Cultural Perspectives on the Efficacy and Adoption of the Crohn’s Disease Exclusion Diet across Diverse Ethnicities: A Case-Based Overview
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The Use of CDED in Korea
Adapting the Diet to Korean Cuisine: Addressing Challenges and Implementation Strategies
3.2. The Use of CDED in an Arab Patient from Israel
3.2.1. Clinical Overview
3.2.2. Adapting the Diet to Arabic Cuisine: Addressing Challenges and Implementation Strategies
3.3. The Use of CDED by a Hispanic South American Patient from Spain
3.3.1. Clinical Overview
3.3.2. Adapting the Diet to Colombian Cuisine: Addressing Challenges and Implementation Strategies
3.4. The Use of CDED in a Patient of Turkish Descent from The Netherlands
3.4.1. Clinical Overview
3.4.2. Adapting the Diet to Turkish Cuisine: Addressing Challenges and Implementation Strategies
3.5. The CDED in a Religious Jewish Patient from Israel
3.5.1. Clinical Overview
3.5.2. Adapting the Diet to Religious Jewish Cuisine: Addressing Challenges and Implementation Strategies
3.6. The Use of CDED among Patients from Argentina
3.6.1. Clinical Overview Case 1
3.6.2. Clinical Overview Case 2
3.6.3. Adapting the Diet to Argentina: Addressing Challenges and Implementation Strategies
3.7. The CDED in a Patient of Indian Descent from Canada
3.7.1. Clinical Overview
3.7.2. Adapting the Diet to Indian Cuisine: Addressing Challenges and Implementation Strategies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2020, 15, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hebuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed]
- Sigall Boneh, R.W.C.; Oseran, I.; Sarbagili-Shabat, C.; Albenberg, L.G.; Lionetti, P.; Manuel Navas-López, V.; Martín-de-Carpi, J.; Yanai, H.; Maharshak, N.; Van Limbergen, J.; et al. The Crohn’s Disease Exclusion Diet: A Comprehensive Review of Evidence, Implementation Strategies, Practical Guidance, and Future Directions. Inflamm. Bowel Dis. 2023, izad255. [Google Scholar] [CrossRef]
- Stein, R.; Daniel, S.G.; Baldassano, R.N.; Feigenbaum, K.; Kachelries, K.; Sigall-Boneh, R.; Weston, S.; Levine, A.; Bittinger, K. Outcomes and Predictors of Sustained Remission After Drug Withdrawal in Pediatric Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 608–615. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- Fliss-Isakov, N.; Aviv Cohen, N.; Bromberg, A.; Elbert, G.; Anbar, R.; Ron, Y.; Hirsch, A.; Thurm, T.; Maharshak, N. Crohn’s Disease Exclusion Diet for the Treatment of Crohn’s Disease: Real-World Experience from a Tertiary Center. J. Clin. Med. 2023, 12, 5428. [Google Scholar] [CrossRef]
- Matuszczyk, M.; Meglicka, M.; Wiernicka, A.; Jarzebicka, D.; Osiecki, M.; Kotkowicz-Szczur, M.; Kierkus, J. Effect of the Crohn’s Disease Exclusion Diet (CDED) on the Fecal Calprotectin Level in Children with Active Crohn’s Disease. J. Clin. Med. 2022, 11, 4146. [Google Scholar] [CrossRef]
- Szczubelek, M.; Pomorska, K.; Korolczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of Crohn’s Disease Exclusion Diet for Induction of Remission in Crohn’s Disease Adult Patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef] [PubMed]
- Jijon Andrade, M.C.; Pujol Muncunill, G.; Lozano Ruf, A.; Alvarez Carnero, L.; Vila Miravet, V.; Garcia Arenas, D.; Egea Castillo, N.; Martin de Carpi, J. Efficacy of Crohn’s disease exclusion diet in treatment -naive children and children progressed on biological therapy: A retrospective chart review. BMC Gastroenterol. 2023, 23, 225. [Google Scholar] [CrossRef] [PubMed]
- Martin-Masot, R.; Herrador-Lopez, M.; Navas-Lopez, V.M. Dietary Habit Modifications in Paediatric Patients after One Year of Treatment with the Crohn’s Disease Exclusion Diet. Nutrients 2023, 15, 554. [Google Scholar] [CrossRef] [PubMed]
- Niseteo, T.; Sila, S.; Trivic, I.; Misak, Z.; Kolacek, S.; Hojsak, I. Modified Crohn’s disease exclusion diet is equally effective as exclusive enteral nutrition: Real-world data. Nutr. Clin. Pract. 2022, 37, 435–441. [Google Scholar] [CrossRef]
- Urlep, D.; Benedik, E.; Brecelj, J.; Orel, R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: Results of a prospective cohort study. Eur. J. Pediatr. 2020, 179, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Scarallo, L.; Banci, E.; Pierattini, V.; Lionetti, P. Crohn’s disease exclusion diet in children with Crohn’s disease: A case series. Curr. Med. Res. Opin. 2021, 37, 1115–1120. [Google Scholar] [CrossRef]
- Scarallo, L.; Banci, E.; De Blasi, A.; Paci, M.; Renzo, S.; Naldini, S.; Barp, J.; Pochesci, S.; Fioretti, L.; Pasquini, B.; et al. A real-life pediatric experience of Crohn’s disease exclusion diet at disease onset and in refractory patients. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 592–601. [Google Scholar] [CrossRef]
- Arcucci, M.S.; Menendez, L.; Orsi, M.; Gallo, J.; Guzman, L.; Busoni, V.; Lifschitz, C. Role of adjuvant Crohn’s disease exclusion diet plus enteral nutrition in asymptomatic pediatric Crohn’s disease having biochemical activity: A randomized, pilot study. Indian J. Gastroenterol. 2024, 43, 199–207. [Google Scholar] [CrossRef]
- Wall, C.L.; Bensley, R.; Glyn, T.; Haines, M.; Rowbotham, D.; Bissett, I.; Eglinton, T.; Gearry, R.B. Preoperative Crohn’s Disease Exclusion Diet and Exclusive Enteral Nutrition in Adults with Crohn’s Disease: A Feasibility Randomised Controlled Trial. Nutrients 2024, 16, 2105. [Google Scholar] [CrossRef]
- Landorf, E.; Hammond, P.; Abu-Assi, R.; Ellison, S.; Boyle, T.; Comerford, A.; Couper, R. Formula modifications to the Crohn’s disease exclusion diet do not impact therapy success in paediatric Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 1279–1286. [Google Scholar] [CrossRef]
- Damas, O.M.; Maldonado-Contreras, A. Breaking Barriers in Dietary Research: Strategies to Diversify Recruitment in Clinical Studies and Develop Culturally Tailored Diets for Hispanic Communities Living With Inflammatory Bowel Disease. Gastroenterology 2023, 165, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Vicentín, R.W.M.; Pais, A.B.; Contreras, M.; Orsi, M. One-year prospective registry of inflammatory bowel disease in the Argentine pediatric population. Arch. Argent Pediatr. 2017, 115, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Larrosa-Haro, A.; Abundis-Castro, L.; Contreras, M.B.; Gallo, M.J.; Peña-Quintana, L.; Targa Ferreira, C.H.; Nacif, P.A.; Vázquez-Frías, R.; Bravo, S.; Muñoz-Urribarri, A.B.; et al. Epidemiologic trend of pediatric inflammatory bowel disease in Latin America: The Latin American Society for Pediatric Gastroenterology, Hepatology and Nutrition (LASPGHAN) Working Group. Gastroenterol. Mex. 2021, 86, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef]
- Guo, A.L.J.; Brantsæter, A.L.; Klingberg, S.; Östensson, M.; Størdal, K.; Mårild, K. Early-life diet and risk of inflammatory bowel disease: A pooled study in two Scandinavian birth cohorts. Gut 2024, 73, 590–600. [Google Scholar] [CrossRef]
- Damas, O.M.; Estes, D.; Avalos, D.; Quintero, M.A.; Morillo, D.; Caraballo, F.; Lopez, J.; Deshpande, A.R.; Kerman, D.; McCauley, J.L.; et al. Hispanics Coming to the US Adopt US Cultural Behaviors and Eat Less Healthy: Implications for Development of Inflammatory Bowel Disease. Dig. Dis. Sci. 2018, 63, 3058–3066. [Google Scholar] [CrossRef]
- Sproesser, G.; Ruby, M.B.; Arbit, N.; Akotia, C.S.; Alvarenga, M.D.S.; Bhangaokar, R.; Furumitsu, I.; Hu, X.; Imada, S.; Kaptan, G.; et al. Understanding traditional and modern eating: The TEP10 framework. BMC Public Health 2019, 19, 1606. [Google Scholar] [CrossRef]
- Zhu, Z.; Lei, Y.; Lin, Z. Effects of Crohn’s disease exclusion diet on remission: A systematic review. Therap. Adv. Gastroenterol. 2023, 16, 17562848231184056. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients with Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Fitzpatrick, J.A.; Melton, S.L.; Yao, C.K.; Gibson, P.R.; Halmos, E.P. Dietary management of adults with IBD—The emerging role of dietary therapy. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 652–669. [Google Scholar] [CrossRef]
- Agrawal, M.; Spencer, E.A.; Colombel, J.F.; Ungaro, R.C. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User’s Guide for Adult and Pediatric Gastroenterologists. Gastroenterology 2021, 161, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.E.; Day, A.S.; Dimitroff, C.; Trakman, G.L.; Silva, H.; Bryant, R.V.; Purcell, L.; Yao, C.K.; Landorf, E.; Fitzpatrick, J.A. Practical application of the Crohn’s disease exclusion diet as therapy in an adult Australian population. J. Gastroenterol. Hepatol. 2024, 39, 446–456. [Google Scholar] [CrossRef] [PubMed]
- van Lingen, E.; van der Marel, S.; Maljaars, J.; Keller, J.; van der Meulen-de Jong, A. Comment on Szczubelek et al. Effectiveness of Crohn’s Disease Exclusion Diet for Induction of Remission in Crohn’s Disease Adult Patients. Nutrients 2021, 14, 4112. [Google Scholar] [CrossRef]
- Gibson, P.R. The evidence base for efficacy of the low FODMAP diet in irritable bowel syndrome: Is it ready for prime time as a first-line therapy? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 32–35. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients with Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188. [Google Scholar] [CrossRef]
- Halmos, E.P.; Gibson, P.R. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef]
- Urlep, D.; Orel, R.; Kunstek, P.; Benedik, E. Treatment of Active Crohn’s Disease in Children Using Partial Enteral Nutrition Combined with a Modified Crohn’s Disease Exclusion Diet: A Pilot Prospective Cohort Trial on Clinical and Endoscopic Outcomes. Nutrients 2023, 15, 4676. [Google Scholar] [CrossRef]
- Herrador-Lopez, M.; Martin-Masot, R.; Navas-Lopez, V.M. EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients 2020, 12, 3793. [Google Scholar] [CrossRef]
- Alsarhan, A.; Aljasmi, R.; Ajaka, N.; Krishnamurthy, B.; Kader, A.; Aljasmi, M.; Nahdi, N.; Malik, E.; Murbati, B.; Aljabri, E.; et al. Challenges in Managing Paediatric Crohn’s Disease With Crohn’s Disease Exclusion Diet (CDED): The First Single-Center Study in the United Arab Emirates. Cureus 2023, 15, e43970. [Google Scholar] [CrossRef]
| Country | Study Reference | Study Population | Diet Adaptation/Cultural Issues |
|---|---|---|---|
| Israel | Sigall-Boneh 2014 [4], Levine 2019 [5], Stein 2022 [7], Yanai 2022 [8], Isakov-Fliss 2003 [9] | Children and adults with active CD | The studies conducted in several centers across Israel reflect diversity and various ethnicities within the population. Additionally, the population exhibits diversity in religious beliefs. |
| USA and Canada | Levine 2019 [5], Stein 2022 [7] | Children and young adults with CD in active disease and in remission | Cultural characteristics include high consumption of fast foods and ultra-processed foods, necessitating the search for suitable alternatives and the adoption of home cooking and meal preparation. |
| Poland | Matuszczyk 2022 [10], Szczubelek 2021 [11] | Children and adults with active CD | Modulife program with recipes and dietetic consultation to assess tolerance and adherence to the diet was sufficient when following the regular CDED guidelines and avoiding ultra-processed foods. Only the amount of obligatory products was increased if necessary. No other adaptations were needed. |
| Spain | Jijon Andrade2023 [12], Martin-Masot 2023 [13] | Children with mild–moderate CD | Following the diet resulted in a reduction in ultra-processed food intake, high diet quality, and high adherence to the Mediterranean diet. No specific cultural or ethnical aspects, or CDED diet adaptations, are mentioned. |
| Croatia | Niseteo 2022 [14] | Pediatric CD patients | No adaptations to CDED were described. |
| Slovenia | Urlep 2020 [15] | Pediatric CD patients | Sometimes AID-CD (anti-inflammatory) is offered, a diet CDED modification with Slovenian products. |
| Italy | Scarallo 2021 [16], Scarallo 2024 [17] | Mild to moderate luminal–colonic CD patients | The CDED was well tolerated, and adherence to the diet regimen was good. No specific cultural or ethnical aspects, or CDED diet adaptations, are mentioned. |
| Argentina | Arcucci 2023 [18] | Asymptomatic children with CD and elevated FCP on medical treatment | Compliance was not a problem in the CDED + PEN group, and no diet adaptations are mentioned. |
| New Zealand | Wall 2024 [19] | Adult CD who required elective gastrointestinal surgery | No adaptations to CDED are described. |
| Australia | Landorf [20] | Children with active CD | No cultural adaptations are described, but adjustments were made for vegan diets and cow’s milk allergy. |
| Center | Country | Population | Number of Patients Treated with CDED per Year |
|---|---|---|---|
| The E. Wolfson Medical Center | Israel | Children | 70–80 |
| Severance Children’s Hospital | Korea | Children | 10 |
| Hospital de niños Ricardo Gutierrez | Argentina | Children | 3–5 |
| Hospital Italiano de Buenos Aires | Argentina | Children | 20–30 |
| Hospital Regional Universitario de Málaga | Spain | Children | 60–70 |
| Haemek Medical Center | Israel | Adults | 20–30 |
| Emma Children’s Hospital/Amsterdam UMC | The Netherlands | Children Adults | 25–35 5–10 |
| University of Alberta | Canada | Children | 20–30 |
| Traditional Korean Meal | CDED Adjustment |
|---|---|
| Original recipe: Gimbap Seasoned rice and stuffings such as hams, crabmeat, and bulgogi (beef marinated with soy sauce, sugars, sesame oils, etc) wrapped with seaweed. Usually, sesame oils and sesames are put on top of gimbap. | We replaced seaweed with rice paper. Rice was seasoned with small amount of vinegars and salts. Instead of hams, crabmeats and bulgogi, we recommended chicken and steamed vegetables to be used as stuffings. |
| Traditional Arabic Meal | CDED Adjustment |
|---|---|
| Pita bread: A staple in Arabic cuisine, often used for dips and sandwiches. Pita is made from yeast and wheat flour. | Substituting yeast and wheat flour with rice flour and olive oil. |
| Synia: Traditional Middle Eastern meat and tahini casserole typically made with lamb or beef. | Using ground chicken breast, potatoes, onions, and tomatoes instead of lamb/beef. For phase 2, incorporating raw tahini and eggplants/cauliflower. |
| Baklava: A dessert of honey-soaked phyllo pastry layered with nuts. | In phase 2, instead of phyllo pastry we used rice paper and Canola oil, and the rest of the ingredients were identical to the original recipe with sugar and honey as the syrup, and walnuts for the filling. |
| Traditional Colombian Recipe | CDED Adjustment |
|---|---|
Arepas
|
|
| Traditional Argentinian Meal | CDED Adjustment |
|---|---|
| Asado: Traditional meal, very frequent in family gatherings or with friends, as well as at birthdays or festivities, where beef is roasted over firewood | Roasted chicken/fish. Chicken brochettes with vegetables. Roasted vegetables (potato, onion, tomato in phase 1; eggplant, pumpkin, sweet potato in phase 2). From stage 2 onwards, one portion of red beef such as loin or peceto is allowed. |
| Pasta: On Sundays, families often have family lunches that include pasta with wheat flour with stew (tomato sauce with vegetables, red meat and sausages) | Gnocchi recipe (cooked with mashed potato, egg, salt) or rice noodles. For the stew, a chicken stew recipe with chicken and vegetables (tomato, onion, carrot, potato) is usually given |
| Traditionnel dessert: Flan with dulce de leche (caramel sauce) | The family is encouraged to cook the flan at home. It is suggested to use formula to cook the flan and the dulce de leche (12 measures of formula plus 50 g of sugar, 1/2 spoonful of baking soda and 1/4 vanilla stick). The same can be done with other traditional desserts such as rice pudding, pancakes with dulce de leche and custard. |
| School lunch: Usually families send ready-to-eat meals, which can be out of the refrigerator for a few hours, or the children eat the food offered in the cafeteria. The snack time is the most complicated since they usually offer alfajores (wheat flour dough with dulce de leche and chocolate coating), sweet cookies, fried potatoes. | If the family is able to send a meal to school, this option is encouraged, with homemade food preferred. Otherwise, a list of permitted foods will be sent to the school cafeteria for adaptation. Recipes are given for potato and egg salad, vegetable or tuna pie or empanadas (rice flour with water and oil), rice croquettes with vegetables, potato tortilla, chicken nuggets/sausages (chicken, carrot, parsley, egg, rice flour). For snacks we suggest puffed rice or meringues (egg white with sugar, lemon or orange zest), and recipes are offered for apple or lemon cookies, banana muffins, fruit muffins, dried fruits in phase 2, olives/cherry tomatoes/carrot sticks. |
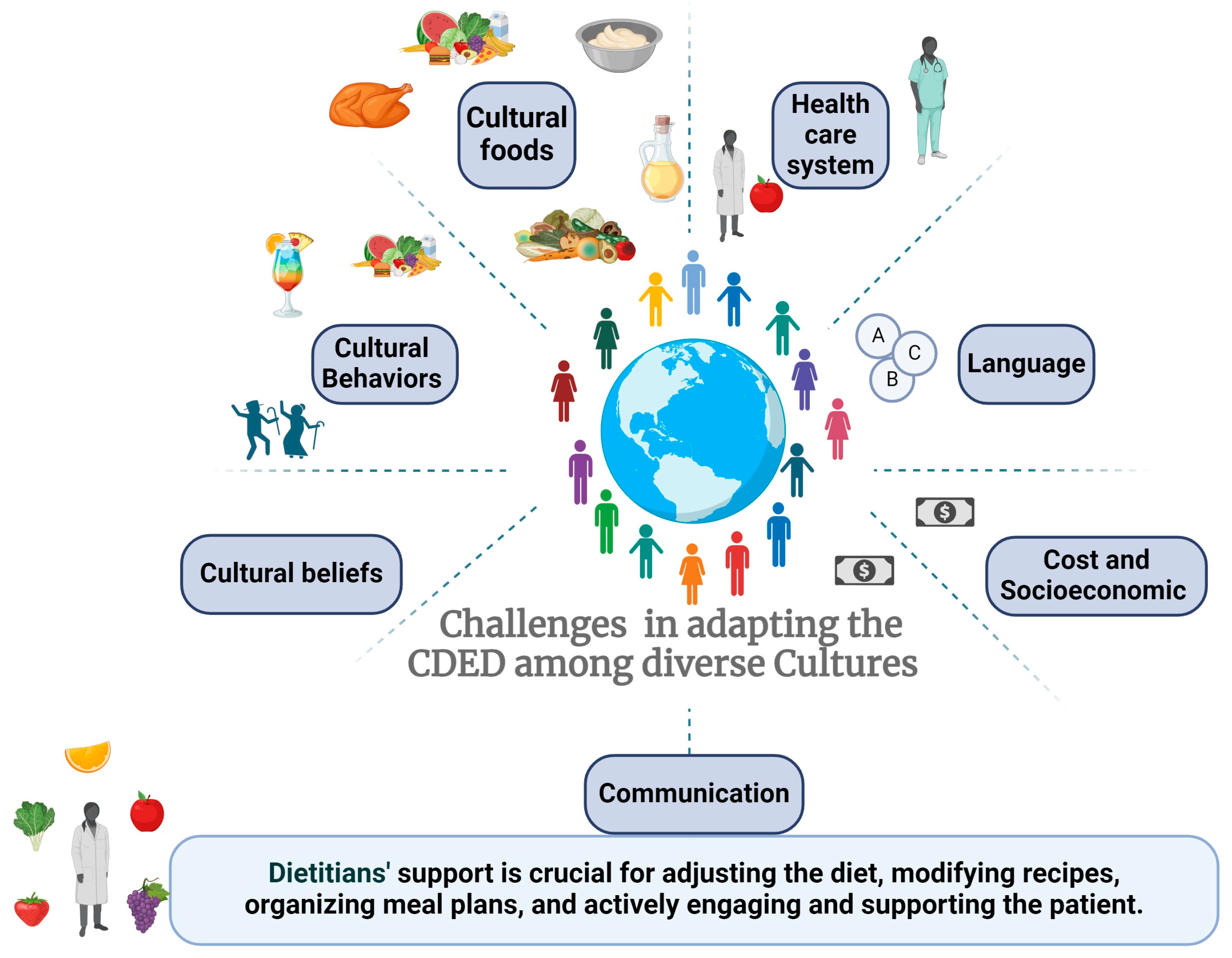
| Barrier | Relevant Points | Solutions for Healthcare Practitioners |
|---|---|---|
| Healthcare system | Lack of multidisciplinary teams and language barriers | Ensure availability of a multidisciplinary team, including a dietitian fluent in the patient’s language. Utilize translation services when necessary to ensure understanding of instructions and rationale. Use international guidelines to advocate for having a dietitian as part of your MDT. |
| Language | Lack of information in native language | Utilize the Modulife app and supportive materials available in 10 different languages. Build local resources to share materials. |
| Costs and Socioeconomic | Healthy foods may be more expensive; lack of knowledge regarding healthy diet | Guide patients in selecting affordable, seasonal fruits and vegetables according to recommendations. Educate patients on reading food labels and making informed choices. Advocate for support from the local medical system for potential cost-saving nutritional therapies. |
| Cultural Behaviors | Food is integral to cultural and social events | Understand cultural foods and gather recipes from patients to share with others. Customize cultural recipes to align with CDED principles, increasing adherence. Recommend more activities not related to foods. |
| Cultural Foods | Traditional foods may not align with CDED | Prepare special meals, organize events with CDED-friendly foods, and adjust recipes for specific occasions. Emphasize the temporary nature of dietary restrictions. |
| School meals | School meal programs may not provide CDED-friendly options, lack of control over ingredients and preparation | Collaborate with school administrators and nutrition staff to develop CDED-compliant meal options. Provide guidelines and recipes to schools for preparing suitable meals. Encourage parents to pack CDED-friendly meals for their children. Educate school staff on the importance of dietary adherence for the child’s health. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigall Boneh, R.; Park, S.; Soledad Arcucci, M.; Herrador-López, M.; Sarbagili-Shabat, C.; Kolonimos, N.; Wierdsma, N.; Chen, M.; Hershkovitz, E.; Wine, E.; et al. Cultural Perspectives on the Efficacy and Adoption of the Crohn’s Disease Exclusion Diet across Diverse Ethnicities: A Case-Based Overview. Nutrients 2024, 16, 3184. https://doi.org/10.3390/nu16183184
Sigall Boneh R, Park S, Soledad Arcucci M, Herrador-López M, Sarbagili-Shabat C, Kolonimos N, Wierdsma N, Chen M, Hershkovitz E, Wine E, et al. Cultural Perspectives on the Efficacy and Adoption of the Crohn’s Disease Exclusion Diet across Diverse Ethnicities: A Case-Based Overview. Nutrients. 2024; 16(18):3184. https://doi.org/10.3390/nu16183184
Chicago/Turabian StyleSigall Boneh, Rotem, Sowon Park, Maria Soledad Arcucci, Marta Herrador-López, Chen Sarbagili-Shabat, Nitzan Kolonimos, Nicolette Wierdsma, Min Chen, Einat Hershkovitz, Eytan Wine, and et al. 2024. "Cultural Perspectives on the Efficacy and Adoption of the Crohn’s Disease Exclusion Diet across Diverse Ethnicities: A Case-Based Overview" Nutrients 16, no. 18: 3184. https://doi.org/10.3390/nu16183184






