A New Strategy for Dietary Nutrition to Improve Intestinal Homeostasis in Diarrheal Irritable Bowel Syndrome: A Perspective on Intestinal Flora and Intestinal Epithelial Interaction
Abstract
1. Introduction
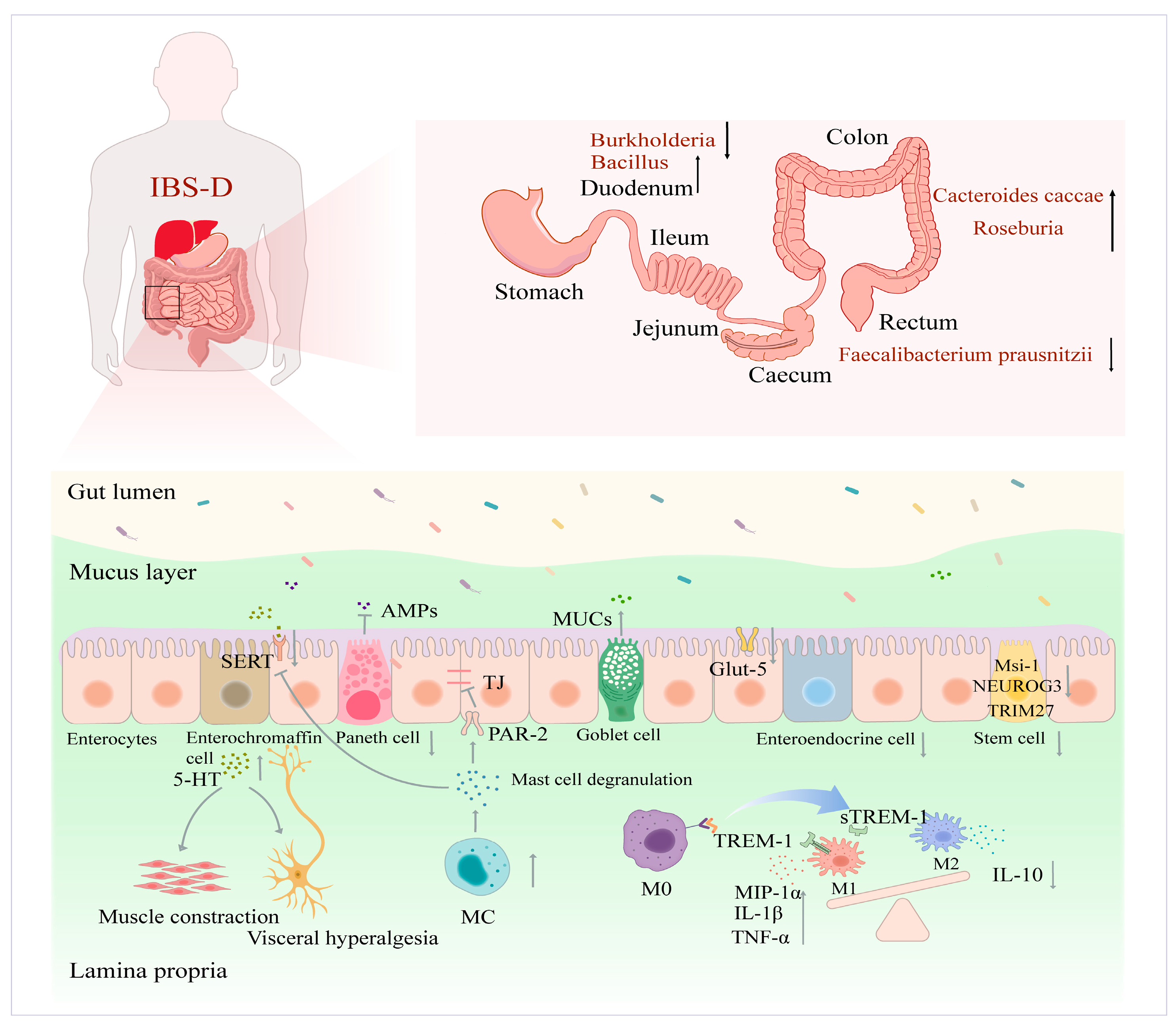
2. IBS-D Intestinal Homeostatic Imbalance and Physiological Properties
2.1. Impaired Functional Populations of IECs
2.1.1. Secretory Cell Populations
2.1.2. Immune Cells
2.1.3. ISCs and Absorptive Cell Populations
2.2. Ecological Imbalance of Intestinal Flora
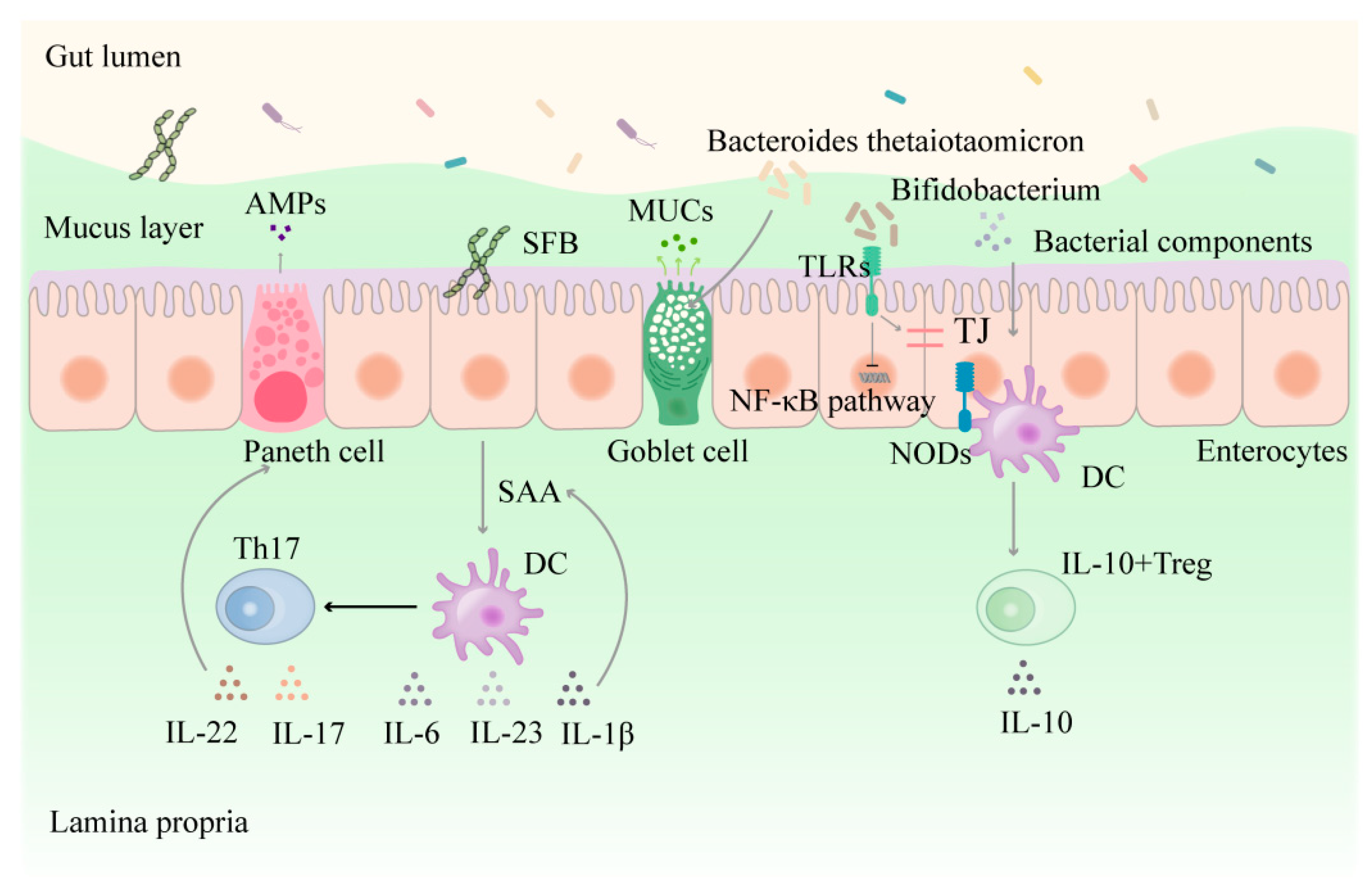
3. The Interaction between the Functional Populations of IECs and Intestinal Flora
3.1. Direct Interaction between Intestinal Flora and Epithelial Cells
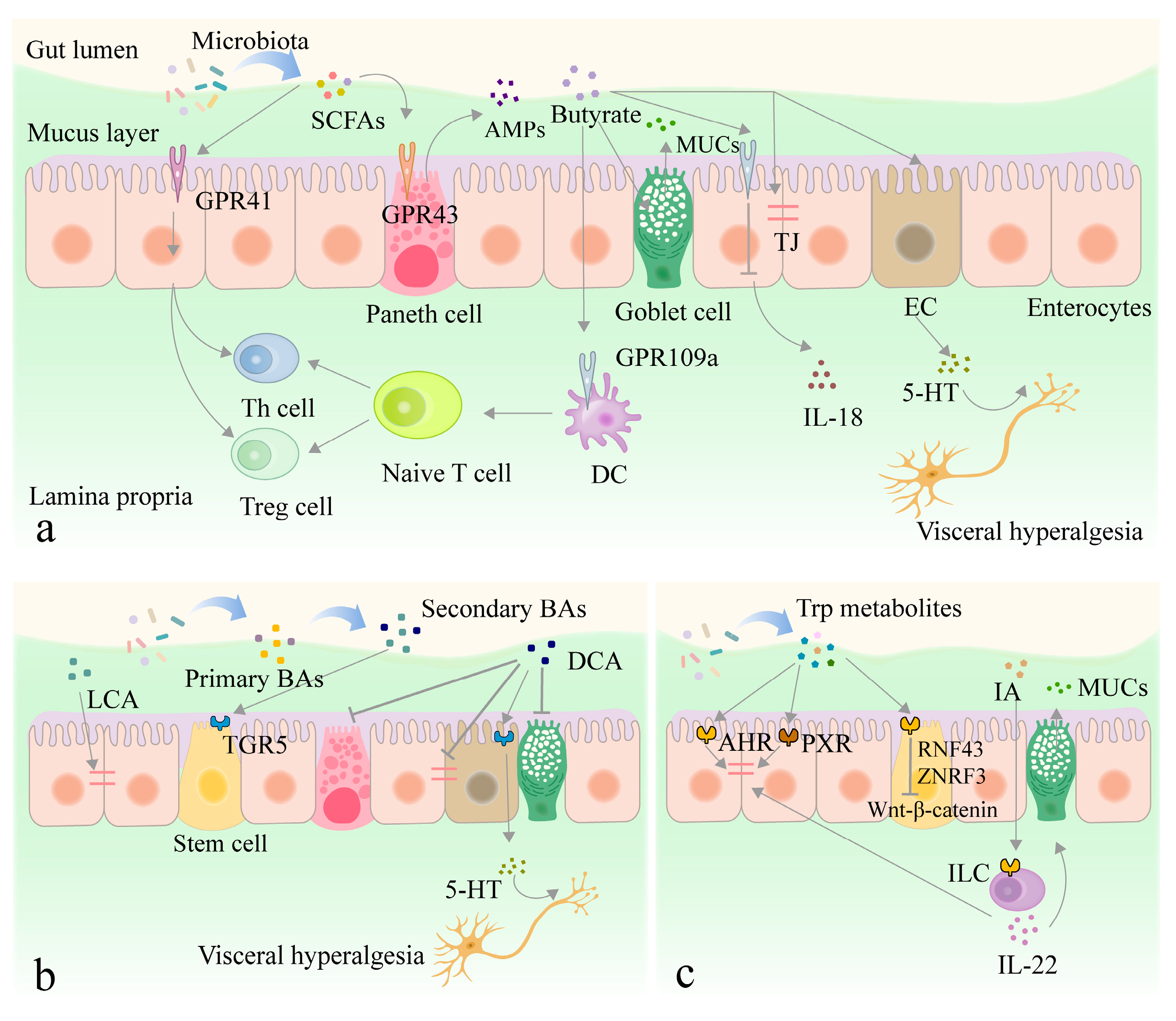
3.2. Interaction between Metabolites Derived from Intestinal Flora and Intestinal Epithelium
3.2.1. SCFAs
3.2.2. Other Metabolites
3.3. The Functional Populations of IECs and the Chemicals They Secrete Affect Intestinal Flora
4. Dietary Nutrient Intervention in IBS-D
4.1. Essential Micronutrients
4.1.1. Vitamins
4.1.2. Minerals
4.2. Probiotics and Prebiotics
4.2.1. Probiotics
4.2.2. Prebiotics
4.3. Polyphenols
4.4. The Synergistic Ability of Different Dietary Nutrients to Improve Intestinal Homeostasis
5. Results and Outlook
Funding
Conflicts of Interest
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D. Review article: Epidemiology of IBS and other bowel disorders of gut–brain interaction (DGBI). Aliment. Pharm. Ther. 2021, 54, S1–S11. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.; Lembo, A.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.; De Man, J.; Hassett, L.; De Winter, B.; Grover, M. Intestinal barrier dysfunction in irritable bowel syndrome: A systematic review. Therap. Adv. Gastroenterol. 2021, 14, 1–31. [Google Scholar] [CrossRef]
- Ami, D.S.; Dan, D.; Shin, F.; Charles, G.; Uday, C.; Kok, A.G.; Pail, S.H.A.; Kang, J.; Chen, M.; Max, S.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Rej, A.; Sanders, D.S.; Shaw, C.C.; Buckle, R.; Trott, N.; Agrawal, A.; Aziz, I. Efficacy and Acceptability of Dietary Therapies in Non-Constipated Irritable Bowel Syndrome: A Randomized Trial of Traditional Dietary Advice, the Low FODMAP Diet, and the Gluten-Free Diet. Clin. Gastroenterol. Hepatol. 2022, 20, 2876–2887.e15. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A.J.; Wolf, R. Improvements over Time in Individual Diarrhea-Predominant Irritable Bowel Syndrome Symptoms (IBS-D) with Rifaximin Repeat Treatment. Am. J. Gastroenterol. 2016, 111, S1. [Google Scholar] [CrossRef]
- Brenner, D.M.; Sayuk, G.S. Current US Food and Drug Administration-Approved Pharmacologic Therapies for the Treatment of Irritable Bowel Syndrome with Diarrhea. Adv. Ther. 2019, 37, 83–96. [Google Scholar] [CrossRef]
- Chen, G.; Xie, X.; Peng, C. Treatment of Irritable Bowel Syndrome by Chinese Medicine: A Review. Chin. J. Integr. Med. 2023, 29, 377–384. [Google Scholar] [CrossRef]
- Wang, M.; Xie, X.; Zhao, S.; Ma, X.; Wang, Z.; Zhang, Y. Fecal microbiota transplantation for irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 2023, 14, 1136343. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Brummer, R.J. Faecal microbiota transplantation in IBS—New evidence for success? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- González-Castro, A.M.; Martínez, C.; Salvo-Romero, E.; Fortea, M.; Pardo-Camacho, C.; Pérez-Berezo, T.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 53–63. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients with Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Bek, S.; Teo, Y.N.; Tan, X.-H.; Fan, K.; Siah, K. Association between irritable bowel syndrome and micronutrients: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1485–1497. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.-T.; Heitkemper, M.M.; Zia, J. Effects of Low-FODMAPS Diet on Irritable Bowel Syndrome Symptoms and Gut Microbiome. Gastroenterol. Nurs. 2019, 42, 150–158. [Google Scholar] [CrossRef]
- Ceccherini, C.; Daniotti, S.; Bearzi, C.; Re, I. Evaluating the Efficacy of Probiotics in IBS Treatment Using a Systematic Review of Clinical Trials and Multi-Criteria Decision Analysis. Nutrients 2022, 14, 2689. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Xiong, Z.; Liao, Z.; Qian, Y.; Song, X.; Ai, L.; Xia, Y. Lactobacillus plantarum AR495 improves stress-induced irritable bowel syndrome in rats by targeting gut microbiota and Mast cell-PAR2-TRPV1 signaling pathway. Food Sci. Hum. Well. 2024, 13, 698–708. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Biotherapy Using Probiotics as Therapeutic Agents to Restore the Gut Microbiota to Relieve Gastrointestinal Tract Inflammation, IBD, IBS and Prevent Induction of Cancer. Int. J. Mol. Sci. 2023, 24, 5748. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Ramió-Pujol, S.; Amoedo, J.; Malagón, M.; Serrano, M.; Bahí, A.; Lluansí, A.; Busquets, D.; Pardo, L.; Srra-Pagès, M.; et al. P686 Novel grape-derived prebiotic selectively enhances abundance and metabolic activity of IBD and IBS faecal butyrate producing bacteria in an in vitro model of intestinal fermentation. J. Crohns Colitis 2021, 15, S605–S606. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, Z.; Yang, L.; Li, R.; Ma, Y. Combined effect of vitamin C and vitamin D3 on intestinal epithelial barrier by regulating Notch signaling pathway. Nutr. Metab. 2021, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Salomon, N.; Lahat, A.; Ungar, B.; Eliakim, R.; Kriger-Sharabi, O.; Reiss-Mintz, H.; Koslowsky, B.; Shitrit, A.B.G.; Tamir-Degabli, N.; et al. Real-world experience with Curcumin–QingDai combination for patients with active ulcerative colitis: A retrospective multicentre cohort study. Aliment. Pharm. Ther. 2023, 58, 175–181. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef]
- Kawai, S.; Iijima, H.; Shinzaki, S.; Hiyama, S.; Yamaguchi, T.; Araki, M.; Iwatani, S.; Shiraishi, E.; Mukai, A.; Inoue, T. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J. Gastroenterol. 2017, 52, 904–919. [Google Scholar] [CrossRef]
- Lu, H.; Liu, P.; Zhang, X.; Bao, T.; Wang, T.; Guo, L.; Li, Y.; Dong, X.; Li, X.; Dong, Y.; et al. Inulin and Lycium barbarum polysaccharides ameliorate diabetes by enhancing gut barrier via modulating gut microbiota and activating gut mucosal TLR2+ intraepithelial γδ T cells in rats. J. Funct. Foods 2021, 79, 104407. [Google Scholar] [CrossRef]
- Shen, X.; Xie, A.; Li, Z.; Jiang, C.; Wu, J.; Li, M.; Yue, X. Research Progress for Probiotics Regulating Intestinal Flora to Improve Functional Dyspepsia: A Review. Foods 2024, 13, 151. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Chiu, P.W.Y. A Review of the Effects of Natural Compounds, Medicinal Plants, and Mushrooms on the Gut Microbiota in Colitis and Cancer. Front. Pharmacol. 2020, 11, 744. [Google Scholar] [CrossRef]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms 2022, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Camacho, C.; Ganda Mall, J.-P.; Martínez, C.; Pigrau, M.; Expósito, E.; Albert-Bayo, M.; Melón-Ardanaz, E.; Nieto, A.; Rodiño-Janeiro, B.; Fortea, M.; et al. Mucosal Plasma Cell Activation and Proximity to Nerve Fibres Are Associated with Glycocalyx Reduction in Diarrhoea-Predominant Irritable Bowel Syndrome: Jejunal Barrier Alterations Underlying Clinical Manifestations. Cells 2022, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ren, P.; Wang, M. Changes in intestinal barrier protein expression and intestinal flora in a rat model of visceral hypersensitivity. Neurogastroenterol. Motil. 2021, 34, e14299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yao, J.; Wang, C.; Zhang, L.; Kong, W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol. Med. Rep. 2015, 12, 3257–3264. [Google Scholar] [CrossRef]
- Eluisa, P.; Javier, A.-L.; Morgane, V.F. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70, 1275. [Google Scholar]
- Riba, A.; Olier, M.; Lacroix-Lamandé, S.; Lencina, C.; Bacquié, V.; Harkat, C.; Gillet, M.; Baron, M.; Sommer, C.; Mallet, C.; et al. Paneth Cell Defects Induce Microbiota Dysbiosis in Mice and Promote Visceral Hypersensitivity. Gastroenterology 2017, 153, 1594–1606.e2. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hausken, T.; Gilja, O.H.; Hatlebakk, J. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2016, 11, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Zhu, Z.; Hu, C.; Long, G.; Chen, B.; Guo, R.; Fang, M.; Jiang, M. Potential Roles of Enterochromaffin Cells in Early Life Stress-Induced Irritable Bowel Syndrome. Front. Cell Neurosci. 2022, 16, 837166. [Google Scholar] [CrossRef]
- Luo, M.; Zhuang, X.; Tian, Z.; Xiong, L. Alterations in short-chain fatty acids and serotonin in irritable bowel syndrome: A systematic review and meta-analysis. BMC Gastroenterol. 2021, 21, 14. [Google Scholar] [CrossRef]
- Coates, M.D.; Mahoney, C.R.; Linden, D.R. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004, 126, 1657–1664. [Google Scholar] [CrossRef]
- Foley, S.; Garsed, K.; Singh, G.; Duroudier, N.; Swan, C.; Hall, I.; Zaitoun, A.; Bennett, A.; Marsden, C.; Holmes, G.; et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology 2011, 140, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, M.; Santos, J.; de Torres, I.; Alonso, C.; Vicario, M.; Ramos, L.; Martínez, C.; Casellas, F.; Saperas, E.; Malagelada, J.-R. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 2007, 56, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Perez Ingles, D.; Myneedu, K.; Deoker, A.; Sarosiek, I.; Zuckerman, M.-J.; Schmulson, M.-J.; Bashashati, M. Mast cells are increased in the small intestinal mucosa of patients with irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol. Motil. 2019, 31, E13718. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Hou, X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J. Neurogastroenterol. Motil. 2016, 22, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Lobo, B.; Pigrau, M.; Ramos, L.; González-Castro, A.; Alonso, C.; Guilarte, M.; Guilá, M.; Terres, I.; Azpiroz, F.; et al. Diarrhoea-predominant irritable bowel syndrome: An organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut 2012, 62, 1160–1168. [Google Scholar] [CrossRef]
- Aguilera-Lizarraga, J.; Hussein, H.; Boeckxstaens, G.E. Immune activation in irritable bowel syndrome: What is the evidence? Nat. Rev. Immunol. 2022, 22, 674–686. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, T.; Grabauskas, G.; Owyang, C. Mucosal Serotonin Reuptake Transporter Expression in Irritable Bowel Syndrome Is Modulated by Gut Microbiota Via Mast Cell–Prostaglandin E2. Gastroenterology 2022, 162, 1962–1974.e6. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Peng, L.; Kou, G.; Wang, P.; Lu, L. Assessment of Serum sTREM-1 as a Marker of Subclinical Inflammation in Diarrhea-Predominant Patients with Irritable Bowel Syndrome. Digest. Dis. Sci. 2018, 63, 1182–1191. [Google Scholar] [CrossRef]
- Katsumata, R.; Ishii, M.; Lee, S.; Handa, Y.; Murao, T.; Fujita, M.; Matsumoto, H.; Otauki, T.; Shiotani, A. Cytokine Profile and Immunoglobulin E-mediated Serological Food Hypersensitivity in Patients with Irritable Bowel Syndrome with Diarrhea. J. Neurogastroenterol. Motil. 2018, 24, 415–421. [Google Scholar] [CrossRef]
- Boyer, J.; Saint-Paul, M.C.; Dadone, B.; Patouraux, S.; Vivinus, M.-H.; Ouvrier, D.; Michiels, J.-F.; Piche, T.; Tulic, M.-K. Inflammatory cell distribution in colon mucosa as a new tool for diagnosis of irritable bowel syndrome: A promising pilot study. Neurogastroenterol. Motil. 2018, 30, e13223. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; Odle, J.; Lin, X.; Zhao, J.; Xiao, K.; Liu, Y. Modulation of intestinal stem cell homeostasis by nutrients: A novel therapeutic option for intestinal diseases. Nutr. Res. Rev. 2022, 35, 150–158. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Reduction in duodenal endocrine cells in irritable bowel syndrome is associated with stem cell abnormalities. World J. Gastroenterol. 2015, 21, 9577–9587. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Lei, Z.; Ge, P.; Lu, Z.; Chai, Q.; Zhang, Y.; Qiang, L.; Yu, Y.; Zhang, X.; et al. TRIM27 maintains gut homeostasis by promoting intestinal stem cell self-renewal. Cell. Mol. Immunol. 2023, 20, 158–174. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Maranda, L.; Candela, N. Vitamin D status in pediatric irritable bowel syndrome. PLoS ONE 2017, 12, e0172183. [Google Scholar]
- Rezazadegan, M.; Soheilipour, M.; Tarrahi, M.J.; Amani, R. Correlation between Zinc Nutritional Status with Serum Zonulin and Gastrointestinal Symptoms in Diarrhea-Predominant Irritable Bowel Syndrome: A Case–Control Study. Digest. Dis. Sci. 2022, 67, 3632–3638. [Google Scholar] [CrossRef]
- Fitzpatrick, L.R.; Jenabzadeh, P. IBD and Bile Acid Absorption: Focus on Pre-clinical and Clinical Observations. Front. Physiol. 2020, 11, 564. [Google Scholar] [CrossRef]
- Melchior, C.; Douard, V.; Coëffier, M.; Gourcerol, G. Fructose and irritable bowel syndrome. Nutr. Res. Rev. 2020, 33, 235–243. [Google Scholar] [CrossRef]
- Oh, A.-R.; Sohn, S.; Lee, J.; Park, J.-M.; Nam, K.-T.; Hahm, K.-B.; Kim, Y.-B.; Lee, H.; Cha, J.-Y. ChREBP deficiency leads to diarrhea-predominant irritable bowel syndrome. Metabolism 2018, 85, 286–297. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Gao, S.; Liu, Y.; Wang, W.; Feng, Y.; Pei, L.; Sun, Z.; Liu, L.; Wang, C. Effect of gut flora mediated-bile acid metabolism on intestinal immune microenvironment. Immunology 2023, 170, 301–318. [Google Scholar] [CrossRef]
- Guo, H.; Gibson, S.A.; Ting, J.P.Y. Gut microbiota, NLR proteins, and intestinal homeostasis. J. Exp. Med. 2020, 217, e20181832. [Google Scholar] [CrossRef]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.-E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef]
- Petitfils, C.; Maurel, S.; Payros, G.; Hueber, A.; Agaiz, B.; Gazzo, G.; Marrocco, R.; Auvray, F.; Langevin, G.; Motta, J.-P.; et al. Identification of bacterial lipopeptides as key players in IBS. Gut 2023, 72, 939–950. [Google Scholar] [CrossRef]
- Jalanka-Tuovinen, J.; Salonen, A.; Nikkilä, J.; Immonen, O.; Kekkonen, R.; Lahti, L.; Palva, A.; Vos, W.M. Intestinal microbiota in healthy adults: Temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE 2011, 6, e23035. [Google Scholar] [CrossRef]
- Zhu, X.; Hong, G.; Li, Y.; Yang, P.; Cheng, M.; Zhang, L.; Li, Y.; Ji, L.; Li, G.; Chen, C.; et al. Understanding of the Site-Specific Microbial Patterns towards Accurate Identification for Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Microbiol. Spectrum. 2021, 9, e01255-21. [Google Scholar] [CrossRef]
- Wedlake, L.; A’Hern, R.; Russell, D.; Thomas, K.; Walters, J.R.F.; Andreyev, H.J.N. Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharm. Ther. 2009, 30, 707–717. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, W.; Chen, Y.; Huang, F.; Lu, L.; Lin, C.; Huang, T.; Ning, Z.; Zhai, L.; Zhao, L.; et al. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J. Clin. Invest 2020, 130, 438–450. [Google Scholar] [CrossRef]
- Zhan, K.; Zheng, H.; Li, J.; Wu, H.; Qin, S.; Luo, L.; Huang, S. Gut Microbiota-Bile Acid Crosstalk in Diarrhea-Irritable Bowel Syndrome. BioMed Res. Int. 2020, 2020, 3828249. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Xu, P.; Cheng, L.; Wang, Q.-Q.; Qiu, H.-Y.; Yan, X.-J. Mucosa-associated microbiota dysbiosis in the terminal ileum correlates with bowel symptoms in diarrhea-predominant irritable bowel syndrome. Clin. Transl. Gastroen. 2023, 10, 14309. [Google Scholar] [CrossRef]
- Hou, Y.; Dong, L.; Lu, X.; Shi, H.; Xu, B.; Zhong, W.; Ma, L.; Wang, S.; Yang, C.; He, X.; et al. Distinctions Between Fecal and Intestinal Mucosal Microbiota in Subgroups of Irritable Bowel Syndrome. Digest. Dis. Sci. 2022, 67, 5580–5592. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2014, 29, 1395–1403. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 10235. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients with Diarrhea-Predominant Irritable Bowel Syndrome and Patients with Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.; Unno, T.; Shin, J. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Maubert, M.; Quévrain, E.; Chain, F.; Marquant, R.; Kharrat, P.; Carlier, I.; Bermudz, L.; Pigneur, B.; Lequin, O.; Bridonneau, C.; et al. Identification of an Anti-Inflammatory Protein from Faecalibacterium prausnitzii, a Deficient Commensal Bacteria Implicated in Crohn’s Disease. Gastroenterology 2014, 65, 415–425. [Google Scholar] [CrossRef]
- Gu, X.; Song, L.; Li, L.; Liu, T.; Zhang, M.; Li, Z.; Wang, P.; Li, M.; Zuo, X. Fusobacterium nucleatum Causes Microbial Dysbiosis and Exacerbates Visceral Hypersensitivity in a Colonization-Independent Manner. Front. Microbiol. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Chung, H.; Kasper, D.L. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Curr. Opin. Immunol. 2010, 22, 455–460. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Atarashi, H.; Nagamori, T.; et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef]
- Beneke, V.; Grieger, K.M.; Hartwig, C.; Müller, J.; Sohn, K.; Blaudszun, A.; Hilger, N.; Schaudien, D.; Fricke, S.; Braun, A.; et al. Homeostatic T helper 17 cell responses triggered by complex microbiota are maintained in ex vivo intestinal tissue slices. Eur. J. Immunol. 2024, 54, 2350946. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselit, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T. Bifidobacterium bifidum Enhances the Intestinal Epithelial Tight Junction Barrier and Protects against Intestinal Inflammation by Targeting the Toll-like Receptor-2 Pathway in an NF-κB-Independent Manner. Int. J. Mol. Sci. 2021, 22, 8070. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2020, 33, e13983. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2020, 80, 37–49. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Couto, M.R.; Gonçalves, P.; Magro, F.; Martel, F. Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease. Pharmacol. Res. 2020, 159, 104947. [Google Scholar] [CrossRef]
- Ma, S.; Yeom, J.; Lim, Y.-H. Specific activation of hypoxia-inducible factor-2α by propionate metabolism via a β-oxidation-like pathway stimulates MUC2 production in intestinal goblet cells. Biomed. Pharmacother. 2022, 155, 113672. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; Sluis, M.; Bouma, J.; Boehm, G.; Goudoever, J.; Seuningen, I.; Renes, I. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.; Alexeev, E.; Wang, R.; Onyiah, J.; Kominsky, D.; Colgan, S. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Digest. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.; Ann, P.; Ma, L.; Nagler, C.; Flsmagilov, S.; Mazmanian, K.; Hsiao, E. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 163, 258. [Google Scholar] [CrossRef]
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Yao, B.; He, J.; Yin, X.; Shi, Y.; Wan, J.; Tian, Z. The protective effect of lithocholic acid on the intestinal epithelial barrier is mediated by the vitamin D receptor via a SIRT1/Nrf2 and NF-κB dependent mechanism in Caco-2 cells. Toxicol. Lett. 2019, 316, 109–118. [Google Scholar] [CrossRef]
- Liu, L.; Dong, W.; Wang, S.; Zhang, Y.; Liu, T.; Xie, R.; Wang, B.; Cao, H. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018, 9, 5588–5597. [Google Scholar] [CrossRef]
- Bromke, M.A.; Krzystek-Korpacka, M. Bile Acid Signaling in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 9096. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pelllicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef] [PubMed]
- Mosińska, P.; Szczepaniak, A.; Fichna, J. Bile acids and FXR in functional gastrointestinal disorders. Dig. Liver Dis. 2018, 50, 795–803. [Google Scholar] [CrossRef]
- Kubota, H.; Ishizawa, M.; Kodama, M.; Nagase, Y.; Kato, S.; Makishima, M.; Sakurai, K. Vitamin D Receptor Mediates Attenuating Effect of Lithocholic Acid on Dextran Sulfate Sodium Induced Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 3517. [Google Scholar] [CrossRef] [PubMed]
- Alemi, F.; Poole, D.P.; Chiu, J.; Schoonjans, K.; Cattaruzza, F.; Grider, J.; Bunnett, N.; Corvera, C. The Receptor TGR5 Mediates the Prokinetic Actions of Intestinal Bile Acids and Is Required for Normal Defecation in Mice. Gastroenterology 2013, 144, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Wu, J.; Ye, B. Gut Microbiota-Derived Metabolites in the Development of Diseases. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6658674. [Google Scholar] [CrossRef]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derived from Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef]
- Beaumont, M.; Neyrinck, A.M.; Olivares, M.; Rodriguez, J.; de Rocca Serra, A.; Roumain, M.; Bindels, L.; Cani, P.; Evenepoel, P.; Muccioli, G.; et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018, 32, 6681–6693. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Metidji, A.; Omenetti, S.; Crotta, S.; Li, Y.; Nye, E.; Ross, E.; Li, V.; Maradana, M.; Schiering, C.; Stockinger, B. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 2018, 49, 353–362.e355. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Dopamine receptor D2 confers colonization resistance via microbial metabolites. Nature 2024, 628, 180–185. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Beck, P.L.; McKay, D.M. Neuroimmunophysiology of the gut: Advances and emerging concepts focusing on the epithelium. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 765–784. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef]
- Cui, C.; Wang, F.; Zheng, Y.; Wei, H.; Peng, J. From birth to death: The hardworking life of Paneth cell in the small intestine. Front. Immunol. 2023, 14, 1122258. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hooper, L.V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Ou, J.; Liang, S.; Guo, X.; He, X. α-Defensins Promote Bacteroides Colonization on Mucosal Reservoir to Prevent Antibiotic-Induced Dysbiosis. Front. Immunol. 2020, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- Drurey, C.; Lindholm, H.T.; Coakley, G.; Poveda, M.; Löser, S.; Doolan, R.; Gerbe, F.; Jay, P.; Harris, N.; Oudhoff, M.; et al. Intestinal epithelial tuft cell induction is negated by a murine helminth and its secreted products. J. Exp. Med. 2021, 219, e20211140. [Google Scholar] [CrossRef] [PubMed]
- Slifer, Z.M.; Blikslager, A.T. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int. J. Mol. Sci. 2020, 21, 972. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 9–21. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Hansson, G.C. Membrane mucins of the intestine at a glance. J. Cell Sci. 2020, 133, jcs.240929. [Google Scholar] [CrossRef]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qin, Y.; Xiong, X.; Wang, Z.; Wang, M.; Wang, Y.; Wang, Q.; Yang, H.; Yin, Y. Effects of iron, vitamin A, and the interaction between the two nutrients on intestinal development and cell differentiation in piglets. J. Anim. Sci. 2021, 99, skab258. [Google Scholar] [CrossRef]
- Mielke, L.A.; Jones, S.A.; Raverdeau, M.; Higgs, R.; Stefanska, A.; Groom, J.R.; Misiak, A.; Dungan, L.S.; Sutton, C.E.; Streubel, G.; et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J. Exp. Med. 2013, 210, 1117–1124. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef]
- Iyer, N.; Grizotte-Lake, M.; Duncan, K.; Gordon, S.R.; Palmer, A.C.S.; Calvin, C.; Zhong, G.; Isoherranen, N.; Vaishnava, S. Epithelium intrinsic vitamin A signaling co-ordinates pathogen clearance in the gut via IL-18. PLoS Pathog. 2020, 16, e1008360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shin, D.; Zhang, X. Protective role of 1,25(OH)2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Chen, G.; Zuo, S.; Zhang, J.; Chen, Z.; Wang, X.; Li, J.; Liu, Y.; Wang, P. 1,25-Dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-α induced injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling pathway. Biochem. Biophys. Res. Commun. 2015, 460, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Singh, T.P.; Wei, X.; Yao, H.; Wang, H. Protective Effect of 1,25-Dihydroxy Vitamin D3 on Pepsin–Trypsin-Resistant Gliadin-Induced Tight Junction Injuries. Digest. Dis. Sci. 2018, 63, 92–104. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Zhong, Y.N.; Zhao, F.; Hao, Z.; Xu, Y.; Lai, R.; Yin, X. Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J. Gastroenterol. Hepatol. 2020, 35, 1023–1031. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radical. Bio. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef]
- Li, J.; Kong, D.; Wang, Q.; Wu, W.; Tang, Y.; Bai, T.; Guo, L.; Wei, L.; Zhang, Q.; Yu, Y.; et al. Niacin ameliorates ulcerative colitis via prostaglandin D2-mediated D prostanoid receptor 1 activation. EMBO Mol. Med. 2017, 9, 571–588. [Google Scholar] [CrossRef]
- Ligaarden, S.C.; Farup, P.G. Low intake of vitamin B6 is associated with irritable bowel syndrome symptoms. Nutr. Res. 2011, 31, 356–361. [Google Scholar] [CrossRef]
- Ge, Y.; Zadeh, M.; Mohamadzadeh, M. Vitamin B12 Regulates the Transcriptional, Metabolic, and Epigenetic Programing in Human Ileal Epithelial Cells. Nutrients 2022, 14, 2825. [Google Scholar] [CrossRef]
- Shao, Y.; Wolf, P.G.; Guo, S.; Guo, Y.; Gaskins, H.R.; Zhang, B. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J. Nutr. Biochem. 2017, 43, 18–26. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Su, W.; Ying, Z.; He, J.; Zhang, L.; Zhong, X.; Wang, T. Zinc oxide nanoparticles as a substitute for zinc oxide or colistin sulfate: Effects on growth, serum enzymes, zinc deposition, intestinal morphology and epithelial barrier in weaned piglets. PLoS ONE 2017, 12, e0181136. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Straubing, S.; Robbe-Masselot, C.; Haase, H. Zinc Deficiency Disturbs Mucin Expression, O-Glycosylation and Secretion by Intestinal Goblet Cells. Int. J. Mol. Sci. 2020, 21, 6149. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, X.; Pi, S.; Chang, J.; Dou, X.; Yan, S.; Song, X.; Chen, Y.; Zeng, X.; Zhu, L.; et al. Dietary supplementation with biogenic selenium nanoparticles alleviate oxidative stress-induced intestinal barrier dysfunction. npj Sci. Food 2022, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Che, H.; Xie, J.; Dong, X.; Song, L.; Xie, W.; Sun, J. Supplementary selenium in the form of selenylation α-D-1,6-glucan ameliorates dextran sulfate sodium induced colitis in vivo. Int. J. Biol. Macromol. 2022, 195, 67–74. [Google Scholar] [CrossRef]
- Nelson, S.; Lei, X.; Prabhu, K. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef]
- Lin, H.; Chen, D.; Du, Q.; Pan, T.; Tu, H.; Xu, Y.; Teng, T.; Tu, J.; Li, J.; Lin, Z.; et al. Dietary Copper Plays an Important Role in Maintaining Intestinal Barrier Integrity during Alcohol-Induced Liver Disease through Regulation of the Intestinal HIF-1α Signaling Pathway and Oxidative Stress. Front. Physiol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Li, Y.; Li, L.; Zhai, W.; Wang, P.; Yang, X.; Gu, X.; Song, L.; Li, Z.; et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018, 8, 2964. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, E.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering 2021, 7, 376–385. [Google Scholar] [CrossRef]
- Ishaque, S.; Khosruzzaman, S.; Ahmed, D.; Sah, M. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Cheng, J.; Zhai, J.; Zhou, L.; Wang, B. Lactobacillus rhamnosus GG Promotes Intestinal Vitamin D Absorption by Upregulating Vitamin D Transporters in Senile Osteoporosis. Calcif. Tissue Int. 2022, 111, 162–170. [Google Scholar] [CrossRef]
- Li, M.-Y.; Duan, J.-Q.; Wang, X.-H.; Liu, M.; Yang, Q.; Li, Y.; Wang, K.; Liu, H.; Wang, F. Inulin Inhibits the Inflammatory Response through Modulating Enteric Glial Cell Function in Type 2 Diabetic Mellitus Mice by Reshaping Intestinal Flora. ACS Omega 2023, 8, 36729–36743. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response. Nutrients 2017, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- Bridgette, W.; Megan, R.; Tokuwa, K.; Gareth, C.P.; Simon, A.; James, M.A.; Peter, M.I.; Miranda, C.L.; Kevin, W. β-Galactooligosaccharide in Conjunction with Low FODMAP Diet Improves Irritable Bowel Syndrome Symptoms but Reduces Fecal Bifidobacteria. Am. J. Gastroenterol. 2020, 115, 906–915. [Google Scholar]
- Guo, L.; Zhang, X.; Lv, N.; Wang, L.; Gan, J.; Jiang, X.; Wang, Y. Therapeutic Role and Potential Mechanism of Resveratrol in Atherosclerosis: Therapeutic Role and Potential Mechanism of Resveratrol in Atherosclerosis: TLR4/NF-κB/HIF-1α. Mediat. Inflamm. 2023, 2023, 1097706. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.; Li, Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct 2020, 11, 1063–1073. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, M.; Li, Z.; Meng, Z. Curcumin inhibits angiotensin II-induced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-γ activity and reducing oxidative stress. Int. J. Mol. Med. 2017, 39, 1307–1316. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- He, Z.; Deng, N.; Zheng, B.; Gu, Y.; Chen, J.; Li, T.; Liu, R.-H.; Yuan, L.; Li, W. Apple peel polyphenol alleviates antibiotic-induced intestinal dysbiosis by modulating tight junction proteins, the TLR4/NF-κB pathway and intestinal flora. Food Funct. 2023, 14, 6678–6689. [Google Scholar] [CrossRef]
- Yu, L.-M.; Mao, L.-Q.; Wu, C.-Y.; Ye, W.; Wang, X. Chlorogenic acid improves intestinal barrier function by downregulating CD14 to inhibit the NF-κB signaling pathway. J. Funct. Foods 2021, 85, 104640. [Google Scholar] [CrossRef]
- Xu, X.; Dong, Q.; Zhong, Q.; Xiu, W.; Chen, Q.; Wang, J.; Zhou, Z. The Flavonoid Kurarinone Regulates Macrophage Functions via Aryl Hydrocarbon Receptor and Alleviates Intestinal Inflammation in Irritable Bowel Syndrome. J. Inflamm. Res. 2021, 14, 4347–4359. [Google Scholar] [CrossRef]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shen, M.; Yu, Q.; Chen, Y.; Wen, H.-L.; Lu, H.-Y.; Chen, S.; Xie, J.-H. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 2022, 397, 133768. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, R.; Wang, N.; Deng, Y.; Tan, B.; Yin, Y.; Qi, M.; Wang, J. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef]
- Meza-Meza, M.R.; Ruiz-Ballesteros, A.I.; de la Cruz-Mosso, U. Functional effects of vitamin D: From nutrient to immunomodulator. Crit. Rev. Food Sci. Nutr. 2020, 62, 3042–3062. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, A.; Amani, R.; Hajiani, E.; Alavinejad, P.; Cheraghian, B.; Ghadiri, A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol. Motil. 2016, 28, 1533–1544. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Amani, R.; Hasanvand, A.; Yousefi Rad, E.; Alipour, M.; Saboori, S.; Choghakhori, R. Association of Serum Vitamin D Concentration with Clinical Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. J. Am. Coll. Nutr. 2019, 38, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Abuelazm, M.; Muhammad, S.; Gamal, M.; Labieb, F.; Amin, M.A.; Abdelazeem, B.; Brašić, J.R. The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2618. [Google Scholar] [CrossRef]
- Khalighi, S.M.; Mokhtare, M.; Janani, L.; Faghihi Kashani, A.H.; Masoodi, M.; Agah, S.; Abbaspour, N.; Dehnad, A.; Shidfar, F. Vitamin D3 Supplementation in Diarrhea-Predominant Irritable Bowel Syndrome Patients: The Effects on Symptoms Improvement, Serum Corticotropin-Releasing Hormone, and Interleukin-6—A Randomized Clinical Trial. Complement. Med. Res. 2020, 27, 302–309. [Google Scholar]
- Krimi, R.B.; Kotelevets, L.; Dubuquoy, L.; Plaisancié, P.; Walker, F.; Lehy, T.; Desreumaux, P.; Seuningen, I.V.; Chastre, E.; Forgue-Lafitte, M.-E.; et al. Resistin-like molecule β regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2008, 14, 931–941. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B. The Impact of Zinc and Zinc Homeostasis on the Intestinal Mucosal Barrier and Intestinal Diseases. Biomolecules 2022, 12, 900. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Tanabe, S.; Suzuki, T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. J. Appl. Physiol. Gastrointest. Liver Physiol. 2016, 311, G105–G116. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Chatterjee, T.; Joshi, P.; Poddar, A.; Bhattacharyya, B.; Singh, S.P.; Gupta, V.; Charkrabarti, P. Structure and Activity of Lysozyme on Binding to ZnO Nanoparticles. Langmuir 2009, 26, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Zhao, C.; Li, F.; Gu, Z.; Liu, L.; Zhang, L.; Wang, Y.; He, L.; Liu, Y.; Liu, Q.; et al. Intestinal HIF-1α Deletion Exacerbates Alcoholic Liver Disease through Inducing Intestinal Dysbiosis and Barrier Dysfunction. J. Hepatol. 2018, 69, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Harris, L.A.; Baffy, N. Modulation of the gut microbiota: A focus on treatments for irritable bowel syndrome. Postgrad. Med. 2017, 129, 872–888. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 75, 306–318. [Google Scholar] [CrossRef]
- Wilson, B.; Rossi, M.; Dimidi, E.; Whelan, K. Prebiotics in Irritable Bowel Syndrome and other functional bowel disorders in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Chiarioni, G.; Popa, S.L.; Ismaiel, A.; Pop, C.; Dumitrascu, D.; Brata, V.D.; Duse, T.A.; Incze, V.; Surdea-Blaga, T. The Effect of Polyphenols, Minerals, Fibers, and Fruits on Irritable Bowel Syndrome: A Systematic Review. Nutrients 2023, 15, 4070. [Google Scholar] [CrossRef]
- He, Z.; Deng, N.; Zheng, B.; Li, T.; Liu, R.; Yuan, L.; Li, W. Changes in polyphenol fractions and bacterial composition after in vitrofermentation of apple peel polyphenol by gut microbiota. Int. J. Food Sci. Tech. 2022, 57, 4268–4276. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Tech. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2019, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, I.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Butelli, E. Combined Dietary Anthocyanins, Flavonols, and Stilbenoids Alleviate Inflammatory Bowel Disease Symptoms in Mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef]
- Marcela, D.; Premysl, L. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar]
- Wang, Y.; Hong, C.; Wu, Z.; Li, S.; Xia, Y.; Liang, Y.; He, X.; Xiao, X.; Tang, W. Resveratrol in Intestinal Health and Disease: Focusing on Intestinal Barrier. Front. Nutr. 2022, 9, 848400. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Li, Y.-Y.; Liu, Z.-J.; Wang, J.-F. Quercetin effectively improves LPS-induced intestinal inflammation, pyroptosis, and disruption of the barrier function through the TLR4/NF-κB/NLRP3 signaling pathway in vivo and in vitro. Food Nutr. Res. 2022, 66, 8948. [Google Scholar] [CrossRef]
- Caban, M.; Lewandowska, U. Polyphenols and the potential mechanisms of their therapeutic benefits against inflammatory bowel diseases. J. Funct. Foods 2022, 95, 105181. [Google Scholar] [CrossRef]
- Zhai, L.; Huang, C.; Ning, Z.; Zhang, Y.; Zhuang, M.; Yang, W.; Wang, X.; Wang, J.; Zhang, L.; Xiao, H.; et al. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe 2023, 31, 33–44.e35. [Google Scholar] [CrossRef]
- Yu, X.-L.; Li, C.-P.; He, L.-P. Vitamin D may alleviate irritable bowel syndrome by modulating serotonin synthesis: A hypothesis based on recent literature. Front. Physiol. 2023, 14, 92958. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Wang, G.; Xiong, Z.; Wei, G.; Liao, Z.; Qian, Y.; Cai, Z.; Ai, L. Lactobacillus plantarum AR495 improves colonic transport hyperactivity in irritable bowel syndrome through tryptophan metabolism. Food Funct. 2024, 14, 7416–7429. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-Y.; Zang, K.-H.; Zuo, X.; Bian, Z.-X. X. Quercetin Attenuates Visceral Hypersensitivity and 5-Hydroxytryptamine Availability in Postinflammatory Irritable Bowel Syndrome Rats: Role of Enterochromaffin Cells in the Colon. J. Med. Food 2019, 22, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Erarslan, A.-S.; Ozmerdivenli, R.; Sirinyıldız, F.; Cevik, O.; Gumus, E.; Cesur, G. Therapeutic and prophylactic role of vitamin D and curcumin in acetic acid-induced acute ulcerative colitis model. Toxicol. Mech. Methods 2023, 33, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, H.; Li, Y.; Yang, H.; Tan, B.; Qin, J. Vitamin D3 and Lactobacillus rhamnosus GG/p40 Synergize to Protect Mice From Colitis by Promoting Vitamin D Receptor Expression and Epithelial Proliferation. Inflamm. Bowel Dis. 2023, 29, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Dhar, P. Amalgamation of polyphenols and probiotics induce health promotion. Crit. Rev. Food Sci. Nutr. 2019, 59, 2903–2926. [Google Scholar] [CrossRef]
- Hu, Q.; Li, J.; Wang, T.; Xu, X.; Duan, Y.; Jin, Y. Polyphenolic Nanoparticle-Modified Probiotics for Microenvironment Remodeling and Targeted Therapy of Inflammatory Bowel Disease. ACS Nano 2024, 18, 12917–12932. [Google Scholar] [CrossRef]

| Nutrient Type | Component | Subjects | Main Findings and Markers | Effects | References |
|---|---|---|---|---|---|
| Essential micronutrients | VA | In vitro | TEER ↑, ZO-1 ↑, occludin ↑, and claudins ↑. | Regulates intestinal barrier function. | [127] |
| Piglets | Induction of ISC differentiation, number of EECs ↑. | Promots intestinal development in weaned piglets. | [128] | ||
| Mice | IL-22 ↑ and AMP ↑. | Inhibits DSS-induced colitis. | [129,130] | ||
| Mice | IL-18 ↑ and IFN-γ ↑. | Limits pathogen invasion and activated immune cells to facilitate pathogen clearance during the early stages of infection. | [131] | ||
| VD | In vitro and mice | TEER ↑, LPS ↑, and TJ ↑. | Repaires intestinal barrier damage. | [132] | |
| In vitro | NF-κB/MLCK-P-MLC ↓ and VDR ↑. | [133] | |||
| In vitro and mice | TEER ↑, MyD88 ↓, and zonulin ↓. | Increases intestinal permeability. | [134] | ||
| Mice | MUC2 ↑, goblet cell count ↑, and RELMβ ↑. | Enhances colonic barrier function and modulates colitis. | [135] | ||
| VE | Mice | LPS ↓ and occludin ↑. | Maintains barrier integrity. | [136] | |
| VB3 | Mice | Activated DP1, reduced apoptosis, and promoted renewal of IECs. | [137] | ||
| VB6 | Humans | Lower VB6 intake disrupts the balance between pro- and anti-inflammatory cytokines. | Severe IBS symptoms associated with lower VB6 intake. | [138] | |
| VB12 | In vitro | TFF1/2/3 ↑, MUC13 ↑, and claudin2/18 ↑. | Reveals critical impact of VB12 in regulating cellular transcription, metabolism, and epigenetic programs. | [139] | |
| Zinc | In vitro | Activates the PI3K/Akt/mTOR signaling pathway, ZO-1 ↑. | Zinc maintains intestinal barrier function, regulated cell signaling and protein kinase activity. | [140] | |
| Piglets | occludin ↑, ZO-1 ↑. | Improves performance and reduces intestinal permeability in weaned piglets. | [141] | ||
| In vitro | Zinc deficiency affected mucin secretion, structure and stability of the mucus layer. | Zinc is important for formation and maintenance of the physical intestinal epithelial barrier. | [142] | ||
| Selenium | Mice | MUC2 ↑ and Reg IIIγ ↑. | Repaires intestinal barrier damage. | [143] | |
| Mice | occludin ↑ and claudin-1 ↑. Ameliorates goblet cell injury. | Maintaines the integrity of the physical and chemical intestinal barriers. | [144] | ||
| In vitro | M2 macrophages ↑. | Optimal Se status is essential for M2 macrophage activation, attenuating pro-inflammatory mediator expression. | [145] | ||
| Copper | In vitro, mice | Activates the HIF-1α signaling pathway. occludin ↑. | Copper homeostasis is essential for maintaining intestinal barrier integrity. | [146] | |
| Probiotics | Clostridium butyricum (CB) | Humans | Clostridium difficile ↓. | Improves clinical symptoms. | [147] |
| Lactobacillus plantarum (L. plantarum) CCFM8610 | Humans | IBS-SSS ↓, IBS-QOL ↓, and the relative abundance of butyric acid-producing strains ↑. | Significant relief of clinical symptoms and intestinal dysbiosis in IBS-D people. | [148] | |
| Multi-strain probiotic preparation (14 probiotic strains) | Humans | Significantly improve the severity of abdominal pain ↓, the number of bowel movements per day, IBS-SSS ↓, and IBS-QOL ↓. | Multi-strain probiotics are associated with significant symptom improvement in IBS-D patients and are well tolerated. | [149] | |
| Lactobacillus rhamnosus GG | Mice | CD36 ↑, NPC1L1 ↑, SR-B1 ↓. | Lactobacillus rhamnosus GG culture supernatant promotes intestinal absorption VD by affecting protein levels of VD transporters. | [150] | |
| Prebiotic | SDF | In vitro, mice | Bacteroidetes/Firmicutes ↑, IL-1β ↓, IL-6 ↓, TNF-α ↓, butyric acid ↑, and acetic acid ↑. | Restores intestinal dysbiosis and normalized the SCFA concentration in the colon. | [151] |
| New prebiotic blend (PB) | In vitro, mice | occludin ↑, activates the PPARγ signaling pathway and regulates intestinal flora. | Significantly reduces IBS symptoms and regulates the gut flora. | [152] | |
| β-GOS | Humans | Bifidobacterium ↑, flatulence ↓, abdominal pain ↓. | Effective relief of IBS clinical symptoms. | [153] | |
| Polyphenol | Resveratrol | In vitro | M1 macrophages ↓, IL-1β ↓, IL-6 ↓, TNF-α ↓ | It has a significant anti-inflammatory effect and prevents disease progression via the TLR4/NF-κB signaling pathway. | [154] |
| Mice | Akkermansia ↓, Dorea ↓, Sutterella ↓, Bilophila ↓, and Bifidobacterium ↑. | Alleviates gut microbiota dysbiosis. | [155] | ||
| Curcumin | Rats | PPAR-γ activity ↑, IL-6 ↓, TNF-α ↓, and NO ↓. | Anti-inflammatory and antioxidative. | [156] | |
| Quercetin | Mice | IL-1β ↓, IL-17 ↓, IL-6 ↓, TNF-α ↓, Bacteroides ↑, Bifidobacterium ↑, Lactobacillus ↑, and Clostridia ↑, Fusobacterium ↓, and Enterococcus ↓. | Dietary quercetin directly stimulates the immune system, reduces inflammation, and restores gut flora balance. | [157] | |
| Apple peel polyphenol | Mice | TLR4 ↓, NF-κB ↓, and pro-inflammatory factors ↓. | Relieves intestinal inflammation. | [158] | |
| Chlorogenic acid | In vitro, mice | IL-1β ↓, IL-6 ↓, TNF-α ↓, and CD14 ↓. Blocks the NF-κB signaling pathway. | Elucidates the mechanism by which CGA inhibits inflammation and protects intestinal barrier function. | [159] | |
| Kurarinone | Humans, mice | Macrophage activation ↓, pro-inflammatory cytokine expression ↓, anti-inflammatory cytokines, such as IL-10 ↑. | Uncovers the mechanism of how KAR regulates macrophage function. | [160] | |
| Caffeic acid | Mice | IL1β, IL-6, TNF-α mRNA, protein levels ↓, IL-10 mRNA and protein levels ↓, ROS ↓, LPS ↓, and butyric acid ↑. | Alleviates DSS-induced colitis and improves defense against oxidative stress and inflammatory response. | [161] | |
| Anthocyanin | Mice | Chorion length, number of goblet cells, SCFAs, Lachnospiraceae, Bacteroidaceae, Ruminococcaceae ↑, and Shigella ↓. | Improves intestinal barrier function impairment and intestinal flora dysbiosis. | [162] | |
| Ellagic acid | Piglets | TJ protein ↑, DAO ↓, and goblet cell count ↑. Improves intestinal damage. | Restores intestinal barrier integrity. | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Cao, Y.; Liu, Y.; Zheng, J. A New Strategy for Dietary Nutrition to Improve Intestinal Homeostasis in Diarrheal Irritable Bowel Syndrome: A Perspective on Intestinal Flora and Intestinal Epithelial Interaction. Nutrients 2024, 16, 3192. https://doi.org/10.3390/nu16183192
Wu X, Cao Y, Liu Y, Zheng J. A New Strategy for Dietary Nutrition to Improve Intestinal Homeostasis in Diarrheal Irritable Bowel Syndrome: A Perspective on Intestinal Flora and Intestinal Epithelial Interaction. Nutrients. 2024; 16(18):3192. https://doi.org/10.3390/nu16183192
Chicago/Turabian StyleWu, Xinyu, Yilong Cao, Yixiang Liu, and Jie Zheng. 2024. "A New Strategy for Dietary Nutrition to Improve Intestinal Homeostasis in Diarrheal Irritable Bowel Syndrome: A Perspective on Intestinal Flora and Intestinal Epithelial Interaction" Nutrients 16, no. 18: 3192. https://doi.org/10.3390/nu16183192
APA StyleWu, X., Cao, Y., Liu, Y., & Zheng, J. (2024). A New Strategy for Dietary Nutrition to Improve Intestinal Homeostasis in Diarrheal Irritable Bowel Syndrome: A Perspective on Intestinal Flora and Intestinal Epithelial Interaction. Nutrients, 16(18), 3192. https://doi.org/10.3390/nu16183192







