The Impact of Body Composition on Mortality and Hospital Length of Stay after Endovascular and Open Aortic Aneurysm Repair: A Retrospective Cohort Study
Abstract
:1. Background
2. Methods
2.1. Study Patients
2.2. Data Collection
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. Length of Hospital Stay
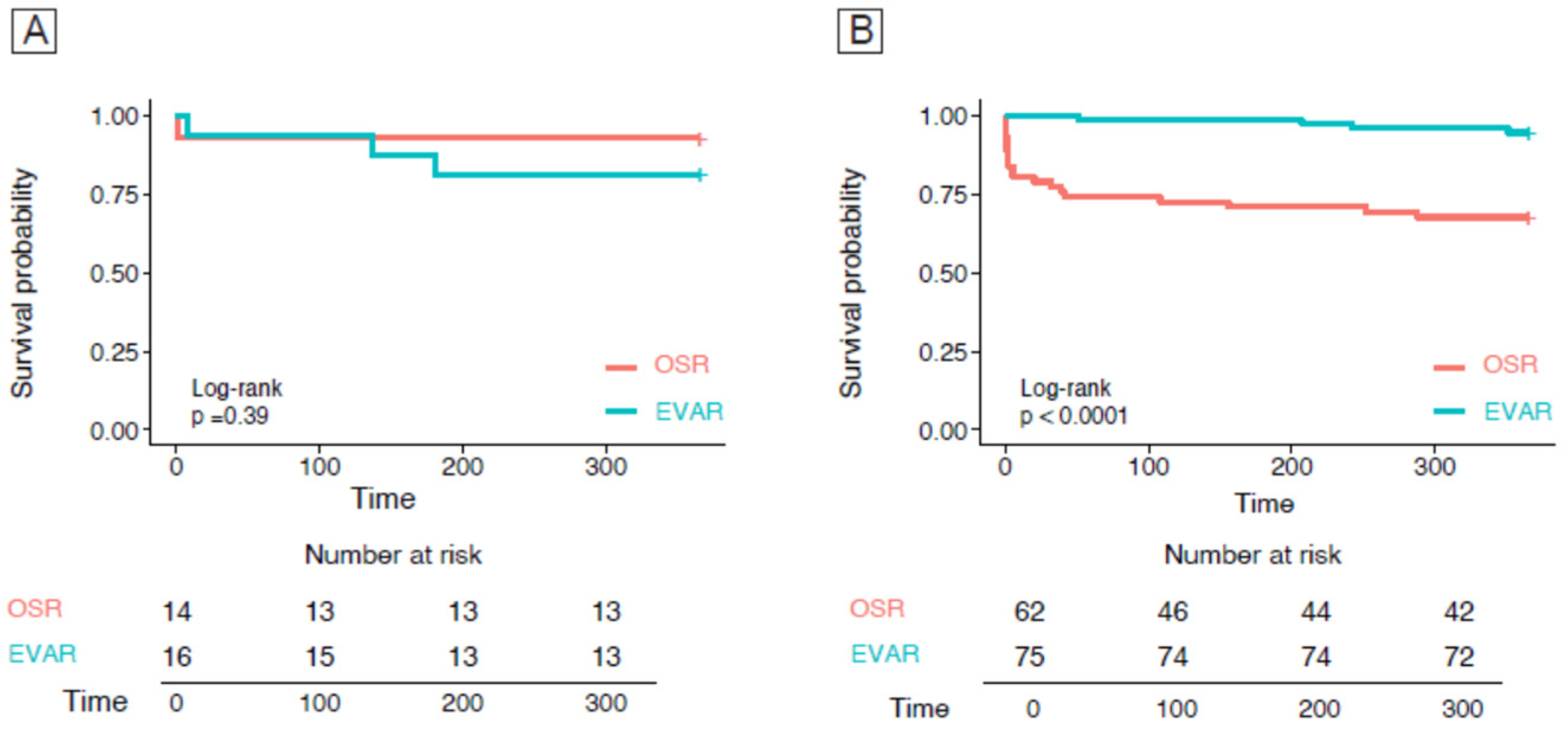
3.3. Overall and 1-Year Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tillman, K.; Lee, O.D.; Whitty, K. Abdominal aortic aneurysm: An often asymptomatic and fatal men’s health issue. Am. J. Men’s Health 2013, 7, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Reimerink, J.J.; van der Laan, M.J.; Koelemay, M.J.; Balm, R.; Legemate, D.A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 2013, 100, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Beller, C.J.; Gebhard, M.M.; Karck, M.; Labrosse, M.R. Usefulness and limitations of computational models in aortic disease risk stratification. J. Vasc. Surg. 2010, 52, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.O.; Holt, P.J.; Hinchliffe, R.; Loftus, I.M.; Thompson, M.M. Predicting risk in elective abdominal aortic aneurysm repair: A systematic review of current evidence. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 637–645. [Google Scholar] [CrossRef]
- Knoedler, S.; Schliermann, R.; Knoedler, L.; Wu, M.; Hansen, F.J.; Matar, D.Y.; Obed, D.; Vervoort, D.; Haug, V.; Hundeshagen, G.; et al. Impact of sarcopenia on outcomes in surgical patients: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 4238–4262. [Google Scholar] [CrossRef]
- Komori, K.; Kano, K.; Aoyama, T.; Hara, K.; Nagasawa, S.; Nakazono, M.; Shimoda, Y.; Maezawa, Y.; Kumazu, Y.; Kawabe, T.; et al. Clinical Impact of Surgical Sarcopenia on Long-term Survival. Anticancer Res. 2022, 42, 4545–4552. [Google Scholar] [CrossRef]
- Dakis, K.; Nana, P.; Brodis, A.; Kouvelos, G.; Behrendt, C.A.; Giannoukas, A.; Kölbel, T.; Spanos, K. Sarcopenia is a Prognostic Biomarker for Long-Term Survival after Endovascular Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2022, 83, 358–368. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. Radiol. Med. 2022, 127, 228–237. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Gao, B.; Zhou, M. Associations of Preoperative Nutritional Status and Sarcopenia with Mortality in Patients with Abdominal Aortic Aneurysm After Open and Endovascular Abdominal Aortic Aneurysm Repair: A Retrospective Study. J. Cardiothorac. Vasc. Anesth. 2024, 38, 1337–1346. [Google Scholar] [CrossRef]
- Indrakusuma, R.; Zijilmans, J.L.; Jalalzadeh, H.; Planken, R.N.; Balm, R.; Koelemay, M.J.W. Psoas muscle area as a prognostic factor for survival in patients with an asymptomatic infrarenal abdominal aortic aneurysm: A retrospective cohort study. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bradley, N.; Walter, A.; Dolan, R.; Wilson, A.; Siddiqui, T.; Roxburgh, C.; McMillan, D.; Guthrie, G. Evaluation of the prognostic value of computed tomography-derived body composition in patients undergoing endovascular aneurysm repair. J. Cachexia Sarcopenia Muscle 2023, 14, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Hale, A.L.; Twomey, K.; Ewing, J.A.; Langan, E.M., 3rd; Cull, D.L.; Gray, B.H. Impact of sarcopenia on long-term mortality following endovascular aneurysm repair. Vasc. Med. 2016, 21, 217–222. [Google Scholar] [CrossRef]
- Matsubara, Y.; Matsumoto, T.; Aoyagi, Y.; Tanaka, S.; Okadome, J.; Morisaki, K.; Shirabe, K.; Maehara, Y. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J. Vasc. Surg. 2015, 61, 945–950. [Google Scholar] [CrossRef]
- Masuda, T.; Shirabe, K.; Ikegami, T.; Harimoto, N.; Yoshizumi, T.; Soejima, Y.; Uchiyama, H.; Ikeda, T.; Baba, H.; Maehara, Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014, 20, 401–407. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Shirabe, K.; Nakagawara, H.; Ikegami, T.; Harimoto, N.; Toshima, T.; Yamashita, Y.; Ikeda, T.; Soejima, Y.; Maehara, Y. Skeletal muscle area correlates with body surface area in healthy adults. Hepatol. Res. 2014, 44, 313–318. [Google Scholar] [CrossRef]
- D’Oria, M.; Grando, B.; Taglialavoro, J.; Gorgatti, F.; Calvagna, C.; Bassini, S.; Riccitelli, F.; Griselli, F.; D’Andrea, A.; Lepidi, S. Association Between Psoas Muscle Sarcopenia and Long-Term Survival Following Elective Endovascular Aortic Repair. J. Surg. Res. 2022, 280, 459–468. [Google Scholar] [CrossRef]
- Warmerdam, B.W.; van Rijswijk, C.S.; Droop, A.; Lucassen, C.J.; Hamming, J.F.; van Schaik, J.; van der Vorst, J.R. The association between sarcopenia and adverse outcomes after complex endovascular aortic repair. J. Cardiovasc. Surg. 2023, 65, 256–264. [Google Scholar] [CrossRef]
- Mezzetto, L.; D’Oria, M.; Mani, K.; Scali, S.; Bastos Gonçalves, F.; Trimarchi, S.; Budtz-Lilly, J.; DeMartino, R.; Veraldi, G.; Mastrorilli, D. Scoping review of radiologic assessment and prognostic impact of skeletal muscle sarcopenia in patients undergoing endovascular repair for aortic disease. J. Vasc. Surg. 2022, 76, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Saucy, F.; Probst, H.; Hungerbühler, J.; Maufroy, C.; Ricco, J.B. Impact of Frailty and Sarcopenia on Thirty-Day and Long-Term Mortality in Patients Undergoing Elective Endovascular Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1935. [Google Scholar] [CrossRef] [PubMed]
- Thurston, B.; Pena, G.N.; Howell, S.; Cowled, P.; Fitridge, R. Low total psoas area as scored in the clinic setting independently predicts midterm mortality after endovascular aneurysm repair in male patients. J. Vasc. Surg. 2018, 67, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Alenezi, A.O.; Tai, E.; Jaberi, A.; Brown, A.; Mafeld, S.; Roche-Nagle, G. Adverse Outcomes after Advanced EVAR in Patients with Sarcopaenia. Cardiovasc. Intervent. Radiol. 2021, 44, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar] [CrossRef]
- Schermerhorn, M.L.; Buck, D.B.; O’Malley, A.J.; Curran, T.; McCallum, J.C.; Darling, J.; Landon, B.E. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N. Engl. J. Med. 2015, 373, 328–338. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. (1985) 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Waduud, M.A.; Wood, B.; Keleabetswe, P.; Manning, J.; Linton, E.; Drozd, M.; Hammond, C.J.; Bailey, M.A.; Scott, D.J.A. Vascular surgeons and interventional radiologists at the Leeds Vascular Institute. Influence of psoas muscle area on mortality following elective abdominal aortic aneurysm repair. Br. J. Surg. 2019, 106, 367–374. [Google Scholar] [CrossRef]
- Lindström, I.; Protto, S.; Khan, N.; Sillanpää, N.; Hernesniemi, J.; Oksala, N.J. Developing sarcopenia predicts long-term mortality after elective endovascular aortic aneurysm repair. Vasc. Surg. 2020, 71, 1169–1178.e5. [Google Scholar] [CrossRef]
- Lo, R.C.; Bensley, R.P.; Hamdan, A.D.; Wyers, M.; Adams, J.E.; Schermerhorn, M.L.; Vascular Study Group of New England. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J. Vasc. Surg. 2013, 57, 1261–1268.e5. [Google Scholar] [CrossRef]

| All (n = 477) | OSR (n = 250) (52.4%) | EVAR (n = 227) (47.6%) | p-Value | |
|---|---|---|---|---|
| Age, years | 73.4 ± 8.0 | 70.8 ± 7.6 | 76.3 ± 7.5 | <0.001 * |
| Male, n (%) | 431 (90.4) | 228 (53) | 203 (47) | 0.308 |
| Weight, Kg | 78.2 ± 14.6 | 78.2 ± 13.9 | 78.3 ± 15.5 | 0.380 |
| Body mass index (Kg/m2) | 26.4 ± 4.6 | 26.5 ± 4.7 | 26.3 ± 4.5 | 0.505 |
| Hospital stay, n | 9.9 ± 9.5 | 12.0 ± 9.4 | 7.7 ± 9.0 | 0.012 * |
| Hospital stay after intervention, n | 8.4 ± 8.9 | 10.6 ± 8.9 | 6.2 ± 8.3 | <0.001 * |
| Coronary diseases, n (%) | 239 (50) | 116 (46) | 123 (54) | 0.089 |
| Dyslipidemia, n (%) | 288 (60.4) | 152 (60.4) | 136 (60) | 0.843 |
| Hypertension, n (%) | 375 (78.6) | 196/250 | 180/227 | 0.730 |
| Neurological diseases (dementia), n (%) | 13 (2.7) | 6 (2.4) | 7 (3) | 0.228 |
| Smoking, n (%) | 221 (46.3) | 120 (48) | 101 (44) | 0.443 |
| Peripheral artery disease, n (%) | 96 (20) | 46 (18) | 50 (22) | 0.312 |
| Cardiac failure, n (%) | 11 (2.3) | 4 (4.4) | 7 (3.1) | 0.281 |
| Cardiac valvular disease, n (%) | 35 (7.3) | 19 (7.6) | 16 (7) | 0.818 |
| COPD, n (%) | 103 (21.6) | 50 (20) | 53 (23.3) | 0.437 |
| Anticoagulant therapy, n (%) | 73 (15.3) | 26 (10.4) | 47 (20.7) | 0.002 * |
| Chronic renal failure, n (%) | 91 (19.1) | 36 (14.4) | 55 (24.2) | 0.006 * |
| Diabetes, n (%) | 75 (15.7) | 34 (13.6) | 41 (18) | 0.107 |
| Stroke, n (%) | 51 (10.7) | 26 (10.4) | 25 (11) | 0.829 |
| Cancer, n (%) | 91 (19.3) | 41 (16.4) | 51 (22.4) | 0.159 |
| Obesity, n (%) | 44 (9.2) | 20 (8) | 24 (10.5) | 0.345 |
| Clinical presentation asymtomatic, n (%) | 407 (85.3) | 202 (80.8) | 205 (90.3) | 0.001 * |
| Clinical presentation symtomatic, n (%) | 70 (14.7) | 48 (19.2) | 22 (9.7) | 0.001 * |
| Diameter, n (%) | 57.2 ± 15.2 | 55.5 ± 12.8 | 58.7 ± 17.1 | 0.034 * |
| Necessity of blood transfusions, n (%) | 69 (14.5) | 58 (23.2) | 11 (4.8) | 0.001 * |
| Creatinin (mg/dL) | 458 ± 82 | 104.3 ± 79.4 | 105.0 ± 85.0 | 0.907 |
| SMA, cm2 | 146.6 ± 33.5 | 149.5 ± 35.1 | 143.5 ± 31.2 | 0.085 |
| SMI, cm2/m2 | 49.5 ± 10.8 | 50.3 ± 11.2 | 48.7 ± 10.3 | 0.173 |
| VAT, cm2 | 212.1 ± 194.2 | 194.4 ± 104.0 | 231.7 ± 259.2 | 0.064 |
| SAT, cm2 | 161.3 ± 59.7 | 161.7 ± 60.6 | 160.9 ± 58.9 | 0.907 |
| OSR | EVAR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude Model | Adjusted Model | |||||||||||
| Β-Coefficient | CI 95% | p-Value | Β-Coefficient | CI 95% | p-Value | Β-Coefficient | CI 95% | p-Value | Β-Coefficient | CI 95% | p-Value | |
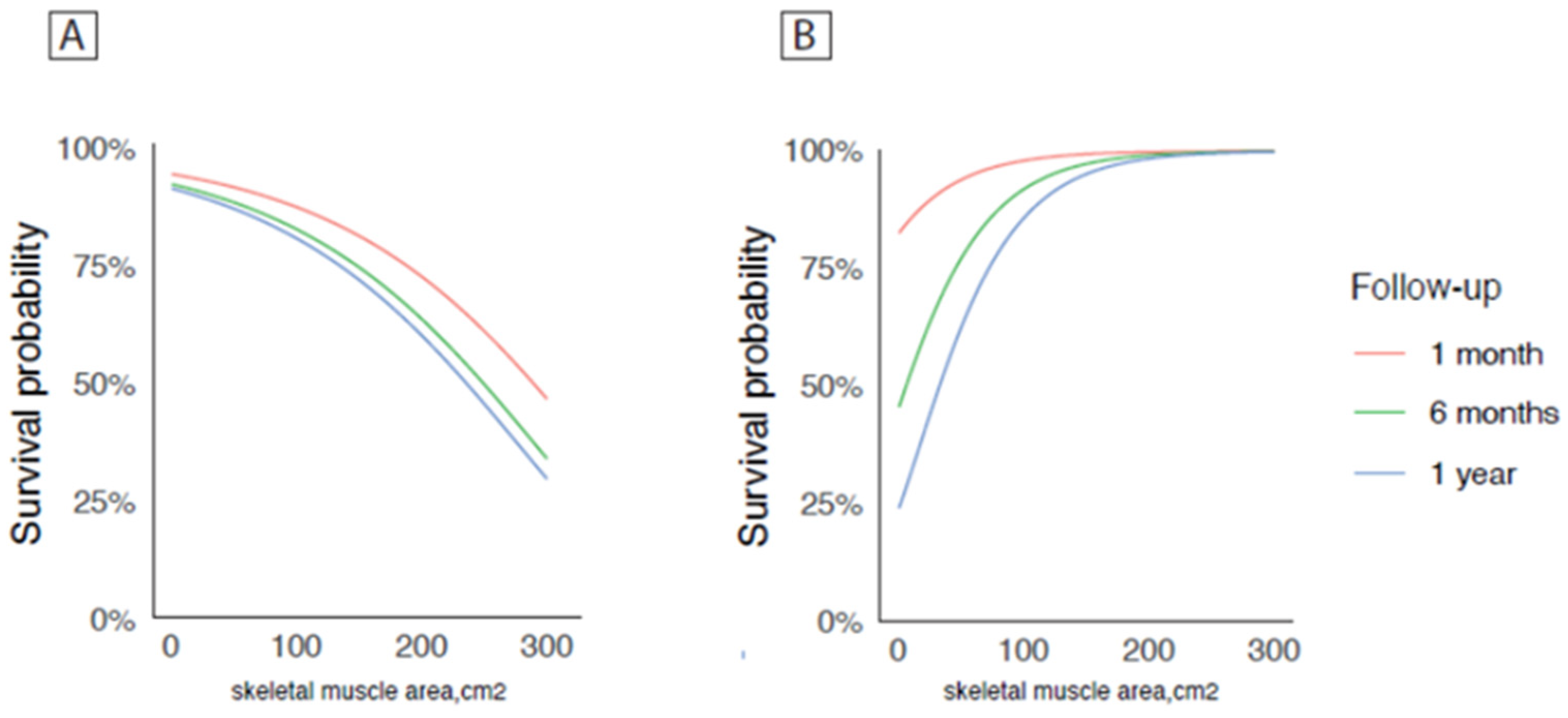
| SMA | −0.032 | −0.050–0.032 | 0.665 | 0.001 | −0.42–0.043 | 0.991 | −0.200 | −0.111; −0.017 | <0.001 | −0.194 | −0.114; −0.010 | 0.019 |
| Sarcopenic | 0.046 | −2.776–5.292 | 0.539 | 0.391 | −3.240–4.841 | 0.696 | −0.247 | −2.853; −11.013 | <0.001 | −3.224 | −2.734–11.370 | 0.002 |
| SMI | −0.006 | −0.137; 0.125 | 0.934 | 0.025 | −0.113–0.157 | 0.747 | −0.156 | −0.305; −0.004 | 0.045 | 0.120 | −0.289–0.034 | 0.120 |
| VAT | 0.020 | −0.012–0.016 | 0.927 | 0.357 | −0.012–0.018 | 0.722 | −0.063 | −0.008–0.003 | 0.409 | 0.231 | −0.009–0.001 | 0.231 |
| SAT | 0.062 | −0.014; 0.034 | 0.402 | 0.081 | −0.011–0.037 | 0.290 | 0.219 | −0.023–0.028 | 0.402 | 0.110 | −0.024–0.027 | 0.912 |
| OSR | EVAR | |||||
|---|---|---|---|---|---|---|
| Biompedentiometry Parameters | HR | CI 95% | p-Value | HR | CI 95% | p-Value |
| SMA | 0.9861 | 1.001–1.027 | 0.030 * | 1.301 | 0.947–0.996 | 0.026 * |
| SMI | 0.9567 | 0.988–1.105 | 0.117 | 1.066 | 0.863–1.020 | 0.134 |
| VAT | 0.9952 | 1.001–1.009 | 0.012 * | 1.002 | 0.990–1.005 | 0.490 |
| SAT | 0.993 | 0.999–1.015 | 0.065 | 1.010 | 0.998–1.021 | 0.094 |
| OSR | EVAR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biompedentiometry Parameters | Univariate | Multivariate | ||||||||||
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | HR | CI 95% | p-Value | HR | CI 95% | p-Value | |
| SMA | 1.003 | 0.99–1.004 | 0.794 | 1.001 | 0.994–1.007 | 0.723 | 0.993 | 0.986–0.999 | 0.004 * | 0.989 | 0.982–0.996 | 0.003 * |
| SMI | 0.995 | 0.972–1.019 | 0.708 | 0.996 | 0.973–1.021 | 0.802 | 0.986 | 0.967–1.003 | 0.178 | 0.977 | 0.957–0.998 | 0.032 * |
| VAT | 1.000 | 0.997–1.002 | 0.977 | 1.005 | 0.997–1.002 | 0.885 | 0.998 | 0.997–1.002 | 0.171 | 0.998 | 0.996–0.999 | 0.034 * |
| SAT | 1.000 | 0.996–1.004 | 0.847 | 1.000 | 0.996–1.004 | 0.811 | 0.997 | 0.994–1.001 | 0.255 | 0.996 | 0.992–1.000 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giorno, R.; Robaldo, A.; Astorino, A.; Gabutti, L.; Chianca, V.; Rizzo, S.; Riva, F.; Ettorre, L.; Stefanelli, K.; Canevascini, R.; et al. The Impact of Body Composition on Mortality and Hospital Length of Stay after Endovascular and Open Aortic Aneurysm Repair: A Retrospective Cohort Study. Nutrients 2024, 16, 3205. https://doi.org/10.3390/nu16183205
Del Giorno R, Robaldo A, Astorino A, Gabutti L, Chianca V, Rizzo S, Riva F, Ettorre L, Stefanelli K, Canevascini R, et al. The Impact of Body Composition on Mortality and Hospital Length of Stay after Endovascular and Open Aortic Aneurysm Repair: A Retrospective Cohort Study. Nutrients. 2024; 16(18):3205. https://doi.org/10.3390/nu16183205
Chicago/Turabian StyleDel Giorno, Rosaria, Alessandro Robaldo, Alessia Astorino, Luca Gabutti, Vito Chianca, Stefania Rizzo, Francesca Riva, Ludovica Ettorre, Kevyn Stefanelli, Reto Canevascini, and et al. 2024. "The Impact of Body Composition on Mortality and Hospital Length of Stay after Endovascular and Open Aortic Aneurysm Repair: A Retrospective Cohort Study" Nutrients 16, no. 18: 3205. https://doi.org/10.3390/nu16183205
APA StyleDel Giorno, R., Robaldo, A., Astorino, A., Gabutti, L., Chianca, V., Rizzo, S., Riva, F., Ettorre, L., Stefanelli, K., Canevascini, R., Giovannacci, L., & Prouse, G. (2024). The Impact of Body Composition on Mortality and Hospital Length of Stay after Endovascular and Open Aortic Aneurysm Repair: A Retrospective Cohort Study. Nutrients, 16(18), 3205. https://doi.org/10.3390/nu16183205







