Transplant-Acquired Food Allergy in Children
Abstract
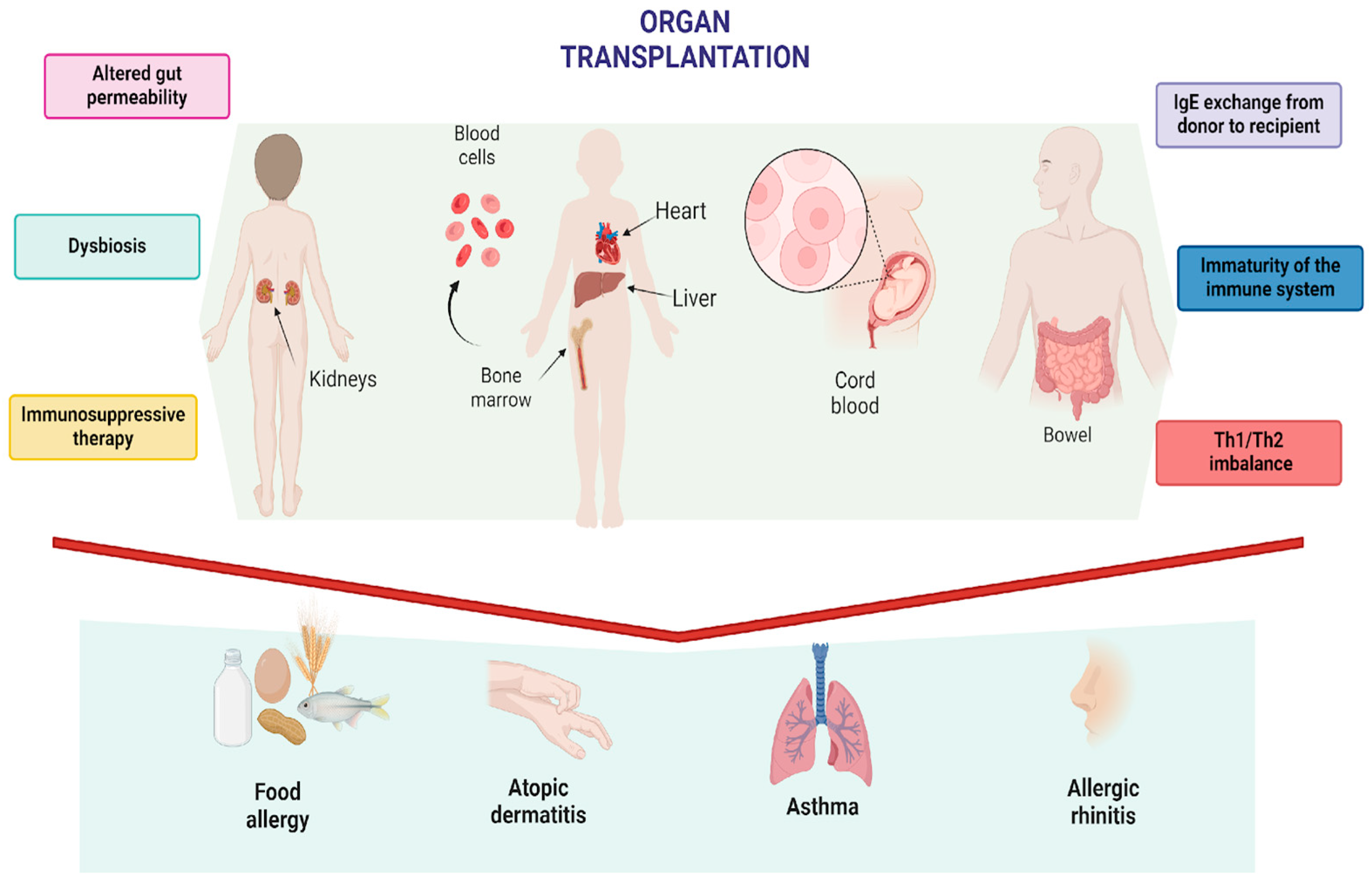
1. Introduction
1.1. Transplant-Related Hypothesis
1.2. Immunosuppressive-Related Hypothesis
1.3. Microbiome-Related Hypothesis
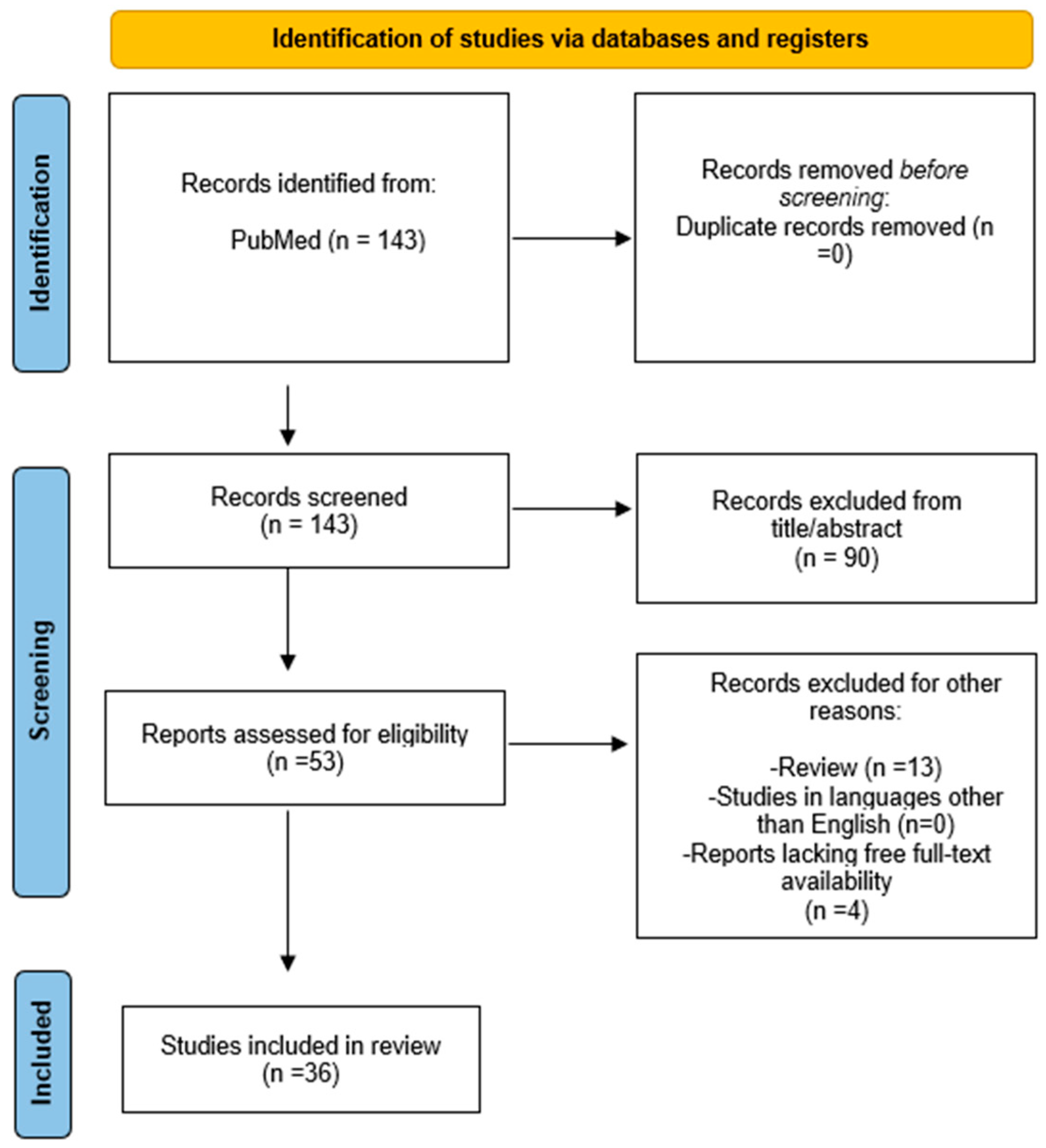
2. Methods
3. Results
4. Discussion
Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food Allergy: Epidemiology, Pathogenesis, Diagnosis, and Treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e5. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; de Silva, D.; Halken, S.; Worm, M.; Khaleva, E.; Arasi, S.; Dunn-Galvin, A.; Nwaru, B.I.; De Jong, N.W.; Rodríguez Del Río, P.; et al. Managing Food Allergy: GA2LEN Guideline 2022. World Allergy Organ. J. 2022, 15, 100687. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGalvin, A.; Garvey, L.H.; Riggioni, C.; de Silva, D.; Angier, E.; Arasi, S.; et al. EAACI Guidelines: Anaphylaxis (2021 Update). Allergy 2022, 77, 357–377. [Google Scholar] [CrossRef]
- Dinardo, G.; Cafarotti, A.; Galletta, F.; Fiocchi, A.; Arasi, S. Omalizumab in Severe Asthma and Food Allergies with IgE Levels >1500 KU/L: Two-Year Evaluation. Pediatr. Allergy Immunol. 2023, 34, e14057. [Google Scholar] [CrossRef]
- Arasi, S.; Ebisawa, M.; Eigenmann, P.; Dinardo, G. Editorial Comment on “Oral Immunotherapy as a Curative Treatment for Food-Allergic Preschool Children: Current Evidence and Potential Underlying Mechanisms”. Pediatr. Allergy Immunol. 2024, 35, e14071. [Google Scholar] [CrossRef]
- Wood, R.A.; Togias, A.; Sicherer, S.H.; Shreffler, W.G.; Kim, E.H.; Jones, S.M.; Leung, D.Y.M.; Vickery, B.P.; Bird, J.A.; Spergel, J.M.; et al. Omalizumab for the Treatment of Multiple Food Allergies. N. Engl. J. Med. 2024, 390, 889–899. [Google Scholar] [CrossRef]
- Abdi, R.; Li, X.C.; Kupiec-Weglinski, J.W. Grand Challenges in Organ Transplantation. Front. Transplant. 2022, 1, 897679. [Google Scholar] [CrossRef]
- Bezinover, D.; Saner, F. Organ Transplantation in the Modern Era. BMC Anesthesiol. 2019, 19, 32. [Google Scholar] [CrossRef]
- Ferreira, L.D.; Goff, C.; Kamepalli, S.; Montgomery, A.E.; Miggins, J.J.; Goss, J.A.; Rana, A. Survival Benefit of Solid-Organ Transplantation: 10-Year Update. Dig. Dis. Sci. 2023, 68, 3810–3817. [Google Scholar] [CrossRef]
- Nakaoka, A.; Nomura, T.; Ozeki, K.; Suzuki, T.; Kusumoto, S.; Iida, S.; Saitoh, S. Two Cases of Transplant-Acquired Food Allergy Who Developed Resensitization after a Negative Oral Food Challenge. Allergy Asthma Clin. Immunol. 2023, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Lim, A.; Bishop, J.R.; Gane, E.; Rakhmanova, E.; Wong, W.; Evans, H.M. Atopy and Allergy Following Solid Organ Transplantation: A 15-Year Experience. J. Paediatr. Child Health 2023, 59, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Catal, F.; Topal, E.; Selimoglu, M.A.; Karabiber, H.; Baskiran, A.; Senbaba, E.; Varol, I.; Yilmaz, S. Acquired IgE-Mediated Food Allergy after Liver Transplantation in Children. Allergol. Immunopathol. 2015, 43, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Marcus, N.; Amir, A.Z.; Grunebaum, E.; Dipchand, A.; Hebert, D.; Ng, V.L.; Walters, T.; Avitzur, Y. De Novo Allergy and Immune-Mediated Disorders Following Solid-Organ Transplantation-Prevalence, Natural History, and Risk Factors. J. Pediatr. 2018, 196, 154–160.e2. [Google Scholar] [CrossRef]
- Hosakoppal, S.S.; Bryce, P.J. Transplant-Acquired Food Allergy: Current Perspectives. J. Asthma Allergy 2017, 10, 307. [Google Scholar] [CrossRef]
- Guo, Y.; Fang, J.; Ma, J.; Li, G.; Zhang, L.; He, J.; Xu, L.; Lai, X.; Yin, W.; Xiong, Y.; et al. Correlation between Use of Immunosuppressive Agents and Transplant-Acquired Allergies in Renal Transplant Recipients. Transl. Androl. Urol. 2019, 8, 442–447. [Google Scholar] [CrossRef]
- Mori, T.; Kato, J.; Sakurai, M.; Hashimoto, N.; Kohashi, S.; Hashida, R.; Saburi, M.; Kikuchi, T.; Yamane, Y.; Hoshino, K.; et al. New-Onset Food Allergy Following Cord Blood Transplantation in Adult Patients. Bone Marrow Transplant. 2016, 51, 295–296. [Google Scholar] [CrossRef]
- Newman, E.N.; Firszt, R. Post-Transplantation Development of Food Allergies. Curr. Allergy Asthma Rep. 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Davidovits, M.; Cleper, R.; Shapiro, R. New-Onset Post-Transplantation Food Allergy in Children—Is It Attributable only to the Immunosuppressive Protocol? Pediatr. Transplant. 2009, 13, 63–69. [Google Scholar] [CrossRef]
- Brown, C.; Haringman, N.; Davies, C.; Gore, C.; Hussain, M.; Mieli-Vergani, G.; Vergani, D.; Warner, J.O.; Marks, S.D.; Boyle, R.J. High Prevalence of Food Sensitisation in Young Children with Liver Disease: A Clue to Food Allergy Pathogenesis? Pediatr. Allergy Immunol. 2012, 23, 770–777. [Google Scholar] [CrossRef]
- De Bruyne, R.; Gevaert, P.; Van Winckel, M.; De Ruyck, N.; Minne, A.; Bogaert, D.; Van Biervliet, S.; Vande Velde, S.; Smets, F.; Sokal, E.; et al. Raised Immunoglobulin A and Circulating T Follicular Helper Cells Are Linked to the Development of Food Allergy in Paediatric Liver Transplant Patients. Clin. Exp. Allergy 2015, 45, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Haflidadottir, S.; Matthews, I.L.; Almaas, R. Cytokine Profile in Children with Food Allergy Following Liver Transplantation. Pediatr. Transplant. 2020, 24, e13657. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Nakazawa, Y.; Yanagisawa, R.; Tanaka, M.; Saito, S.; Yoshikawa, K.; Hirabayashi, K.; Kusakari, M.; Kato, S.; Hidaka, N.; et al. Food Allergy after Cord Blood Transplantation in Children. Br. J. Haematol. 2012, 158, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Haflidadottir, S.; Østensen, A.B.; Matthews, I.L.; Line, P.D.; Almaas, R. Mycophenolate Mofetil Use Is Associated with Reduced Incidence of Food Allergy in Liver Transplanted Children. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 138–144. [Google Scholar] [CrossRef]
- Frischmeyer-Guerrerio, P.A.; Wisniewski, J.; Wood, R.A.; Nowak-Wegrzyn, A. Manifestations and Long-Term Outcome of Food Allergy in Children after Solid Organ Transplantation. J. Allergy Clin. Immunol. 2008, 122, 1031–1033.e1. [Google Scholar] [CrossRef]
- Öztürk, H.; Bozbulut, N.E.; Sari, S.; Gürkan, Ö.E.; Sözen, H.; Sapmaz, A.; Dalgiç, A.; Dalgiç, B. Predictable and Unusual Adverse Effects of Immunosuppression in Pediatric Liver Transplant Patients. Exp. Clin. Transplant. 2019, 17 (Suppl. S1), 230–233. [Google Scholar] [CrossRef]
- Saalman, R.; Sundell, S.; Kullberg-Lindh, C.; Lövsund-Johannesson, E.; Jontell, M. Long-Standing Oral Mucosal Lesions in Solid Organ-Transplanted Children-a Novel Clinical Entity. Transplantation 2010, 89, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Needham, J.M.; Nicholas, S.K.; Davis, C.M. Food Allergies Developing after Solid Organ Transplant. Pediatr. Transplant. 2015, 19, 827–835. [Google Scholar] [CrossRef]
- Šari, J.P.; Zenko, J.; Bevanda, M.; Bevanda, D. liver transplantation and allergy: Transplant-acquired food allergy. Med. Acad. Most. 2019, 31, 63–65. [Google Scholar]
- Kawahara, A.; Nakanishi, T.; Goto, M.; Akao, K.; Katsuragi, T.; Tsukada, J. Post-Transplant Food Anaphylaxis in an Adult Cord Blood Transplant Recipient (Ms. No. IJHM-D-20-01037R1). Int. J. Hematol. 2021, 114, 292–296. [Google Scholar] [CrossRef]
- Boyle, R.J.; Hardikar, W.; Tang, M.L.K. The Development of Food Allergy after Liver Transplantation. Liver Transplant. 2005, 11, 326–330. [Google Scholar] [CrossRef]
- Trotter, J.F.; Everson, G.T.; Bock, S.A.; Wachs, M.; Bak, T.; Kam, I. Transference of Peanut Allergy through Liver Transplantation. Liver Transpl. 2001, 7, 1088–1089. [Google Scholar] [CrossRef]
- Aggarwal, A.; Balogun, R.; Carr, T.F.; Desai, A.P.; Jie, T.; Pan, J.J. Transfer of Peanut Allergy from Donor to Recipient after Liver Transplant. Ann. Hepatol. 2019, 18, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Doğruel, D.; Noyan, A.; Dursun, H.; Cengiz, N. Allergic Sensitization in Kidney Transplanted Patients—Is It a Result of Immunosuppressive Agents or the Sensitization of Living Donor? Asian Pac. J. Allergy Immunol. 2017, 35, 132–136. [Google Scholar] [CrossRef]
- Hinds, R.; Dhawan, A. Food Allergy after Liver Transplantation—Is It the Result of T-Cell Imbalance? Pediatr. Transplant. 2006, 10, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Giovannini, M.; Barni, S.; Trapani, S.; Indolfi, G. De Novo Food Allergy in Pediatric Recipients of Liver Transplant. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 175–179. [Google Scholar] [CrossRef]
- Debiasi, M.; Pichler, H.; Klinglmüller, F.; Boztug, H.; Schmidthaler, K.; Rech, J.; Scherer, D.; Lupinek, C.; Valenta, R.; Kacinska-Pfaller, E.; et al. Transfer and Loss of Allergen-Specific Responses via Stem Cell Transplantation: A Prospective Observational Study. Allergy 2020, 75, 2243–2253. [Google Scholar] [CrossRef]
- Gruber, S.; Tiringer, K.; Dehlink, E.; Eiwegger, T.; Mayer, E.; Konstantin, H.; Kikic, Z.; Graf, A.; Szépfalusi, Z. Allergic Sensitization in Kidney-Transplanted Patients Prevails under Tacrolimus Treatment. Clin. Exp. Allergy 2011, 41, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Atkins, F.M. Systemic FK506 and Post Transplant Food Allergy in Children. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 525–526. [Google Scholar] [CrossRef]
- de Goër de Herve, M.G.; Gonzales, E.; Hendel-Chavez, H.; Décline, J.L.; Mourier, O.; Abbed, K.; Jacquemin, E.; Taoufik, Y. CD25 Appears Non Essential for Human Peripheral T(Reg) Maintenance In Vivo. PLoS ONE 2010, 5, e11784. [Google Scholar] [CrossRef][Green Version]
- De Bruyne, R.; Dullaers, M.; Van Biervliet, S.; Vande Velde, S.; Raes, A.; Gevaert, P.; Van Winckel, M. Post-Transplant Food Allergy in Children Is Associated with Liver and Not with Renal Transplantation: A Monocentric Comparative Study. Eur. J. Pediatr. 2013, 172, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Gabe, S.M.; Bjarnason, I.; Tolou-Ghamari, Z.; Tredger, J.M.; Johnson, P.G.; Barclay, G.R.; Williams, R.; Silk, D.B.A. The Effect of Tacrolimus (FK506) on Intestinal Barrier Function and Cellular Energy Production in Humans. Gastroenterology 1998, 115, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.; Brener, A.; Granot, E. Cytokine Profile of Food-Allergic Post-Liver Transplant Children Is Identified by High Levels of IL-5 and Low IL-10 Secretion from Patients’ Peripheral Blood Mononuclear Cells. Pediatr. Transplant. 2015, 19, 716–721. [Google Scholar] [CrossRef]
- Manuyakorn, W.; Sinitkul, R.; Treepongkaruna, S.; Kamchaisatian, W.; Vilaiyuk, S.; Srisala, S.; Benjaponpitak, S. In Vitro Cytokine Changes after Pediatric Liver Transplantation. Asian Pac. J. Allergy Immunol. 2015, 33, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Angelucci, C.; Cianferoni, A.; Barni, S.; Indolfi, G.; Casini, A.; Mangone, G.; Materassi, M.; Pucci, N.; Azzari, C.; et al. Increase of Natural Killer Cells in Children with Liver Transplantation-Acquired Food Allergy. Allergol. Immunopathol. 2018, 46, 447–453. [Google Scholar] [CrossRef]
- De Paepe, E.; Plekhova, V.; Vangeenderhuysen, P.; Baeck, N.; Bullens, D.; Claeys, T.; De Graeve, M.; Kamoen, K.; Notebaert, A.; Van de Wiele, T.; et al. Integrated Gut Metabolome and Microbiome Fingerprinting Reveals That Dysbiosis Precedes Allergic Inflammation in IgE-Mediated Pediatric Cow’s Milk Allergy. Allergy 2024, 79, 949–963. [Google Scholar] [CrossRef]
- Lai, Z.; Chen, Z.; Zhang, A.; Niu, Z.; Cheng, M.; Huo, C.; Xu, J. The Gut Microbiota in Liver Transplantation Recipients During the Perioperative Period. Front. Mater. 2022, 13, 854017. [Google Scholar] [CrossRef]
- D’Amico, F.; Rinaldi, M.; Pascale, R.; Fabbrini, M.; Morelli, M.C.; Siniscalchi, A.; Laici, C.; Coladonato, S.; Ravaioli, M.; Cescon, M.; et al. Gut Microbiome Dynamics and Enterobacterales Infection in Liver Transplant Recipients: A Prospective Observational Study. JHEP Rep. Innov. Hepatol. 2024, 6, 101039. [Google Scholar] [CrossRef]
- Almaas, R.; Haflidadottir, S.; Kaldestad, R.H.; Matthews, I.L. Asthma, Eczema, and Food Allergy in Children Following Liver Transplantation. J. Pediatr. 2019, 204, 263–269. [Google Scholar] [CrossRef]
- Barış, Z.; Köksal, B.; Özbek, Ö.; Özçay, F.; Haberal, M. Incidence, Clinical Features, and Outcomes of Food Allergy in Children Who Underwent Liver Transplant: 16-Year Experience. Pediatr. Transplant. 2019, 23, e13399. [Google Scholar] [CrossRef]
- Wisniewski, J.; Lieberman, J.; Nowak-Weogonekgrzyn, A.; Kerkar, N.; Arnon, R.; Iyer, K.; Miloh, T. De Novo Food Sensitization and Eosinophilic Gastrointestinal Disease in Children Post-Liver Transplantation. Clin. Transplant. 2012, 26, E365–E371. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, O.Y.; Ozcay, F.; Avci, Z.; Haberal, A.; Haberal, M. Food Allergy after Liver Transplantation in Children: A Prospective Study. Pediatr. Allergy Immunol. 2009, 20, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Sinitkul, R.; Manuyakorn, W.; Kamchaisatian, W.; Vilaiyuk, S.; Benjaponpitak, S.; Lertudompholwanit, C.; Treepongkaruna, S. De Novo Food Allergy in Pediatric Liver Transplantation Recipients. Asian Pac. J. Allergy Immunol. 2018, 36, 166–174. [Google Scholar] [CrossRef]
- Ozbek, O.Y.; Ozcay, F. Long-Term Outcome of Food Allergy after Liver Transplantation in Children. Pediatr. Transplant. 2015, 19, 436–437. [Google Scholar] [CrossRef]
- Mitsui, M.; Shoda, T.; Natsume, O.; Nomura, I.; Narita, M.; Fukuda, A.; Sakamoto, S.; Kasahara, M.; Ohya, Y. Factors Associated with Development of Food Allergy in Young Children after Liver Transplantation: A Retrospective Analysis of 10 Years’ Experience. J. Allergy Clin. Immunol. Pract. 2017, 5, 1698–1706. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Arrey-Mensah, A.; Sorensen, R.U. Development of Multiple Food Allergies in Children Taking Tacrolimus after Heart and Liver Transplantation. Pediatr. Transplant. 2006, 10, 380–383. [Google Scholar] [CrossRef]
- Hernández-Ojeda, A.; Rojas, N.; Barriga, F.; Wietstruck, M.A.; Morales, P.S.; Borzutzky, A. Incidence and Risk Factors of Food Allergy after Umbilical Cord Blood Transplantation in Children. J. Allergy Clin. Immunol. Pract. 2017, 5, 1789–1791. [Google Scholar] [CrossRef]
- Noble, C.; Peake, J.; Lewindon, P.J. Increase in de Novo Allergies after Paediatric Liver Transplantation: The Brisbane Experience. Pediatr. Transplant. 2011, 15, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Wasuwanich, P.; Batsis, I.; Thawillarp, S.; Alford, M.K.; Mogul, D.; Wood, R.A.; Karnsakul, W. Post-Transplant Eosinophilic Gastrointestinal Disorders and Lymphoproliferative Disorder in Pediatric Liver Transplant Recipients on Tacrolimus. Transpl. Immunol. 2021, 68, 101438. [Google Scholar] [CrossRef]
- Lebel, M.J.; Chapdelaine, H.; Paradis, L.; Des Roches, A.; Alvarez, F. Increase in de Novo Food Allergies after Pediatric Liver Transplantation: Tacrolimus vs. Cyclosporine Immunosuppression. Pediatr. Transplant. 2014, 18, 733–739. [Google Scholar] [CrossRef]
- Maarof, G.; Krzysiek, R.; Décline, J.L.; Cohen, J.; Habes, D.; Jacquemin, E. Management of Post-Liver Transplant-Associated IgE-Mediated Food Allergy in Children. J. Allergy Clin. Immunol. 2011, 127, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Shroff, P.; Mehta, R.S.; Chinen, J.; Karpen, S.J.; Davis, C.M. Presentation of Atopic Disease in a Large Cohort of Pediatric Liver Transplant Recipients. Pediatr. Transplant. 2012, 16, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Lykavleris, P.; Frauger, E.; Habes, D.; Bernard, O.; Debray, D. Angioedema in Pediatric Liver Transplant Recipients under Tacrolimus Immunosuppression. Transplantation 2003, 75, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.M.; Kim, M.J.; Lee, S.K.; Choe, Y.H. Long-Term Follow-up of de Novo Allergy in Pediatric Liver Transplantation—10 Yr Experience of a Single Center. Pediatr. Transplant. 2013, 17, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Kehar, M.; Grunebaum, E.; Jimenez-Rivera, C.; Mozer-Glassberg, Y.; Jamal, A.; Ng, V.L.; Avitzur, Y. Conversion from Tacrolimus to Sirolimus as a Treatment Modality in de Novo Allergies and Immune-Mediated Disorders in Pediatric Liver Transplant Recipients. Pediatr. Transplant. 2020, 24, e13737. [Google Scholar] [CrossRef]
- Granot, E.; Yakobovich, E.; Bardenstein, R. Tacrolimus Immunosuppression—An Association with Asymptomatic Eosinophilia and Elevated Total and Specific IgE Levels. Pediatr. Transplant. 2006, 10, 690–693. [Google Scholar] [CrossRef]
- Arikan, C.; Kilic, M.; Tokat, Y.; Aydogdu, S. Allergic Disease after Pediatric Liver Transplantation with Systemic Tacrolimus and Cyclosporine a Therapy. Transplant. Proc. 2003, 35, 3039–3041. [Google Scholar] [CrossRef]
- Happel, C.S.; Stone, K.D.; Freeman, A.F.; Shah, N.N.; Wang, A.; Lyons, J.J.; Guerrerio, P.A.; Hickstein, D.D.; Su, H.C. Food Allergies Can Persist After Myeloablative Hematopoietic Stem Cell Transplantation in DOCK8-Deficient Patients. J. Allergy Clin. Immunol. 2016, 137, 1895. [Google Scholar] [CrossRef]
- Hourihane, J.O.B.; Rhodes, H.L.; Jones, A.M.; Veys, P.; Connett, G.J. Resolution of Peanut Allergy Following Bone Marrow Transplantation for Primary Immunodeficiency. Allergy 2005, 60, 536–537. [Google Scholar] [CrossRef]
- Narumoto, S.; Sakamoto, S.; Uchida, H.; Sasaki, K.; Shigeta, T.; Fukuda, A.; Nosaka, S.; Irie, R.; Yoshioka, T.; Kasahara, M. Necrotizing Enterocolitis in the Setting of Milk Allergy after Pediatric Living Donor Liver Transplantation. Pediatr. Transplant. 2018, 22, e13096. [Google Scholar] [CrossRef]
- Blanchard, S.S.; Gerrek, M.; Czinn, S.; Chelimsky, G.; Seaman, D.; Siegel, C.; Splawski, J. Food Protein Sensitivity with Partial Villous Atrophy after Pediatric Liver Transplantation with Tacrolimus Immunosuppression. Pediatr. Transplant. 2006, 10, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.A.; Integlia, M.J.; Pleskow, R.G.; Calenda, K.A.; Rohrer, R.J.; Dayal, Y.; Grand, R.J. Tacrolimus-Associated Eosinophilic Gastroenterocolitis in Pediatric Liver Transplant Recipients: Role of Potential Food Allergies in Pathogenesis. Pediatr. Transplant. 2006, 10, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, G.; Cafarotti, A.; Fierro, V.; Artesani, M.C.; Indolfi, C.; Miraglia Del Giudice, M.; Fiocchi, A. Role of Biologics in Severe Food Allergy. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, G.; Fierro, V.; Del Giudice, M.M.; Urbani, S.; Fiocchi, A. Food-Labeling Issues for Severe Food-Allergic Consumers. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, L.; Mastrorilli, C.; Arasi, S.; Barni, S.; Caimmi, D.; Chiera, F.; Dinardo, G.; Gracci, S.; Miraglia Del Giudice, M.; Bernardini, R.; et al. Nutritional and Psychosocial Impact of Food Allergy in Pediatric Age. Life 2024, 14, 695. [Google Scholar] [CrossRef]
- Bergamo, C.; Argento, E.C.; Giampetruzzi, S.; Cutini, M.; Ciabattoni, F.; Faggian, G.; Gaio, P.; Bosa, L.; Cananzi, M. De Novo Food Allergy After Pediatric Liver Transplantation: A Systematic Review. Front. Pediatr. 2022, 10, 885942. [Google Scholar] [CrossRef]

| Reference | Study Population | Age at Transplant | Study Type | Transplant | Immunosuppressive Therapy | Food Allergy | Atopic Dermatitis | Asthma/ Rhinitis | Time Point to a Diagnosis of TAFA after Transplantation | Donor Characteristics | Serum IgE | Management | Duration of Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roberts et al. (2023) [12] | 232 pediatric and adult patients | Median age: Liver: 1.6 (0.75–4) years Kidney: 9.2 (3.7–12.3) years | Retrospective | Liver, Kidney | Tacrolimus, Mycophenolate, Corticosteroids | Yes (33% pediatric liver) | Yes (73% pediatric liver) | Yes (19% rhinitis, 17% asthma pediatric liver) | 1.8 years (1.3–4.0) | Type of donor (living vs. deceased) not significantly associated with TAFA development | ND | ND | ND |
| Almaas et al. (2019) [49] | 59 liver transplant recipients, 56 with chronic liver disease (control) | Median age: 0.8 years (0.6–5.2) | Cross-sectional | Liver | Tacrolimus, Mycophenolate, Prednisolone | Yes (39% in transplanted children) | Yes (41% in transplanted children) | Yes (24% asthma in transplanted children) | 1.5 years (0.5–3.0) | A total of 57 patients received organs from deceased donors, while 2 were recipients of living-donor transplants | Median: 32 IU/mL (9–165) | ND | ND |
| Catal et al. (2015) [13] | 49 pediatric liver transplant recipients | Median age: 5 (0.3–16.5) years | Retrospective | Liver | Tacrolimus, Cyclosporine | Yes (12.2%) | Yes (6.1%) | Yes (12.2% asthma, 4.1% rhinitis) | The reaction occurred within the first year post-transplant in 5 out of 6 patients. | Cadaveric organ: 13 (26.5) Living-related: 33 (67.4) Living non-related: 3 (6.1) | 12 patients (24.5%) had serum total IgE levels > 100 IU/mL | ND | 16 months (1–47) |
| Marcus et al. (2018) [14] | 273 pediatric solid-organ transplant recipients | Median age: Liver: 1.7 years (0.8–6.9) Heart: 1.2 years (0.4–9.2) Kidney: 10.8 years (6.3–15.5) Multivisceral: 1.2 years (0.9–1.6) Total: 2.9 years (0.7–10.3) | Cross-sectional cohort | Liver, Heart, Kidney, Multivisceral | Tacrolimus, Steroids | Yes (25.3%) | Yes (16.1%) | Yes (10.3% asthma, 5.5% rhinitis) | ND | A total of 47 (42.3%) liver and 30 (57.7%) kidney recipients were transplanted with living-donor organs. Organ type (living donor vs. cadaveric), donor/recipient blood type and compatibility were not associated with TAFA development. | ND | Of the 92 children, 10 (11%) were managed conservatively, 65 (71%) received standard medical treatment, and 17 (18%) had their immunosuppressive therapy adjusted after medical treatment failure. Following this change, 9 patients showed improvement, 7 achieved full resolution, 13 remained unaffected, and 4 worsened, including 2 fatalities. | Liver: 3.2 years (1.6–5.8) Heart: 4.5 years (2.8–6.8) Kidney: 2.4 years (1.2–6.0) Multivisceral: 4.8 years (1.6–5.5) Total 3.6 years (1.7–6.3) |
| Barış et al. (2019) [50] | 236 pediatric liver transplant recipients | The mean age: 7.92 ± 2.64 years (range, 4.8–15.6) | Retrospective | Liver | Tacrolimus, Steroids | Yes, 8% | ND | ND | ND | A total of 18 patients underwent living-related liver transplants, with the donor being the mother in 11 cases, the father in 6 cases, and a secondary relative in 2 cases. One patient, who received a deceased-donor liver transplant at 1 year of age due to acute liver failure, required a retransplant at age 5.5 from his mother because of chronic rejection. | Mean level: 350 ± 411 IU/mL | All food allergens were successfully reintroduced in 7 patients (36.8%), while 8 patients with multiple FAs were able to reintroduce only some foods. Milk was reintroduced after an average of 22.8 ± 14.5 months (range 7–54 months), egg after 15.8 ± 8 months (range 7–29 months), and wheat after 30.5 ± 35.7 months (range 11–84 months). The immunosuppressive regimen was changed to a combination of tacrolimus and everolimus in 2 patients, to sirolimus in another 2, and to cyclosporine in 2 patients. | 4.76 ± 3.97 years |
| Wisniewski et al. (2012) [51] | 352 pediatric liver transplant recipients | Median age: 0.9 (0.6–2.0) years | Retrospective | Liver | Tacrolimus, Cyclosporine, Prednisone | Yes (8.5%) | Yes (43%) | Yes (20% asthma, 20% rhinitis) | Median 1.0 ( 0.5–8.2) years post-liver transplantation | The median donor age was 32 years (range 1–71) in the FA group. Female donors (both living and deceased) provided 40% of the organs for FA recipients compared to 50% for controls (p = 0.48). CMV+ donors made up 44% of the FA livers and 56% of the controls (p = 0.43). 12 were living-related transplants, and 17 cadaveric ones. No statistically significant associations were found regarding donor age or sex or type of transplantation. | ND | A total of 14 children remained on avoidance diets, 10 followed unrestricted diets, and 6 were lost to follow-up. Immune suppression was lowered or discontinued in 11% of FAs. | Median 10 (7.3–4.0) years |
| Levy et al. (2009) [19] | 297 pediatric transplant recipients | Mean age: Kidney: 10.8 years (range: 2–18). Liver: 5.5 years (range: 0.5–17.5) | Retrospective | Liver, Kidney | Tacrolimus, Cyclosporine, Prednisone | Yes (4 out of 65 liver recipients) | ND | ND | 1.5–6 years | ND | IgE levels were: 224,155 and 3900 UI/mL. | Tacrolimus therapy was switched to cyclosporin A in 2 patients, but there was no change in their food-induced allergic reactions. All patients were advised to eliminate the allergenic foods from their diets, which led to symptom resolution. No efforts were made to reintroduce the allergenic foods during the follow-up period | 2–4 years |
| Ozbek et al. (2009) [52] | 28 pediatric liver transplant recipients | Mean age: 4.96 +/− 0.76 years | Prospective | Liver | Tacrolimus, Cyclosporine, Sirolimus | Yes (21%) | ND | ND | The time between transplantation and onset of FA was 3, 6 (in 2 patients), 11, 12, and 20 months (mean 9.7 months). | None of the donors had a history of FAs. Food-specific IgE tests or skin prick tests were negative in all 28 donors, including those linked to children who later developed FAs. All the patients received a living-related donor organ transplantation. | Before transplantation: 44.88 UI/mL After 3 months PT: 116.63 UI/mL After 6 months PT: 98.40 UI/mL After 12 months PT: 276.63 UI/mL | Patients who developed FAs after liver transplantation were placed on elimination diets, resolving their symptoms, with no reintroduction of allergenic foods. In some cases, immunosuppressive therapy was switched (tacrolimus-> cyclosporine A), but this did not impact the allergic reactions. | Mean follow-up time: 25.4 months (range 12–40 months) |
| Sinitkul et al. (2018) [53] | 46 pediatric liver transplant recipients | Median age: 19.1 months (15.3–34.2) | Retrospective | Liver | Tacrolimus, Corticosteroids | Yes (54.3%) | ND | ND | 12.2 months (6.2–21.3 months) | Some donors had allergic rhinitis, but only one had a history of shellfish allergy. However, this did not significantly influence the development of FAs in recipients | The levels of IgE ranged from very low values just above positivity to values exceeding 100. | Patients who developed FAs were placed on elimination diets, avoiding allergenic foods. Reintroduction of foods was attempted after 3 years of elimination, with about 19% of patients developing tolerance to at least one allergen. No changes in the immunosuppressive therapy were associated with the resolution of FAs. | Median 59.5 months (57.2, 92.8) |
| Sakashita et al. (2012) [23] | 14 pediatric cord blood transplant recipients | Mean age: 1.6 ± 1.3 years in symptomatic patients, 5.6 ± 4.5 years in asymptomatic ones | Retrospective | Cord Blood (CB) | Tacrolimus, Cyclosporine, Methotrexate, Methylprednisolone | Yes (5 out of 14) | No | No | 3 to 6 months after CBT | Four patients received transplants of unrelated CB cells; one was related. | Total IgE levels reached more than 3000 UI/mL) | Eliminating the suspected food(s) resolved the symptoms in all 5 patients. | ND |
| Ozbek et al. (2015) [54] | 28 pediatric liver transplant recipients | Mean age: 10.16 years | Retrospective | Liver | Tacrolimus, Cyclosporine, Sirolimus | Yes (21%) | ND | Yes (asthma in one patient) | Mean 9.66 months (33 +/− 19 months) | ND | ND | The systematic elimination of allergens from the diet was maintained in all cases. An oral challenge with each allergen was conducted. The allergens were successfully reintroduced in 4 children within 7 to 38 months of starting the elimination diet. | 5 years |
| De Bruyne et al. (2013) [41] | 49 liver, 21 renal transplant recipients | Median (and range) age: Liver: 22 Months (3 weeks–16 years) Renal: 8.9 (2–15) years | Retrospective | Liver, Renal | Tacrolimus, Cyclosporine, Mycophenolate mofetil, Steroids | Yes (26.5% liver, 0% renal) | Yes (In the non-food-allergic group, 7 of 36 atopic dermatitis) | Yes (In the non-food-allergic group, 2 of 36 children have asthma or allergic rhinitis) | Median 8 months post-transplant (range 1–48 months) | Liver: Living-related (7) Liver: Cadaveric organ (42) Renal: Living-related (2) Renal: Cadaveric organ (19) | Mean IgE levels 5.84 kU/L. Total IgE was higher in liver transplanted patients (24.4 kU/L (0–9930) versus 7 kU/L (0–157)) (p = 0.08). | All patients with FAs systematically eliminated the identified allergens from their diets. In 4 patients, the allergens were successfully reintroduced within 7 to 38 months after starting the elimination diet | Liver: 67 (19–230) Months Renal: 74 (10–166) Months |
| Mitsui et al. (2017) [55] | 206 pediatric liver transplant recipients | Median age: 9 months (6.0–14.3) | Retrospective | Liver | Tacrolimus, Steroids | Yes (20.4%) | Yes | ND | Median 3 months post-transplant (range 1–8 months) | Median age: 33.0 years (30.0–38.0) | Ig-E mediated FA: Median 129 (15.6–1048) Non-IgE-mediated FA: median 52.7 (22.4–131) | ND | ND |
| Haflidadottir et al. (2022) [24] | 107 pediatric liver transplant recipients | Median age: 1.9 years (0.7–8.3 years) | Retrospective | Liver | Tacrolimus, Mycophenolate Mofetil (MMF), Prednisolone | Yes (22%) | Yes | Yes (asthma) | Median 1.6 (0.6–3.3) years. | A total of 124 patients underwent orthotopic liver transplantation. Three patients underwent living-donor liver transplantation; the rest of the patients received split or whole liver from deceased donor. | ND | The introduction of mycophenolate mofetil in the transplantation program led to a reduction in FAs following liver transplantation in children. Additionally, treatment with mycophenolate mofetil at 1 and 2 years post-liver transplantation, alongside tacrolimus, was linked to decreased FAs and food sensitization. | Median 7.6 years (2.5–13.6 years) |
| Frischmeyer-Guerrerio et al. (2008) [25] | 25 pediatric solid organ transplant recipients | Median age: 8.7 months (5.8–13.3 months) | Retrospective | Liver, Small Bowel, Heart, Kidney | Tacrolimus, Mycophenolate Mofetil, Corticosteroids | Yes | Yes | Yes (n = 11 rhinitis; n = 4 asthma) | Median 6.0 months (4.4–10.4 months) | Eleven donors had a history of atopy, but none had a history of FAs. Twelve transplants were living-related liver. | ND | Elimination diet. Of 25 patients, 3 followed unrestricted diets at the time of last follow-up | Median 2.4 years (1.4- 4.7 years) in the clinic and 6.1 years (3.9–7.5 years) by telephone |
| Noble et al. (2011) [58] | 78 pediatric liver transplant recipients | Range 0.1–17.3 years | Retrospective | Live | Tacrolimus, Cyclosporine | Yes (20%) | Yes | Yes (n = 1 rhinitis; n = 5 asthma) | Range from 2 months to 6 years | A total of 78 children received liver transplants from 85 cadaveric donors | ND | All children were treated with the appropriate medications or allergen avoidance measures, including dietary restrictions, and for those with eosinophilic esophagitis (EE), oral steroids and swallowed topical steroid sprays were used. | ND |
| Brown et al. (2012) [20] | 50 pediatric liver transplant recipients | Median age: 12.1 (7.9–20.6) months | Retrospective | Liver | Tacrolimus, Cyclosporine, Mycophenolate Mofetil | Yes (20%) | Yes | Yes (23% asthma and allergic rhinitis) | ND | ND | Median 15.0 (4.0–105.5) UI/mL | ND | ND |
| Wasuwanich et al. (2021) [59] | 98 pediatric liver transplant recipients | Median age: 3.3 years (1.1–9.3) | Retrospective | Liver | Tacrolimus | Yes (7%) | ND | Yes (asthma) | Median time: 1.9 years (0.8–3.5 years), while the median time to diagnose eosinophilic colitis was 0.5 years (0.4–1.4 years) | A total of 28 (29%) of the 96 children had live-donor liver transplantation | ND | ND | At least one year after transplantation |
| Öztürk et al. (2019) [26] | 60 pediatric liver transplant recipients | Mean age: 6.1 years (3 months to 17 years) | Retrospective | Liver | Tacrolimus, Mycophenolate Mofetil, Steroids | Yes (3.3%) | ND | ND | ND | Thirty-nine patients (65%) received livers from living donors, while 21 patients (35%) received livers from deceased donors. | ND | ND | ND |
| Lebel et al. (2014) [60] | 154 pediatric liver transplant recipients | Range: one month to 19 yr | Retrospective | Liver | Tacrolimus, Cyclosporine | Yes (17% Tacrolimus, 3% Cyclosporine) | ND | ND | Median 25 months post-transplant (range 6–94 months) | ND | Mean levels 1082 kU/L | ND | ND |
| Shroff et al. (2012) [62] | 176 pediatric liver transplant recipients | Median age: 16 (3–127; IQR, 7–30) months. Mean age: 26.6 months | Retrospective | Liver | Tacrolimus, Cyclosporine | Yes (40%) | Yes (56%) | Yes (Allergic rhinitis: 64%, asthma 44%) | Median 11.5 (6–28) months post-transplantation Mean: 27.1 months post-transplantation | ND | ND | ND | Median 63 (17–127; IQR, 42–110) months. Mean: 79.0 months |
| Lykavieris et al. (2003) [63] | 121 pediatric liver transplant recipients | Mean age 1.32 years | Retrospective | Liver | Tacrolimus | Yes (10%) | ND | ND | ND | ND | Mean levels 2454 kIU/L | In addition to eliminating food allergens, 8 children were transitioned from tacrolimus to cyclosporine, while the tacrolimus dosage was reduced in 4. Successful reintroduction of food allergens occurred only in those who were switched to cyclosporine. | Mean: 3.75 years; (range 2.8–4.2 years) |
| Saalman et al. (2010) [27] | 39 pediatric liver, 38 pediatric kidney transplant recipients | Median age: 22 months (range 1 month–16 years) | Retrospective | Liver, Kidney | Tacrolimus, Cyclosporine, Azathioprine, Prednisolone | Yes | Long-standing oral lesions including angioedema (7 patients) | ND | Median 21 months (range 6 months to 4.5 years) post-transplant for angioedema | 5 living donors, 3 cadavers | ND | Eliminations diet | Mean 6.84 years |
| Lee et al. (2013) [64] | 93 pediatric liver transplant recipients | Median age: 11 months (8–34 months) | Retrospective | Liver | Tacrolimus | Yes (37.6%) | ND | Asthma (3.2%) | Median 5 months post-transplant (IR 2.3–9.5 months) | Donor allergy was not found to be a risk factor for the development of allergy in the recipient (HR 95% CI: 1.271 (0.307–5.271) p-value: 0.741) | ND | The management ranged from simple observation to strict antigen restriction or an elemental diet, depending on the patients’ age, clinical symptoms, and feeding methods | Median follow-up: 70 months (41–90 months) |
| Kehar et al. (2020) [65] | 8 pediatric liver/multivisceral transplant recipients | Median age: 1 year (0.5–2.4 years) | Retrospective | Liver (7), Multivisceral (1) | Tacrolimus switched to Sirolimus | Yes (2 out of 8) | Yes (2 out of 8) | ND | Median: 1.3 (0.25–8) years after transplantation | Of the 7 isolated liver transplants, 3 (43%) received an allograft from a living donor, while 4 (57%) received one from a deceased donor | ND | Elimination diet. Eight recipients who underwent either liver (n = 7) or multivisceral transplants (n = 1) experienced severe, treatment-resistant PTAID (Post-transplant allergy or immune-mediated disease) and were transitioned from tacrolimus to sirolimus. | Median follow-up of 5 years |
| Granot et al. (2006) [66] | 30 pediatric liver transplant recipients | Mean age: 10.6 years (1.9–21 years) | Retrospective | Liver | Tacrolimus, Cyclosporine, Prednisone | Yes (13.3%) | ND | Yes (asthma in 1 patient) | ND | ND | Five patients were < 3 years of age and IgE levels ranged from 54 to 111 IU/mL (mean: 83), Five patients were > or =9 years and IgE levels ranged from 134 to 1606 IU/mL (mean: 557) | ND | ND |
| Arikan et al. (2003) [67] | 50 pediatric liver transplant recipients | Mean age: 9 years (13–5 years) | Retrospective | Liver | Tacrolimus, Cyclosporine, Prednisone | Yes (4%) | Yes | Yes (asthma in 1 patient) | Mean 6.3 months (range 4–9 months) | 1 cadaveric donor, 2 living-related donors | Mean 1020 IU/L (range 400–1800 IU/L) | Symptoms resolved with appropriate elimination diets. | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indolfi, C.; Klain, A.; Dinardo, G.; Grella, C.; Perrotta, A.; Colosimo, S.; Decimo, F.; Miraglia del Giudice, M. Transplant-Acquired Food Allergy in Children. Nutrients 2024, 16, 3201. https://doi.org/10.3390/nu16183201
Indolfi C, Klain A, Dinardo G, Grella C, Perrotta A, Colosimo S, Decimo F, Miraglia del Giudice M. Transplant-Acquired Food Allergy in Children. Nutrients. 2024; 16(18):3201. https://doi.org/10.3390/nu16183201
Chicago/Turabian StyleIndolfi, Cristiana, Angela Klain, Giulio Dinardo, Carolina Grella, Alessandra Perrotta, Simone Colosimo, Fabio Decimo, and Michele Miraglia del Giudice. 2024. "Transplant-Acquired Food Allergy in Children" Nutrients 16, no. 18: 3201. https://doi.org/10.3390/nu16183201
APA StyleIndolfi, C., Klain, A., Dinardo, G., Grella, C., Perrotta, A., Colosimo, S., Decimo, F., & Miraglia del Giudice, M. (2024). Transplant-Acquired Food Allergy in Children. Nutrients, 16(18), 3201. https://doi.org/10.3390/nu16183201







