Abstract
Individuals with dementia and neurodegenerative diseases (NDDs) often suffer from cardiovascular diseases (CVDs). Neuroinflammation driven by conditions involved in CVDs is linked to disruptions in the central nervous system triggering immune reactions, perpetuating an “inflammatory-like” environment. The Mediterranean diet (MedDiet), known for its anti-inflammatory and antioxidant properties, has been proposed as a key factor to attenuate these risks. Blood nuclear cell samples were collected from 134 participants of the PREDIMED trial, which randomized participants to three diets: one supplemented with extra-virgin olive oil (MedDiet-EVOO), another with nuts (MedDiet-Nuts), and a low-fat control diet. These samples were analyzed at baseline and 12-month follow-up to assess the impact of these dietary interventions on gene expression markers. We first selected target genes by analyzing intersections between NDD and CVD associations. Significant gene expression changes from baseline to 12 months were observed in the participants allocated to the MedDiet-EVOO, particularly in CDKN2A, IFNG, NLRP3, PIK3CB, and TGFB2. Additionally, TGFB2 expression changed over time in the MedDiet-Nuts group. Comparative analyses showed significant differences in TGFB2 between MedDiet-EVOO and control, and in NAMPT between MedDiet-Nuts and control. Longitudinal models adjusted for different covariates also revealed significant effects for TGFB2 and NAMPT. In conclusion, our results suggest that one year of traditional MedDiet, especially MedDiet-EVOO, modulates gene expression associated with CVD risk and NDDs in older adults at high CV risk.
1. Introduction
A significant proportion of patients affected by dementia have common comorbidities such as cardiovascular diseases (CVDs), type 2 diabetes, hypertension, dyslipidemia or excess body weight. CVDs and neurodegenerative diseases (NDDs) share risk factors that predispose and accelerate both pathologies [1]. One critical non-modifiable pro-inflammatory factor that must be considered is inflammaging [2], a term referring to the chronic progressive stress caused by aging, which increases inflammatory status. Inflammaging added to cardio metabolic risk factors first triggers immune responses in peripheral organs, initiating a systemic inflammatory state that later affects the central nervous system (CNS) and eventually disrupts homeostasis in both areas [3,4]. Once inflammatory cells, molecules, or cytokines infiltrate the CNS, resident glial cells are activated, perpetuating neuroinflammation [3,5,6,7].
Neuroinflammation is characterized by a primary immune reaction to brain injury mediated by key pro-inflammatory cytokines, particularly interleukin (IL)-1b, IL-6, and tumor necrosis factor (TNFα). The neuroinflammatory process involves the activation and priming of glial cells, during which microglia exert macrophage-like functions such as vital surveillance, scavenging, antigen presentation, and cell repair [8]. The released cytokines increase blood–brain barrier (BBB) permeability [9], thereby increasing their own concentrations in the brain, which intensify microglia’s pro-inflammatory responses [10]. The type, degree, and duration of the stimulus determine the neuroinflammatory damage responsible for the destruction of brain tissues characteristic of NDDs [10,11,12]. NDDs are influenced by a wide range of factors such as age, sex, nutrition, socio-economic, and genetic determinants, in addition to lifestyle habits (physical activity, metal health, and wellbeing) that interplay in triggering or accelerating the disease [12,13,14].
It is known that there are components in Mediterranean dietary patterns that exert a protective effect, contributing to attenuate the neuroinflammatory state [12,15]. Resveratrol, tyrosol, anthocyanins, and isoflavones, found in common sources of the Mediterranean diet (such as olive oil, fruits, and legumes), have been associated with varying degrees of consistency in improving cognitive function. From a mechanistic point of view, they exert anti-inflammatory, anti-apoptotic, and neuroprotective effects by modulating various pathways involved in oxidative stress, inflammatory mediators, and promoting cell survival mechanisms [16]. Further, the gut–brain axis has recently been proved as a key entity influencing multiple states or diseases, being susceptible to modification by a variety of factors including diet, drug intake, or medical procedures [17].
Epidemiological studies have tried to bridge the gap between CVDs and Alzheimer’s disease (AD) through association studies, identifying common variants of both diseases [18,19,20]. Additionally, research on the transcriptional profile has inferred genetic information from blood cell analysis, reflecting primary alterations occurring in tissue. Cell-free RNA has been demonstrated to distinguish AD patients versus age-matched controls by comparing transcript levels of AD-related genes [21]. In this regard, advancements in this field have revealed correlations between disease severity and the characteristics of circulating transcriptomes [22].
Thus, systemic inflammation and neuroinflammation play pivotal roles in the progression of NDDs, wherein both established chronic inflammatory processes contribute to brain damage and cognitive impairment. MedDiets may benefit brain function by attenuating both neuroinflammation and systemic inflammation, measured through different gene biomarkers linked to these pathological processes.
The aim of this sub-study of the PREDIMED randomized trial [21] was to determine the effect of a long-term Mediterranean diet (MedDiet) intervention on the gene expression of transcriptomic biomarkers related to neuroinflammation and cardiovascular risk in an older population at high cardiovascular risk.
2. Materials and Methods
2.1. Study Design and Population Recruitment
The study population was a random subsample of volunteers (n = 134, 67 men and 67 women) recruited in different sites of the large-scale multicenter randomized, controlled trial PREDIMED (flow chart in Figure S1 Supplementary Material). PREDIMED assessed the effect of MedDiets on the primary prevention of CVD [23]. In this trial, two traditional MedDiets were tested for long-term effects on CVD risk, one enriched with extra-virgin olive oil (MedDiet-EVOO) and another enriched with raw nuts (MedDiet-Nuts) vs. a control diet based on advice to reduce the fat content of the diet.
Eligible participants were women and men, aged 60–80 years and 55–80 years, respectively, who met at least one of the following criteria: (1) type 2 diabetes or (2) ≥3 major cardiovascular risk factors: current smoking (>1 cig/day during the last month); hypertension (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or antihypertensive medication); LDL cholesterol ≥ 160 mg/dL or lipid-lowering therapy; HDL cholesterol ≤ 40 mg/dL in men or ≤50 mg/dL in women; body mass index ≥ 25 kg/m2; and family history of early-onset coronary heart disease [23]. Exclusion criteria were a prior history of CVD, severe chronic illnesses, substance abuse, allergies or intolerance to olive oil or nuts, a low predicted likelihood of changing dietary habits based on the stages of change model [24], or any condition that might hinder study participation.
2.2. Blood Chemistry Analysis
Sample collection was performed after an overnight fast at baseline and after a 12-month follow-up. Collected samples were centrifuged immediately after extraction, both for 15 min at 1.700× g room temperature. The following analytes were quantified in serum with an ABX Pentra-400 auto-analyzer (Horiba-ABX, Montpellier, France): glucose (mg/dL), triglycerides (mg/dL), HDL-cholesterol (mg/dL), and total cholesterol (mg/dL). LDL-cholesterol was calculated according to the Friedewald formula when triglycerides were <300 mg/dL.
2.3. Cardiovascular and Lifestyle Factors
Dyslipidemia was defined as meeting any of the following criteria: HDL-cholesterol < 40 mg/dL or 50 mg/dL (for men and women respectively), LDL-cholesterol > 200 mg/dL, triglycerides > 150 mg/dL or taking any lipid-lowering drugs. Adherence to the MedDiet was assessed by a validated 14-item questionnaire [25]. Physical activity was recorded via the Minnesota leisure time physical activity questionnaire [26,27].
2.4. RNA Extraction, Reverse Transcription, and Gene Expression Analysis
Nuclear cells were extracted from peripheral blood by using tubes for purification of intracellular RNA from human whole blood (range of white blood cells 4.8 × 106–1.1 × 107 leukocytes/mL) for in vitro diagnostics applications (PAXgene Blood RNA Tube, BRT, Hombrechtikon, Switzerland). The RNA concentration (A260) and purity were calculated spectrophotometrically (NanoDrop ND-1000; NanoDrop Technologies, v3.5, Wilmington, NC, USA). RNA integrity was assessed by using microcapillary gel electrophoresis (Bioanalyzer, NanoChip; Agilent Technologies, version 2.6, Waldbronn, Germany) and the RNA integrity number value (RIN) was calculated with Agilent 2100 Expert Software (Agilent Technologies).
Low-input samples (50–200 ng/µL) underwent preamplification because recommendations pointed target levels should be above 200 ng/µL. Amplification was performed using TaqMan® PreAmp Master Mix (Applied Biosystems, Vilnius, Lithuania). Reverse transcription to cDNA was carried out with a High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Life Technologies, Vilnius, Lithuania). Microarray RT-PCR step was performed using a QuantStudio™ 12K Flex Real-Time PCR System (Life Technologies) and TaqMan® OpenArray™ Real-Time PCR Master Mix (Aplied Biosystems, Vilnius, Lithuania). Finally, results were analyzed with QuantStudio™ 12K Flex Software version 1.3.
2.5. Selection of Gene Targets
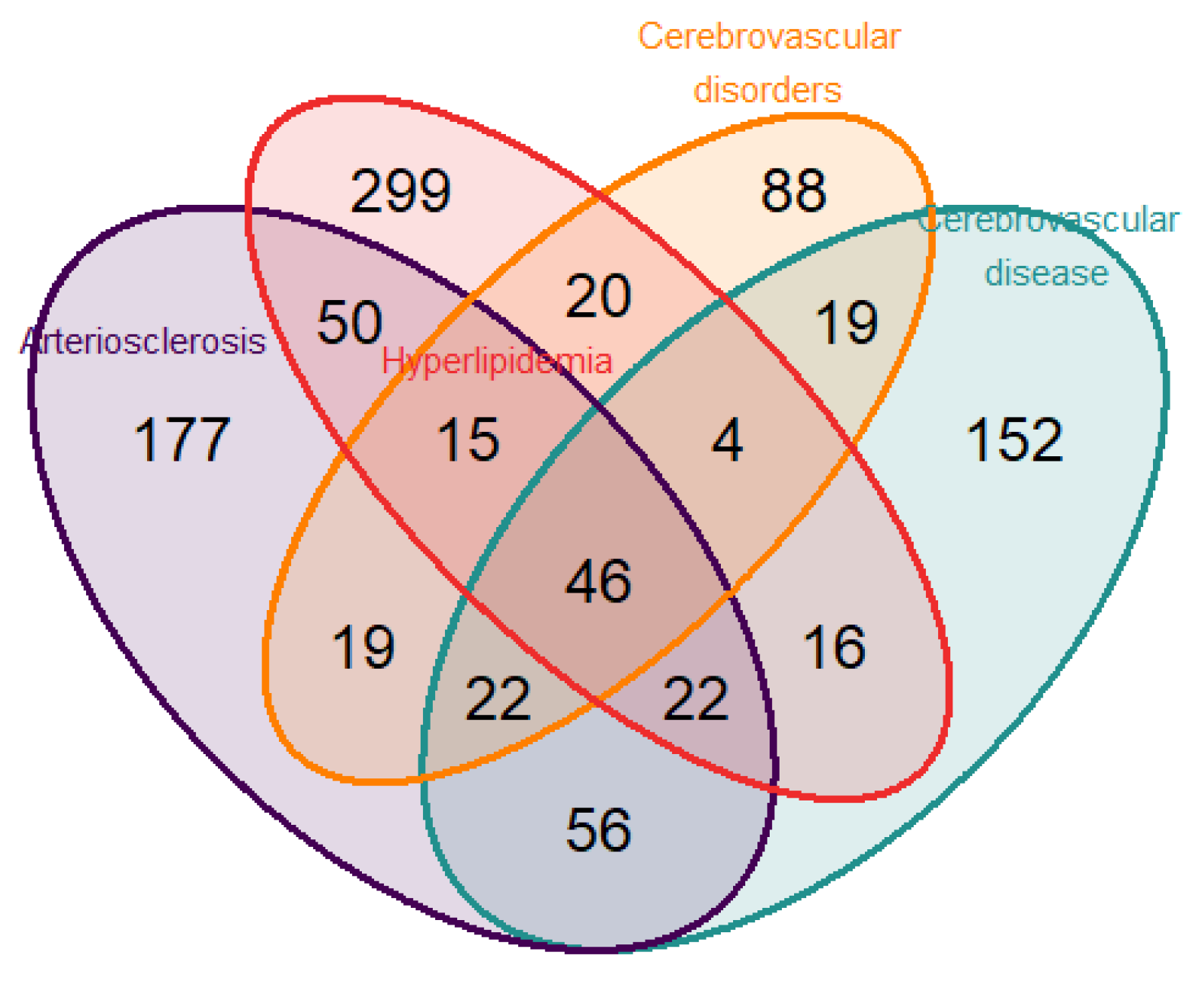
To identify genes related to both neurodegenerative disease and CVD, we performed a disease enrichment analysis to search human gene–disease interactions. The search was executed including the following terms: “atherosclerosis”, “cognitive”, “cholesterol”, “lipid”, “metabolism”, “cerebrovascular”, “dementia”, and “arteriosclerosis”. From the intersection of the selected diseases, “Arteriosclerosis” and “Cerebrovascular disease” in DisGeNET (Database of Gene-Disease Associations) v6 and v7 [28] and “Hyperlipidemia” and “Cerebrovascular disorders” in the Human Disease Ontology [29] (Figure 1), a total of 46 genes were identified. For the present study, the nine genes selected from the intersection according to their biological functions were (a) IFNG (Interferon Gamma); IL10 (Interleukin 10); NFE2L2 (Nuclear Factor, Erythroid 2 Like 2); NLRP3 (NLR Family Pyrin Domain Containing 3); TGFB2 (Transforming Growth Factor Beta 2); (b) Regulators of the senescence and cell cycle: CDKN2A (Cyclin Dependent Kinase Inhibitor 2A); (c) Metabolic and Cell Signaling Regulators: PIK3CB (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Beta); and NAMPT (Nicotinamide Phosphoribosyltransferase).
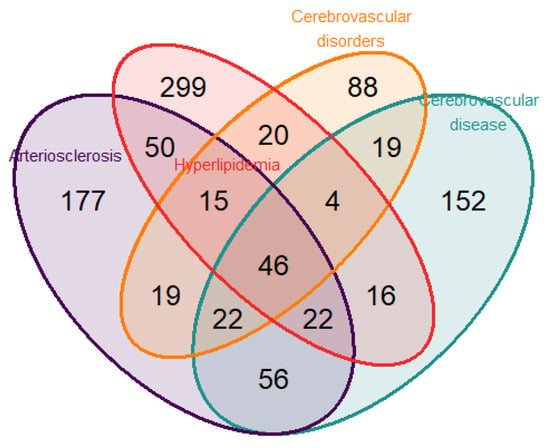
Figure 1.
Venn diagram displaying the number of genes found from the overlap of the 4 selected pathologies (atherosclerosis, hyperlipidemia, cerebrovascular disorders, and cerebrovascular disease) employing the public databases DisGeNET and Disease Ontology.
2.6. Reference Genes and Relative Quantification
We employed the relative quantification approach to present the analysis of gene expression data. We tested 3 potential candidates as control genes (also known as reference or housekeeping genes). The reference genes were chosen using the geNorm algorithm, which conducted a preliminary analysis to distinguish among 21 candidates. The algorithm tested gene expression variation across different samples, resulting in genes with lower M values, or those that were more stably expressed and, therefore, better candidates: we selected GAPDH as a reference. To study differences between baseline value and 12-month follow-up we compared ∆Ct.
The efficiency of the target and reference genes was 100% ± 10% [30,31,32], we applied the 2−ΔΔCt method to quantify changes in gene expression. Therefore, the data are presented as a fold change value normalized to the reference gene and relative to the baseline value. Each pair of patient samples was allocated in the same plate to remove potential run-to-run variation [33,34,35,36].
2.7. Statistical Analyses
The sample size of 36 participants allowed at least 80% power to detect statistically significant differences in PIK3CB gene expression among the 3 groups, within the PREDIMED study of 0.4 units of the relative quantification log2FC, assuming a 2-sided type error of 0.05. A common standard deviation (SD) of 0.6 was estimated.
The assessment of the normality distribution was conducted using normality probability plots and boxplots. Descriptive statistics (mean and standard values) and comparison were calculated at baseline, post-intervention, and 12-month change to display nutritional parameters, energy intake, and key food components. The MedDiets were compared with the control diet using independent Student T-tests. We compared individuals’ changes along the intervention. To assess inter-group differences, independent Student T-tests were calculated employing ΔCt values. To account for the effect of relevant covariates, we performed two models: the first one was adjusted for age, sex, time, and education level; and the second one was additionally adjusted for diabetes, BMI, physical activity, smoking status, hypertension, and dyslipidemia. Diabetes and hypertension were treated as binary qualitative variables, categorized as either ‘Yes’ or ‘No’. Smoking status was stratified as a qualitative variable with three categories: current smoker, former smoker, and never smoker. Dyslipidemia was previously defined as a composite variable based on HDL-cholesterol, LDL-cholesterol, triglyceride levels, or lipid-lowering drugs. Educational level was a qualitative variable comprising 3 categories: higher education or equivalent, secondary education, or primary education. Physical activity was measured as a continuous variable in MET-minutes per week (METmin/week). BMI was also treated as a continuous quantitative variable, expressed in kg/m2. The interaction term time:group of intervention to explain inter-group variability across the trial. Inter-individual variability was assessed through random intercept. The linear mixed-effect model was estimated using restricted maximum likelihood. We used the lme function from the package nlme, using R software version 4.3.2 [37].
3. Results
The mean age and standard deviation of the study population was 66.1 (±6.34) years. Table 1 displays cardiovascular risk factors stratified per group of intervention. Among the subsample, 53% of participants had diabetes, 57% had dyslipidemia, 79% had hypertension, and 15% were smokers. Baseline characteristics of the groups were similar to those of the entire PREDIMED cohort (Supplementary Table S1). We discarded a total of 17 samples due to technical issues during the experimental phase of RT-qPCR.

Table 1.
General characteristics of the study population. Values are expressed as a percentage (for categorical variables) and mean (and standard deviation) for quantitative continuous variables. MedDiet-EVOO, Mediterranean diet supplemented extra virgin olive oil; MedDiet-Nuts, Mediterranean diet supplemented with nuts.
Energy intake, nutritional parameters, and key food components are summarized in Supplementary Table S2. No between-group differences in 12-month change in energy intake were found. Between-group 12-month differences in changes were observed for total fat intake, mainly due to monounsaturated fatty acids (MUFA), between MedDiet-EVOO and MedDiet-Nuts compared to the control diet. We also observed differences in polyunsaturated fatty acids (PUFA) changes between the MedDiet-Nuts and control diet groups. The overall consumption of virgin olive oil and nuts were respectively increased in the MedDiet-EVOO and MedDiet-Nuts interventions compared to the control diet.
Gene Expression
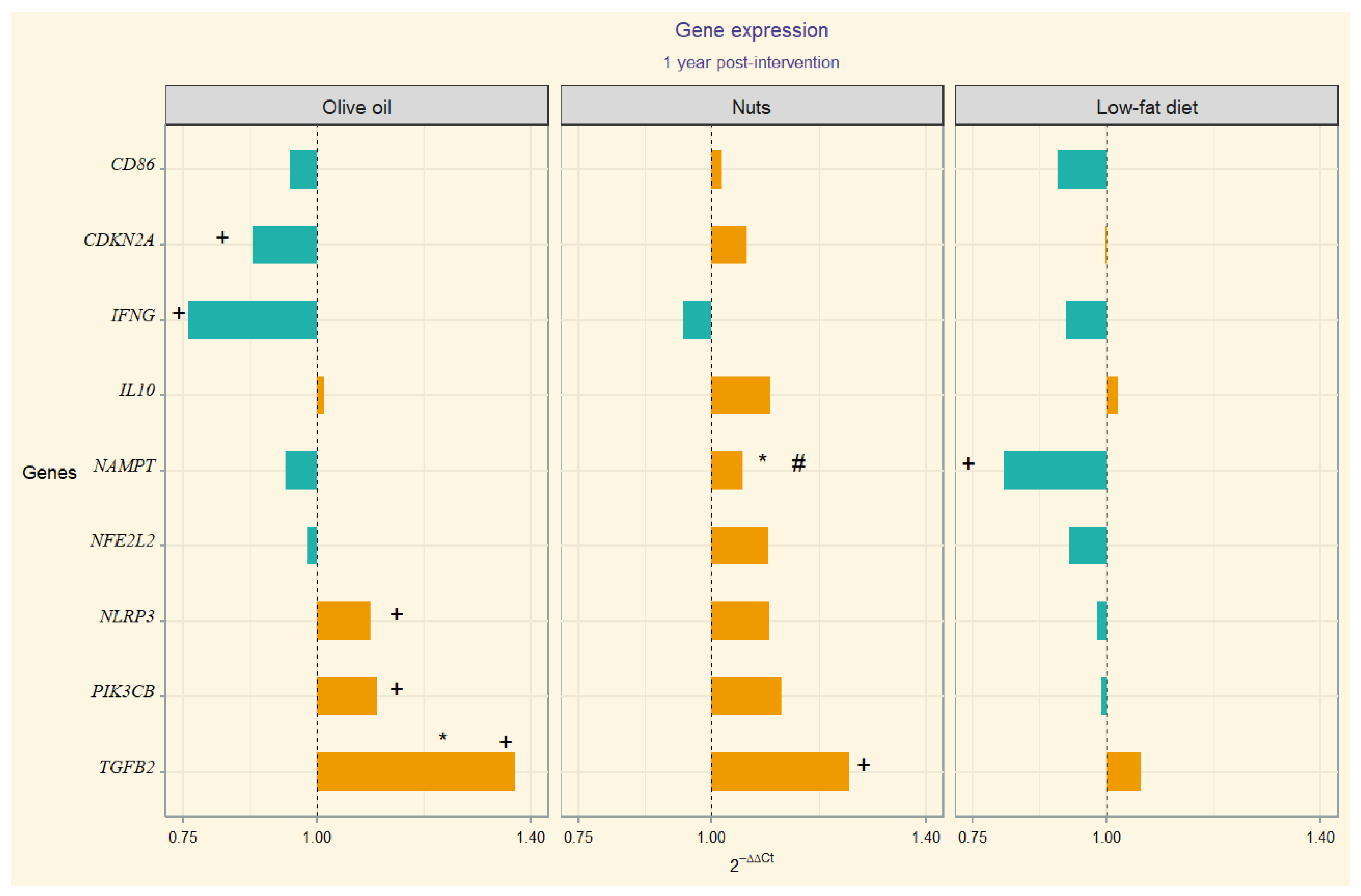
The 2−ΔΔCt values correspondent to gene expression quantification (upregulation or downregulation) are depicted through divergent bars in a visual chart (Figure 1). In the MedDiet-EVOO intervention group, we observed significant 12-month changes in the following gene expression values: CDKN2A, IFNG, NLRP3, PIK3CB, and TGFB2. In this line, we observed temporal change in TGB2 in MedDiet-Nuts. Significant between-group differences in TGFB2 expression were found between the MedDiet-EVOO and control diet. Additionally, significant differences in NAMPT expression were observed in between-group comparisons of MedDiet-Nuts and the control diet group. Model 1 (adjusted for sex, age, and education level) resulted in statistically significant differences for the interaction term time-group of intervention in TGFB2 between the MedDiet-EVOO and control diet. The comparison between the MedDiet-Nuts and control diet disclosed significant results in NAMPT. Meanwhile, model 2 only showed significant differences by statistical criterion in NAMPT in MedDiet-Nuts. The results of the statistical analyses, including within-group, between-group comparisons, and model outputs, are presented in Supplementary Table S3.
4. Discussion
In the search of the molecular mechanisms by which a cardioprotective diet like the Mediterranean one benefits brain health, we analyzed neuroinflammation- and systemic inflammation-related genes’ behavior after a Mediterranean diet intervention. Specifically, the MedDiet-EVOO modulated the expression of the CDKN2A, IFNG, NLRP3, PIK3CB, and TGFB2 genes in the peripheral blood mononuclear cells of older adults at high cardiovascular risk, whereas the MedDiet-Nuts resulted in a different expression of the NAMPT gene compared to the control diet (Figure 2).
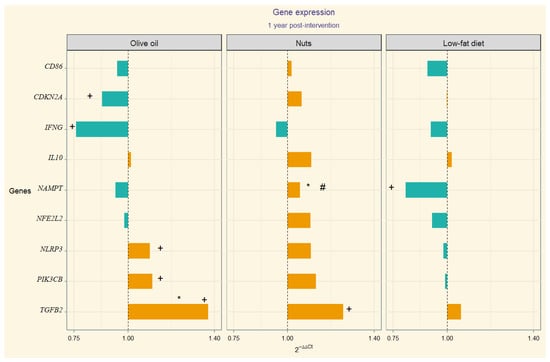
Figure 2.
Divergent bar showing 2−∆∆Ct per group (Orange bars represent upregulation of gene expression, while blue bars indicate downregulation). Statistically significant (p-value < 0.05): + baseline to post-intervention change; * individual comparison between MedDiet-EVOO and MedDiet-Nuts versus control; # time:group interaction (p-value) from mixed-effects of the fully adjusted model compared to control. Numerical p-values are presented in Supplementary Table S3.
There is evidence that AD patients display high plasma levels of numerous pro-inflammatory markers [38,39], including IFN-γ protein levels [40]. In this line, it is known that TGFβ interacts with IFN-γ, indicating a crosstalk between these pleiotropic cytokines, functioning as reciprocal regulation [41]. TGF-β’s signaling is involved in multiple neurological pathways, regulating synaptic growth, neurotrophic functions, and cell survival, although their role is not completely understood, especially in AD [42,43,44]. Research on the role of TGF-β1 in dementia [41,42,44] has shown reduced plasma concentrations in patients with AD [45].
Despite that Aβ42 levels rise at the onset of AD, as the disease progresses, they typically decrease [46,47]. In the present study, we observed an increased expression of TGF-β2 (Figure 2), in particular after the MedDiet interventions, suggesting a possible protective role in this older population. This is aligned with some limited evidence that points at a neurotrophic effect [48], although TGF-β2 functions remain unclear. However, prior research has shown that in AD patients, neurons bearing neurofibrillary tangles exhibit increased expression of TGF-β2 compared to cognitive age-matched controls [49,50,51]. There is limited evidence, based on small studies, that TGF-β2 may play a deleterious role in AD, justified by the higher protein levels found in the brains of AD patients [50,52], but on the other hand, there seems to be a reduced presence of receptors in the neurons of AD patients [51,53]. Our results may be in favor of the effect of the Mediterranean diet through the TGFβ2 anti-apoptotic effect described in other cell types [54].
The inflammasome is a multiprotein complex of the innate immune system that detects danger signals such as tissue damage, cellular stress, or infection. Cytokines become active by inflammasome mediation, triggering local and systemic inflammatory responses essential for immune defense but also involved in chronic inflammatory diseases when deregulated [55]. The NLRP3 inflammasome plays a pivotal role in the onset and progression of Aβ in mice, and may participate in protein tau pathology as well [56]. Prior research conducted to assess the effect of MedDiet adherence counteracting neuroinflammation and age-related diseases revealed that a 3-month intervention with a MedDiet supplemented with different olive oils was associated with downregulation of IFNG transcriptomic levels [57,58], among other inflammatory and proatherogenic biomarkers [59]. In a long-term (3 years) transcriptomic PREDIMED sub-study, a downregulation of both IFN-γ and NLRP3 was reported [60].
Hormesis is based on exposure to a substance exerting a biphasic response depending on the dose [61,62]. This may explain possible differences regarding the gene expression modulation by diet, specifically the upregulation pattern observed in MedDiet-EVOO (Figure 2). The inter-species hormesis theory postulates that the stress-induced synthesis of plant polyphenols, among other phytochemicals, can produce an environmental chemical signature that leads to stress resistance in other species [63,64,65]. In this regard, an hormetic dose-response behavior regarding NLRP3 has been described considering polyphenol intake [66,67,68] that aligns with this hypothesis, suggesting that hormesis may explain these findings.
NAMPT is the gene encoding visfatin, a dual-form ubiquitously expressed, whose functionality encompasses multiple processes related to insulin sensitivity, NDDs, lipid metabolism, atherosclerosis, and pro-inflammatory effects [69,70,71], including NLRP3 inflammasome activation [72,73]. It has also been described that NAMPT promotes IFN-γ secretion in CD4+ T lymphocytes, but paradoxically the IFNG transcript was upregulated when NAMPT was inhibited, suggesting a posttranslational level regulation [74]. An influence of diet on NAMPT expression has been described [75,76]. With regard to visfatin circulating levels, a decrease after a hypocaloric diet with significant weight loss has been described [77]. It has also been reported that the fat type can influence the visfatin response [78,79]. In this regard, no significant weight change was apparent with the MedDiet interventions in the full PREDIMED cohort [80]. Nevertheless, we have observed a significant downregulation of NAMPT in the control diet group compared to the MedDiet supplemented with nuts (Figure 2).
In the present study, the MedDiet-EVOO was the unique group in which an upregulation of PIK3CB and downregulation of CDKN2A versus baseline was observed (Figure 2). In one RNA-seq data study, the mean expression of PIK3CB in AD patients was lower than that of controls, suggesting a role in AD pathogenesis through apoptosis [81]. Similarly, altered blood expression of CDKN2A, measured by qRT-PCR, was reported in preclinical AD patients compared to controls [82]. The CDKN2A protein has previously been described to be upregulated in the brains of patients with AD [83]. These findings suggest that whole blood could be an emerging valuable tissue for unraveling the pathophysiology of AD and differentiating it from normal aging [84].
Several meta-analyses conducted in older adults (60–80 y) have concluded that greater MedDiet adherence usually correlates with an overall better cognitive performance [85,86,87]. Nevertheless, the evidence for causality is weak. The Three-City cohort is a French longitudinal (4-year follow-up) study designed to assess the risk of dementia, with almost 10,000 older participants (mean age, 74 years) [88,89], which did not demonstrate an association between adherence to the MedDiet and the risk of developing dementia [90].
The disbalance between oxidative stress and antioxidant systems has been suggested to play a role in the pathophysiology of NDDs. Further evidence is required to demonstrate if MedDiet can exert a protective effect on dementia or delay its onset [91,92] and explore the underlying molecular mechanisms of these benefits.
Strengths and Limitations
The first limitation is the relatively small sample size, which may result in limited statistical power. More repeated measurements are required to study the dynamic behavior of molecular mechanisms, and therefore improve the strength of the conclusions. As a consequence, the study may not have sufficient sensitivity to identify relevant associations between the variables of interest. The second limitation is that cognitive impairment and risk of dementia were not primary endpoints in the PREDIMED study. No cognitive tests were performed, even though alternative subcohorts within the PREDIMED study have evaluated such outcomes. The third limitation is the biological specimen employed in the transcriptomic study, taking into account that gene expression varies depending on the tissue analyzed. In this regard, PBMCs from blood are useful for studying cardiovascular biomarkers such as inflammation and peripheral cholesterol efflux. However, blood is not a good indicator for selective gene expression in other tissues. Although neurons and cerebral microvasculature cells are closely related to neurodegenerative risk, collecting these cells in population-based research is impractical. Finally, our cohort comprised participants at high cardiovascular risk. Thus, results may not be generalized to the average elderly population.
5. Conclusions
Our results suggested that the expression of inflammatory pathways-related genes linked to both CVD and NDDs is moderately modulated by a traditional MedDiet, especially when supplemented with extra-virgin olive oil, in circulating peripheral blood nuclear cells of older adults at high cardiovascular risk. In particular, the MedDiet-EVOO modulated the expression of CDKN2A, IFNG, NLRP3, PIK3CB, and TGFB2 genes, whereas the MedDiet-Nuts differently expressed NAMPT compared to the control diet. The underlying molecular mechanisms could explain the brain benefits of a cardioprotective diet, such as the Mediterranean diet, although more evidence on large-sized and long-term lifestyle interventions is needed. The research on biomarkers may discover specific molecules able to assess neuroinflammatory process in an accessible way. The combination with imaging techniques may enhance the detection and monitoring of these processes, offering a more comprehensive approach to diagnosis and treatment strategies for neuroinflammatory conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16183147/s1, Figure S1: Flowchart of participant enrollment, randomization, and analysis in the PREDIMED; Table S1: Baseline characteristics of the subsample participants of PREDIMED (N = 134) and comparison with the correspondent baseline characteristics of the complete PREDIMED Study (N = 7237). Values are expressed as a percentage (for categorical variables), mean (standard deviation). Chi-Square test was performed for categorical variables and Student-T test for quantitative continuous variables. Table S2: Mean Consumption and Dietary Changes at Baseline, 12 Months, and Follow-up Comparison. Table S3: Student-T Test and Mixed-Effects Model Comparison of Gene Expression Changes Over 12 Months.
Author Contributions
M.F., J.S.-S., M.Á.M.-G., D.C., E.R. and R.E. were responsible for the development and planning of the clinical trial. O.C. and M.F. conceptualized the sub-study. J.H.-R. carried out the formal analysis, data curation, and software management, while also contributing to the visualization and methodology. O.C., M.F. and J.H.-R. prepared the initial draft of the manuscript. M.M., K.A.P.-V., I.P.-G. and I.B.-C. contributed to the manuscript by reviewing and editing the content. M.Á.M.-G., D.C. and R.E. were also involved in the investigation and conceptualization of the study. X.P., F.A., J.L. and E.R. contributed to the investigation and writing, focusing on reviewing and editing the article. D.R. and R.C.-G. assisted in the writing, review, and editing processes. All authors contributed to the content review and editing of the article and approved the final submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Project “PI20/00012“, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union. It was also supported by Instituto de Salud Carlos III (PI24/00326, PI18/00020; PI19/00017; PI16/00533; PI13/00233; PI20/00012; CP21/00097; PI17/00214) and by Agència de Gestió d’Ajuts Universitaris i de Recerca (2021 SGR 00144). The funders played no role in the study design, collection, analysis, or interpretation of data, nor in the process of writing the manuscript and its publication. J.H.-R. had received the Contrato Rio Hortega CM20/00085 grant. This work was supported by the Instituto de Salud Carlos III through specific network granted to Ramon Estruch (2003–2005) and Miguel A. Martínez-González (2006–2013) for developing the PREDIMED trial.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Hospital del Mar d’Investigacions Mèdiques: protocol code 2017/7482/I in Barcelona, 7 March 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset analyzed during the current study cannot be made publicly available due to national data regulations and ethical considerations, including the absence of explicit written consent from study participants to make their deidentified data available upon study completion. However, data described in the manuscript will be shared with bona fide investigators for collaboration upon request. Requests for collaboration can be made by sending a letter to the PREDIMED Steering Committee (predimed-steering-committee@googlegroups.com).
Acknowledgments
The authors extend their gratitude to all participants of the PREDIMED study. They also acknowledge the support of CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and CIBER de Epidemiología y Salud Pública, both initiatives of the Instituto de Salud Carlos III (Madrid, Spain), and funded by FEDER funds (CB06/03). Special thanks are due to the PREDIMED-Plus participants for their enthusiastic collaboration, and to the PREDIMED-Plus team for their exceptional support. The authors are also grateful to the staff of all associated primary care centers for their remarkable effort. Additionally, they thank the PREDIMED-Plus Biobank Network, part of the National Biobank Platform of ISCIII, for their role in storing and managing the PREDIMED-Plus biological samples.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Huang, L.Y.; Ou, Y.N.; Yang, Y.X.; Wang, Z.T.; Tan, L.; Yu, J.T. Associations of cardiovascular risk factors and lifestyle behaviors with neurodegenerative disease: A Mendelian randomization study. Transl. Psychiatry 2023, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflammaging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood–Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Generoso, J.S.; Collodel, A.; Sayana, P.; Barichello, T. The Impact of Systemic Inflammation on Neuroinflammation. In Translational Neuroimmunology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 7, pp. 169–188. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323858410000109 (accessed on 6 August 2024).
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart–Brain Axis. J. Am. Heart Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Brain, J.; Greene, L.; Tang, E.Y.H.; Louise, J.; Salter, A.; Beach, S.; Turnbull, D.; Siervo, M.; Stephan, B.C.; Tully, P.J. Cardiovascular disease, associated risk factors, and risk of dementia: An umbrella review of meta-analyses. Front. Epidemiol. 2023, 3, 1095236. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Traub, J.; Frey, A.; Störk, S. Chronic Neuroinflammation and Cognitive Decline in Patients with Cardiac Disease: Evidence, Relevance, and Therapeutic Implications. Life 2023, 13, 329. [Google Scholar] [CrossRef]
- Simon, E.; Obst, J.; Gomez-Nicola, D. The Evolving Dialogue of Microglia and Neurons in Alzheimer’s Disease: Microglia as Necessary Transducers of Pathology. Neuroscience 2019, 405, 24–34. [Google Scholar] [CrossRef]
- Kip, E.; Parr-Brownlie, L.C. Healthy lifestyles and wellbeing reduce neuroinflammation and prevent neurodegenerative and psychiatric disorders. Front. Neurosci. 2023, 17, 1092537. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Liu, G.; Yao, L.; Liu, J.; Jiang, Y.; Ma, G.; Chen, Z.; Zhao, B.; Li, K. Cardiovascular disease contributes to Alzheimer’s disease: Evidence from large-scale genome-wide association studies. Neurobiol. Aging 2014, 35, 786–792. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- ENGAGE Consortium; Surakka, I.; Horikoshi, M.; Mägi, R.; Sarin, A.-P.; Mahajan, A.; Lagou, V.; Marullo, L.; Ferreira, T.; Miraglio, B.; et al. The impact of low-frequency and rare variants on lipid levels. Nat. Genet. 2015, 47, 589–597. [Google Scholar] [CrossRef]
- Koh, W.; Pan, W.; Gawad, C.; Fan, H.C.; Kerchner, G.A.; Wyss-Coray, T.; Blumenfeld, Y.J.; El-Sayed, Y.Y.; Quake, S.R. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7361–7366. [Google Scholar] [CrossRef]
- Toden, S.; Zhuang, J.; Acosta, A.D.; Karns, A.P.; Salathia, N.S.; Brewer, J.B.; Wilcock, D.M.; Aballi, J.; Nerenberg, M.; Quake, S.R.; et al. Noninvasive characterization of Alzheimer’s disease by circulating, cell-free messenger RNA next-generation sequencing. Sci. Adv. 2020, 6, eabb1654. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort Profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Nigg, C.R.; Burbank, P.M.; Padula, C.; Dufresne, R.; Rossi, J.S.; Velicer, W.F.; Laforge, R.G.; Prochaska, J.O. Stages of Change across Ten Health Risk Behaviors for Older Adults; The Cerontologist: Washington, DC, USA, 1999; Volume 39, pp. 473–482. Available online: http://gerontologist.oxfordjournals.org/ (accessed on 17 June 2024).
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among OlderSpanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; I Furlong, L. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef]
- Baron, J.A.; Johnson, C.S.-B.; A Schor, M.; Olley, D.; Nickel, L.; Felix, V.; Munro, J.B.; Bello, S.M.; Bearer, C.; Lichenstein, R.; et al. The DO-KB Knowledgebase: A 20-year journey developing the disease open science ecosystem. Nucleic Acids Res. 2024, 52, D1305–D1314. [Google Scholar] [CrossRef]
- Gene Expression Assay Performance Guaranteed with the TaqMan ® Gene Expression Assays QPCR Guarantee Program; Life Technologies Corporation: Carlsbad, MA, USA, 2010.
- Gene Expression Assay Performance Guaranteed with the TaqMan Assays QPCR Guarantee Program; Themo Fisher Scientific: Waltham, MA, USA, 2015.
- Publish with Confidence Using TaqMan Assays; Thermo Fisher Scientific: Waltham, MA, USA, 2020.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.M.; Pinheiro, J.C.; Bates, D.M. Linear and nonlinear mixed-effects models. In Proceedings of the Conference on Applied Statistics in Agriculture, Manhattan, KS, USA, 26–28 April 1998. [Google Scholar]
- Lee, K.S.; Chung, J.H.; Lee, K.H.; Shin, M.-J.; Oh, B.H.; Hong, C.H. Bioplex analysis of plasma cytokines in Alzheimer’s disease and mild cognitive impairment. Immunol. Lett. 2008, 121, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-N.; Niu, L.-D.; Wang, Y.-J.; Cao, X.-P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.-T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.P.; Liu, C.S.; Rau, A.; Lanctôt, K.L.; Köhler, C.A.; Pakosh, M.; Carvalho, A.F.; Herrmann, N. Peripheral inflammatory markers in Alzheimer’s disease: A systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 876–882. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Cornejo, F.; Parada, G.E.; Eugenín, J. Role of TGFβ signaling in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci. 2015, 9, 426. [Google Scholar] [CrossRef]
- Kashima, R.; Hata, A. The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim. Biophys. Sin. 2018, 50, 106–120. [Google Scholar] [CrossRef]
- Unsicker, K.; Krieglstein, K. TGF-betas and their roles in the regulation of neuron survival. Adv. Exp. Med. Biol. 2002, 513, 353–374. [Google Scholar]
- Dobolyi, A.; Vincze, C.; Pál, G.; Lovas, G. The Neuroprotective Functions of Transforming Growth Factor Beta Proteins. Int. J. Mol. Sci. 2012, 13, 8219–8258. [Google Scholar] [CrossRef]
- Mocali, A.; Cedrola, S.; Della Malva, N.; Bontempelli, M.; Mitidieri, V.; Bavazzano, A.; Comolli, R.; Paoletti, F.; La Porta, C. Increased plasma levels of soluble CD40, together with the decrease of TGFβ1, as possible differential markers of Alzheimer disease. Exp. Gerontol. 2004, 39, 1555–1561. [Google Scholar] [CrossRef]
- Perez-Grijalba, V.; Romero, J.; Pesini, P.; Sarasa, L.; Monleon, I.; San-Jose, I.; Arbizu, J.; Martinez-Lage, P.; Munuera, J.; Ruiz, A.; et al. Plasma Aβ42/40 Ratio Detects Early Stages of Alzheimer’s Disease and Correlates with CSF and Neuroimaging Biomarkers in the AB255 Study. J. Prev. Alzheimers Dis. 2018, 6, 34–41. [Google Scholar]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker sig-nature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Ineffective levels of transforming growth factors and their receptor account for old age being a risk factor for Alz-heimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2019, 5, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.R.; Chai, Y.L.; Lee, J.H.; Howlett, D.; Attems, J.; Ballard, C.G.; Aarsland, D.; Francis, P.T.; Chen, C.P.; Lai, M.K.P. Increased Transforming Growth Factor β2 in the Neocortex of Alzheimer’s Disease and Dementia with Lewy Bodies is Correlated with Disease Severity and Soluble Aβ42 Load. J. Alz-heimers Dis. 2017, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Flanders, K.C.; Lippa, C.F.; Smith, T.W.; Pollen, D.A.; Sporn, M.B. Altered expression of transforming growth factor-beta in Alzheimer’s disease. Neurology 1995, 45, 1561–1569. [Google Scholar] [CrossRef]
- Lippa, C.; Flanders, K.; Kim, E.; Croul, S. TGF-β receptors-I and -II immunoexpression in Alzheimer’s disease: A comparison with aging and progressive supranuclear palsy. Neurobiol. Aging 1998, 19, 527–533. [Google Scholar] [CrossRef]
- Noguchi, A.; Nawa, M.; Aiso, S.; Okamoto, K.; Matsuoka, M. Transforming Growth Factor β2 Level is Elevated in Neurons of Alzheimer’s Disease Brains. Int. J. Neurosci. 2010, 120, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Zou, K.; Esposito, L.; Bard, F.; Berber, E.; Van Can, J.; Lin, A.H.; Crews, L.; Tremblay, P.; Mathews, P.; et al. Deficiency in neuronal TGF-β signaling promotes neurodegen-eration and Alzheimer’s pathology. J. Clin. Investig. 2006, 116, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Ben-Lulu, S.; Pollak, Y.; Mogilner, J.; Bejar, J.; G Coran, A.; Sukhotnik, I. Dietary Transforming Growth Factor-Beta 2 (TGF-β2) Supplementation Reduces Methotrexate-Induced Intestinal Mucosal Injury in a Rat. PLoS ONE 2012, 7, e45221. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Bellenguez, C.; Kucukali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.; Muñoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos, M.D.C.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Khymenets, O.; Fito, M.; De La Torre, R.; Anglada, R.; Dopazo, A.; Covas, M.I. Characterization of Human Gene Ex-pression Changes after Olive Oil Ingestion: An Exploratory Approach. 2009. Available online: https://pubmed.ncbi.nlm.nih.gov/19545487/ (accessed on 13 June 2022).
- Llorente-Cortés, V.; Estruch, R.; Mena, M.P.; Ros, E.; González, M.A.M.; Fitó, M.; Lamuela-Raventós, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Casas, R.; Sacanella, E.; Corella, D.; Andrés-Lacueva, C.; Llorach, R.; Garrabou, G.; Cardellach, F.; Sala-Vila, A.; Ros, E.; et al. The 3-year effect of the Mediterranean diet intervention on inflammatory biomarkers related to cardiovascular disease. Biomedicines 2021, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007, 61, 147–159. [Google Scholar] [CrossRef]
- Hooper, P.L.; Tytell, M.; Vígh, L. Xenohormesis: Health benefits from an eon of plant stress response evolution. Cell Stress Chaperon 2010, 15, 761–770. [Google Scholar] [CrossRef]
- Surh, Y. Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann. N. Y. Acad. Sci. 2011, 1229, 1–6. [Google Scholar] [CrossRef]
- Testa, G.; Biasi, F.; Poli, G.; Chiarpotto, E. Calorie Restriction and Dietary Restriction Mimetics: A Strategy for Improving Healthy Aging and Longevity. Curr. Pharm. Des. 2014, 20, 2950–2977. [Google Scholar] [CrossRef]
- Calabrese, V.; Wenzel, U.; Piccoli, T.; Jacob, U.M.; Nicolosi, L.; Fazzolari, G.; Failla, G.; Fritsch, T.; Osakabe, N.; Calabrese, E.J. Investigating hormesis, aging, and neuro-degeneration: From bench to clinics. Open Med. 2024, 19, 20240986. [Google Scholar] [CrossRef]
- Murakami, A. Impact of hormesis to deepen our understanding of the mechanisms underlying the bioactivities of polyphenols. Curr. Opin. Biotechnol. 2024, 86, 103074. [Google Scholar] [CrossRef]
- Fitó, M.; Konstantinidou, V. Nutritional Genomics and the Mediterranean Diet’s Effects on Human Cardiovascular Health. Nutrients 2016, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.B.; Holm, S.; Aukrust, P.; Halvorsen, B. Visfatin/NAMPT: A Multifaceted Molecule with Diverse Roles in Physiology and Pathophysiology. Annu. Rev. Nutr. 2012, 32, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Dakroub, A.; A Nasser, S.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Audrito, V.; Messana, V.G.; Deaglio, S. NAMPT and NAPRT: Two Metabolic Enzymes With Key Roles in Inflammation. Front. Oncol. 2020, 10, 358. [Google Scholar] [CrossRef]
- Martínez-Morcillo, F.J.; Cantón-Sandoval, J.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Mesa-Del-Castillo, P.; Pérez-Oliva, A.B.; García-Moreno, D.; Mulero, V. Non-canonical roles of NAMPT and PARP in inflammation. Dev. Comp. Immunol. 2021, 115, 103881. [Google Scholar] [CrossRef]
- Li, M.; Lai, Y.; Chen, B.; Guo, C.; Zhou, M.; Zhao, S.; Wang, S.; Li, J.; Yang, N.; Zhang, H. NAMPT is a metabolic checkpoint of IFNγ-producing CD4+ T cells in lupus nephritis. Mol. Ther. 2023, 31, 193–210. [Google Scholar] [CrossRef]
- Wei, X.; Wei, C.; Tan, Y.; Dong, X.; Yang, Z.; Yan, J.; Luo, X. Both prolonged high-fat diet consumption and calorie restriction boost hepatic NAD plus metabolism in mice. J. Nutr. Biochem. 2023, 115, 109296. [Google Scholar] [CrossRef]
- Kärberg, K.; Forbes, A.; Lember, M. Unlocking the Dietary Puzzle: How Macronutrient Intake Shapes the Relationship be-tween Visfatin and Atherosclerosis in Type 2 Diabetes. Medicina 2024, 60, 438. [Google Scholar] [CrossRef]
- de Luis, D.A.; Sagrado, M.G.; Conde, R.; Aller, R.; Izaola, O.; Romero, E. Effect of a hypocaloric diet on serum visfatin in obese non-diabetic patients. Nutrition 2008, 24, 517–521. [Google Scholar] [CrossRef]
- Ohlsson, B. An Okinawan-based Nordic diet improves glucose and lipid metabolism in health and type 2 diabetes, in alignment with changes in the endocrine profile, whereas zonulin levels are elevated (Review). Exp. Ther. Med. 2019, 17, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Haghighatdoost, F.; Hosseinzadeh-Attar, M.J.; Kabiri, A.; Eshraghian, M.; Esmaillzadeh, A. Effect of substituting saturated with monounsaturated fatty acids on serum visfatin levels and insulin resistance in overweight women: A randomized cross-over clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Bai, J.; Zhong, S.; Zhang, R.; Kang, K.; Zhang, X.; Xu, Y.; Zhao, C.; Zhao, M. Downregulation of PIK3CB Involved in Alzheimer’s Disease via Apoptosis, Axon Guidance, and FoxO Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Antonell, A.; Lladó, A.; Sánchez-Valle, R.; Sanfeliu, C.; Casserras, T.; Rami, L.; Muñoz-García, C.; Dangla-Valls, A.; Balasa, M.; Boya, P.; et al. Altered Blood Gene Expression of Tumor-Related Genes (PRKCB, BECN1, and CDKN2A) in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5902–5911. [Google Scholar] [CrossRef] [PubMed]
- McShea, A.; Harris, P.L.; Webster, K.R.; Wahl, A.F.; A Smith, M. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am. J. Pathol. 1997, 150, 1933–1939. [Google Scholar]
- Mori, H.; Funahashi, Y.; Yoshino, Y.; Kumon, H.; Ozaki, Y.; Yamazaki, K.; Ochi, S.; Tachibana, A.; Yoshida, T.; Shimizu, H.; et al. Blood CDKN2A gene expression in aging and neurodegenerative diseases. J. Alzheimer’s Dis. 2021, 82, 1737–1744. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive Oil, the Mediterranean Diet, and Arterial Blood Pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) Study 1–3. 2004. Available online: https://academic.oup.com/ajcn/article/80/4/1012/4690349 (accessed on 17 June 2024).
- Berr, C.; Portet, F.; Carriere, I.; Akbaraly, T.N.; Feart, C.; Gourlet, V.; Combe, N.; Barberger-Gateau, P.; Ritchie, K. Olive oil and cognition: Results from the three-city study. Dement. Geriatr. Cogn. Disord. 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Larrieu, S.; Letenneur, L.; Berr, C.; Dartigues, J.F.; Ritchie, K.; Alperovitch, A.; Tavernier, B.; Barberger-Gateau, P. Sociodemographic differences in dietary habits in a population-based sample of elderly subjects: The 3C study. J. Nutr. Health Aging 2004, 8, 497–502. [Google Scholar]
- Antoniak, M.; Pugliatti, M.; Hubbard, R.; Britton, J.; Sotgiu, S.; Sadovnick, A.D.; Yee, I.M.; Cumsille, M.A.; Bevilacqua, J.A.; Burdett, S.; et al. Vascular Factors and Risk of Dementia: Design of the Three-City Study and Baseline Characteristics of the Study Population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar]
- Féart, C. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA 2009, 302, 638. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Reinón, M.E.; Chirlaque, M.D.; Gavrila, D.; Amiano, P.; Mar, J.; Tainta, M.; Ardanaz, E.; Larumbe, R.; Colorado-Yohar, S.M.; Navarro-Mateu, F.; et al. Mediterranean diet and risk of dementia and Alzheimer’s disease in the EPIC-Spain dementia cohort study. Nutrients 2021, 13, 700. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease—A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).