Reduce Ochratoxin A in Foods. Toxins 2024, 16, 58. [Google Scholar] [CrossRef]
- Escrivá, L.; Agahi, F.; Vila-Donat, P.; Mañes, J.; Meca, G.; Manyes, L. Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin. Toxins 2022, 14, 6. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Rosende, M.; Miró, M. Combining in vitro oral bioaccessibility methods with biological assays for human exposome studies of contaminants of emerging concern in solid samples. TrAC Trends Anal. Chem. 2020, 132, 116040. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef]
- Manyes, L.; Escrivá, L.; Ruiz, M.J.; Juan-García, A. Beauvericin and Enniatin B Effects on a Human Lymphoblastoid Jurkat T-Cell Model. Food Chem. Toxicol. 2018, 115, 127–135. [Google Scholar] [CrossRef]
- Skrzydlewski, P.; Twarużek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 2004, 248–252. [Google Scholar] [CrossRef]
- Lavefve, L.; Brownmiller, C.; Howard, L.; Reeves, D.; Adams, S.H.; Chen, J.-R.; Diaz, E.C.; Mauromoustakos, A. Changes in Polyphenolics during Storage of Products Prepared with Freeze-Dried Wild Blueberry Powder. Foods 2020, 9, 466. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in Optimizing the Extraction of (Poly)Phenolic Compounds from Vaccinium myrtillus Berries. J. Chromatogr. A 2022, 1677, 463329. [Google Scholar] [CrossRef] [PubMed]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2023, 12, 91. [Google Scholar] [CrossRef]
- Solcan, M.-B.; Fizeșan, I.; Vlase, L.; Vlase, A.-M.; Rusu, M.E.; Mateș, L.; Petru, A.-E.; Creștin, I.-V.; Tomuțǎ, I.; Popa, D.-S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes nigrum L.), European Blueberry (Vaccinium myrtillus L.), and Mountain Cranberry (Vaccinium vitis-idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Sánchez, D.; Cimbalo, A.; Mañes, J.; Manyes, L. Effect of Bioactive Ingredients on Urinary Excretion of Aflatoxin B1 and Ochratoxin A in Rats, as Measured by Liquid Chromatography with Fluorescence Detection. Toxins 2024, 16, 363. [Google Scholar] [CrossRef]
- Mangiapelo, L.; Frangiamone, M.; Vila-Donat, P.; Paşca, D.; Ianni, F.; Cossignani, L.; Manyes, L. Grape pomace as a novel functional ingredient: Mitigating ochratoxin A bioaccessibility and unraveling cytoprotective mechanisms in vitro. Curr. Res. Food Sci. 2024, 9, 100800. [Google Scholar] [CrossRef]
- Lázaro, Á.; Frangiamone, M.; Maietti, A.; Cimbalo, A.; Vila-Donat, P.; Manyes, L. Allium sativum L. var. Voghiera Reduces Aflatoxin B1 Bioaccessibility and Cytotoxicity In Vitro. Foods 2024, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Shafiur Rahman, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef]
- Akan, Z.; Garip, A.I. Antioxidants may protect cancer cells from apoptosis signals and enhance cell viability. Oxid. Med. Cell. Longev. 2013, 14, 4611–4614. [Google Scholar] [CrossRef]
- Jovancevic, M.; Balijagic, J.; Menkovic, N.; Šavikin, K.; Zdunic, G.; Jankovic, T.; Dekic-Ivankovic, M. Analysis of phenolic compounds in wild populations of bilberry (Vaccinium myrtillus L.) from Montenegro. J. Med. Plants Res. 2011, 5, 910–914. [Google Scholar]
- Šavikin, K.; Zdunić, G.; Janković, T.; Tasić, S.; Menković, N.; Stević, T.; Đorđević, B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum. Nutr. 2009, 64, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.V.; Khazaei, H.; Momtaz, S.; Farzaei, M.H.; Echeverría, J. Natural Products in the Treatment of Pulmonary Emphysema: Therapeutic Effects and Mechanisms of Action. Phytomedicine 2022, 99, 153988. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.-M.; Deaconu, M.; Mitran, R.-A.; Sedky, N.K.; Schiets, F.; Marote, P.; Voicu, I.-S.; Matei, C.; Ziko, L.; Berger, D. The Antioxidant and Anti-Inflammatory Properties of Wild Bilberry Fruit Extracts Embedded in Mesoporous Silica-Type Supports: A Stability Study. Antioxidants 2024, 13, 250. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Rebellato, A.P.; dos Santos Caramês, E.T.; Pallone, J.A.L.; de Oliveira Rocha, L. Mycotoxin bioaccessibility in baby food through in vitro digestion: An overview focusing on risk assessment. Curr. Opin. Food Sci. 2021, 41, 107–115. [Google Scholar] [CrossRef]
- Bhat, P.V.; Pandareesh; Khanum, F.; Tamatam, A. Cytotoxic Effects of Ochratoxin A in Neuro-2a Cells: Role of Oxidative Stress Evidenced by N-acetylcysteine. Front. Microbiol. 2016, 7, 1142. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; González-Arias, C.A.; Ramos, A.J.; Sanchis, V.; Fernández-Cruz, M.L. Cytotoxicity of the Mycotoxins Deoxynivalenol and Ochratoxin A on Caco-2 Cell Line in Presence of Resveratrol. Toxicol. In Vitro 2015, 29, 1639–1646. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Rojas-García, A.E.; Sanchis, V.; Ramos, A.J. UPLC-MS/MS analysis of ochratoxin A metabolites produced by Caco-2 and HepG2 cells in a co-culture system. Food Chem. Toxicol. 2017, 109, 333–340. [Google Scholar] [CrossRef]
- Frangiamone, M.; Alonso-Garrido, M.; Font, G.; Cimbalo, A.; Manyes, L. Pumpkin Extract and Fermented Whey Individually and in Combination Alleviated AFB1- and OTA-Induced Alterations on Neuronal Differentiation in Vitro. Food Chem. Toxicol. 2022, 164, 113011. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.D.; Ryu, D. Protective Effect of alpha-Tocopherol Against Ochratoxin A in Kidney Cell Line HK-2. J. Food Prot. 2023, 86, 100082. [Google Scholar] [CrossRef] [PubMed]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In vitro and in vivo Evaluation of AFB1 and OTA-Toxicity through Immunofluorescence and Flow Cytometry Techniques: A Systematic Review. Food Chem. Toxicol. 2022, 160, 112798. [Google Scholar] [CrossRef]
- Cassioli, C.; Balint, S.; Compeer, E.B.; Felce, J.H.; Gamberucci, A.; Della Bella, C.; Felce, S.L.; Brunetti, J.; Valvo, S.; Pende, D.; et al. Increasing LFA-1 Expression Enhances Immune Synapse Architecture and T Cell Receptor Signaling in Jurkat E6.1 Cells. Front. Cell Dev. Biol. 2021, 9, 673446. [Google Scholar] [CrossRef]
- Frangiamone, M.; Lozano, M.; Cimbalo, A.; Lazaro, A.; Font, G.; Manyes, L. The protective effect of pumpkin and fermentedwhey mixture against AFB1 and OTA immune toxicity in vitro. A transcriptomic approach. Mol. Nutr. Food Res. 2023, 68, 2200902. [Google Scholar] [CrossRef]
- Çelik, D.A.; Gurbuz, N.; Togay, V.A.; Özçelik, N. Ochratoxin A causes cell cycle arrest in G1 and G1/S phases through p53 in HK-2 cells. Toxicon 2020, 180, 11–17. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, L.; Dai, Y.; Li, H.; Huang, K.; Luo, Y.; Xu, W. An in vitro attempt at precision toxicology reveals the involvement of DNA methylation alteration in ochratoxin A-induced G0/G1 phase arrest. Epigenetics 2020, 15, 199–214. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- García-Pérez, E.; Ryu, D.; Lee, C.; Lee, H.J. Ochratoxin A Induces Oxidative Stress in HepG2 Cells by Impairing the Gene Expression of Antioxidant Enzymes. Toxins 2021, 13, 271. [Google Scholar] [CrossRef]
- Longobardi, C.; Ferrara, G.; Andretta, E.; Montagnaro, S.; Damiano, S.; Ciarcia, R. Ochratoxin A and Kidney Oxidative Stress: The Role of Nutraceuticals in Veterinary Medicine—A Review. Toxins 2022, 14, 398. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhai, N.; Chen, X.; Gan, F.; Li, H.; Huang, K. Ochratoxin A-Induced Apoptosis of IPEC-J2 Cells through ROS-Mediated Mitochondrial Permeability Transition Pore Opening Pathway. J. Agric. Food. Chem. 2017, 65, 10630–10637. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Ayvaz, H.; Ruginǎ, D.; Leopold, L.; Stǎnilǎ, A.; Socaciu, C.; Tǎbǎran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma Inhibition by Anthocyanins Is Associated with the Reduction of Oxidative Stress Biomarkers and Changes in Mitochondrial Membrane Potential. Plant Foods Hum. Nutr. 2017, 72, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Costanzini, A.; Sgarbi, G.; Maresca, A.; Del Dotto, V.; Solaini, G.; Baracca, A. Mitochondrial Mass Assessment in a Selected Cell Line under Different Metabolic Conditions. Cells 2019, 8, 1454. [Google Scholar] [CrossRef] [PubMed]
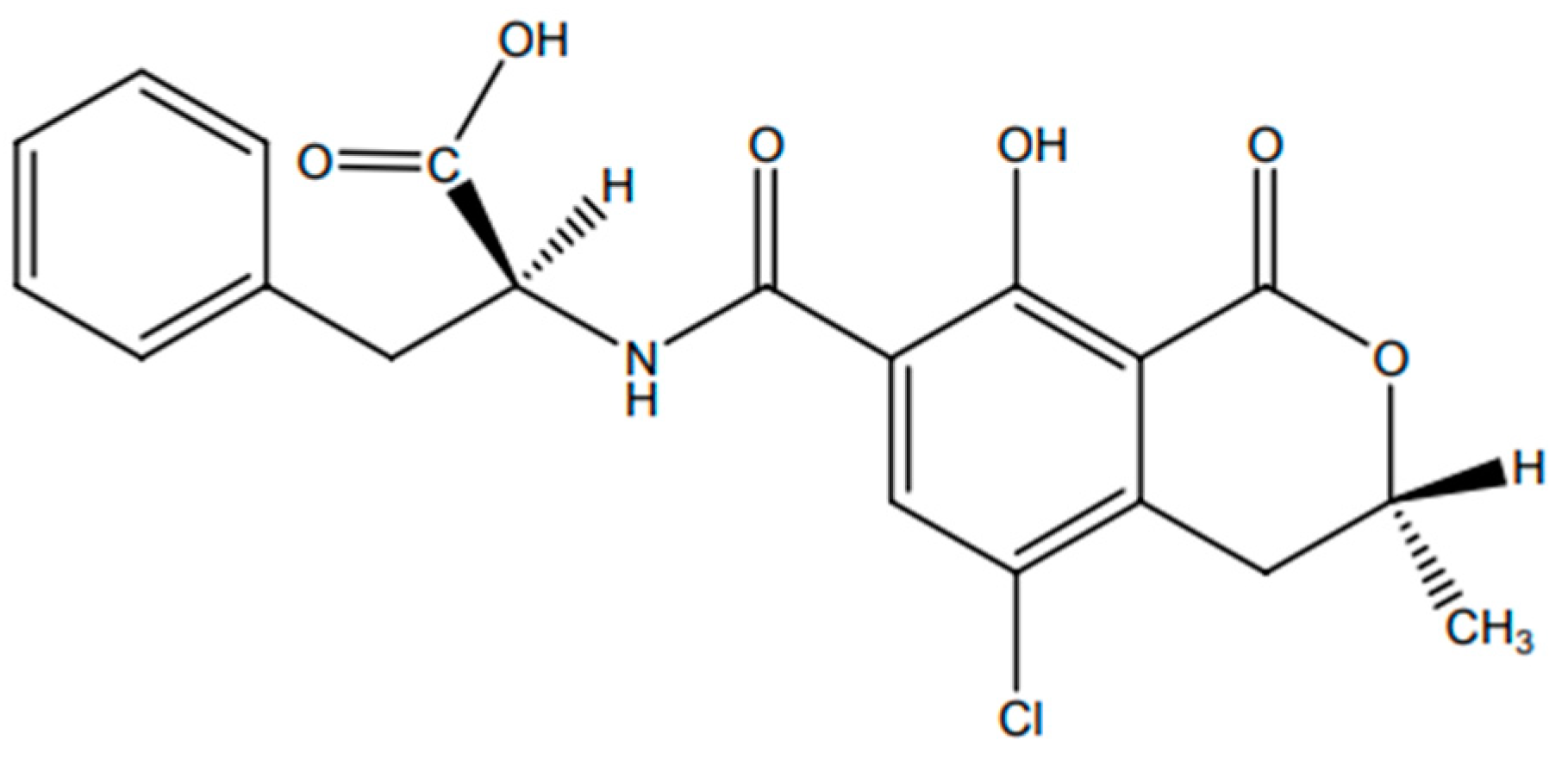

| Matrices | Linearity Range | r2 | Matrix Calibration Curve | LOD/LOQ | ||
|---|---|---|---|---|---|---|
| Barley Flour | 0.05–5 | (µg/g) | 0.999 | y = 224.14x + 2.1586 | 0.1/0.3 | (ng/g) |
| Bread | 0.3–5 | 1 | y =219.18x + 4.0963 | 0.1/0.3 | ||
| Gastric digest | 7.8–500 | (µg/L) | 0.991 | y = 385.29x + 21.437 | 0.2/0.6 | (µg/L) |
| Intestinal digest | 156–2500 | 0.997 | y = 419.11x + 19.094 | 0.2/0.6 |
| Ingredient | C (g) | VM (g) | OTA (g) | OTA-VM (g) |
|---|---|---|---|---|
| Wheat flour | 59.7 | 57.7 | 52.7 | 50.7 |
| Contaminated barley flour | - | - | 7 | 7 |
| Water | 33 | 33 | 33 | 33 |
| Salt | 1.3 | 1.3 | 1.3 | 1.3 |
| Sugar | 2 | 2 | 2 | 2 |
| Fresh yeast | 4 | 4 | 4 | 4 |
| Lyophilized bilberry | - | 2 | - | 2 |
| Total Quantity | 100 | 100 | 100 | 100 |
| Compound | Concentration | |
|---|---|---|
| TPC | mg/g | 62.29 ± 2.32 |
| DPPH | 114.48 ± 2.99 |
| Category | Compound | Concentration | |
|---|---|---|---|
| Hydroxycinnamic Acids | Chlorogenic acid | (µg/g) | 1350.33 ± 27 |
| Hydroxybenzoic Acids | Gallic acid | (µg/g) | 45.85 ± 2.75 |
| Protocatechuic acid | 25.18 ± 1.00 | ||
| Flavanols | (+)-Epicatechin | (µg/g) | 15.67 ± 0.78 |
| (−)-Catechin | 0.22 ± 0.01 | ||
| Procyanidin A1 | - | ||
| Procyanidin B1 | 5.85 ± 0.06 | ||
| Procyanidin B2 | 46.56 ± 6.98 | ||
| Procyanidin B3 | 1.79 ± 0.05 | ||
| Procyanidin B4 | 9.73 ± 1.46 | ||
| Procyanidin C1 | 41.29 ± 1.24 | ||
| Procyanidin C2 | - | ||
| Flavonols | Hyperoside | (µg/g) | 210.31 ± 27.34 |
| Isoquercitrin | 66.30 ± 0.66 | ||
| Quercitrin | 24.84 ± 1.49 | ||
| Quercetol | 5.10 ± 0.25 | ||
| Anthocyanins | Delphinidin 3-galactoside | (mg/g) | 2.15 ± 0.24 |
| Delphinidin 3-glucoside | 3.37 ± 0.23 | ||
| Cyanidin-3-O-galactoside | 2.53 ± 0.18 | ||
| Cyanidin 3-glucoside | - | ||
| Cyanidin 3-arabinoside | 1.37 ± 0.05 | ||
| Petunidin 3-glucoside | 1.84 ± 0.11 | ||
| Malvidin 3-glucoside | 1.82 ± 0.13 |
| Matrices | OTA Concentration | |
|---|---|---|
| Contaminated Barley Flour | (mg/kg) | 149.05 ± 7.89 |
| OTA-Bread | 15.89 ± 0.13 | |
| OTA-VM-Bread | 16.79 ± 0.55 | |
| OTA Gastric Digest | (µg/L) | 15.22 ± 4.93 |
| OTA-VM Gastric Digest | 7.13 ± 3.18 | |
| OTA Intestinal Digest | 1500.50 ± 1.22 | |
| OTA-VM Intestinal Digest | 1287.58 ± 28.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pașca, D.; Frangiamone, M.; Mangiapelo, L.; Vila-Donat, P.; Mîrza, O.; Vlase, A.-M.; Miere, D.; Filip, L.; Mañes, J.; Loghin, F.; et al. Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products. Nutrients 2024, 16, 3137. https://doi.org/10.3390/nu16183137
Pașca D, Frangiamone M, Mangiapelo L, Vila-Donat P, Mîrza O, Vlase A-M, Miere D, Filip L, Mañes J, Loghin F, et al. Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products. Nutrients. 2024; 16(18):3137. https://doi.org/10.3390/nu16183137
Chicago/Turabian StylePașca, Denisia, Massimo Frangiamone, Luciano Mangiapelo, Pilar Vila-Donat, Oana Mîrza, Ana-Maria Vlase, Doina Miere, Lorena Filip, Jordi Mañes, Felicia Loghin, and et al. 2024. "Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products" Nutrients 16, no. 18: 3137. https://doi.org/10.3390/nu16183137
APA StylePașca, D., Frangiamone, M., Mangiapelo, L., Vila-Donat, P., Mîrza, O., Vlase, A.-M., Miere, D., Filip, L., Mañes, J., Loghin, F., & Manyes, L. (2024). Phytochemical Characterization of Bilberries and Their Potential as a Functional Ingredient to Mitigate Ochratoxin A Toxicity in Cereal-Based Products. Nutrients, 16(18), 3137. https://doi.org/10.3390/nu16183137








