Balancing the Oral–Gut–Brain Axis with Diet
Abstract
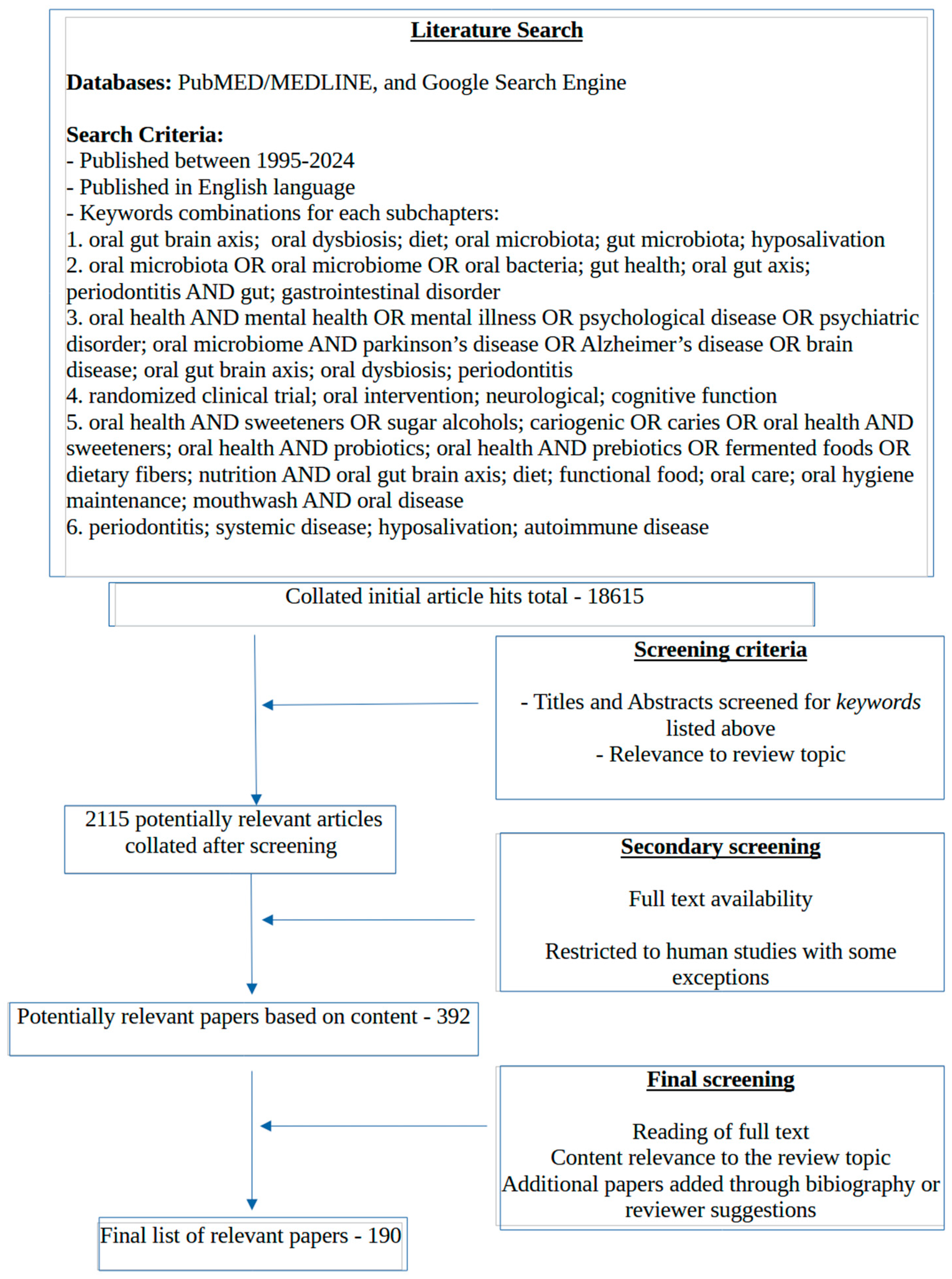
1. Introduction
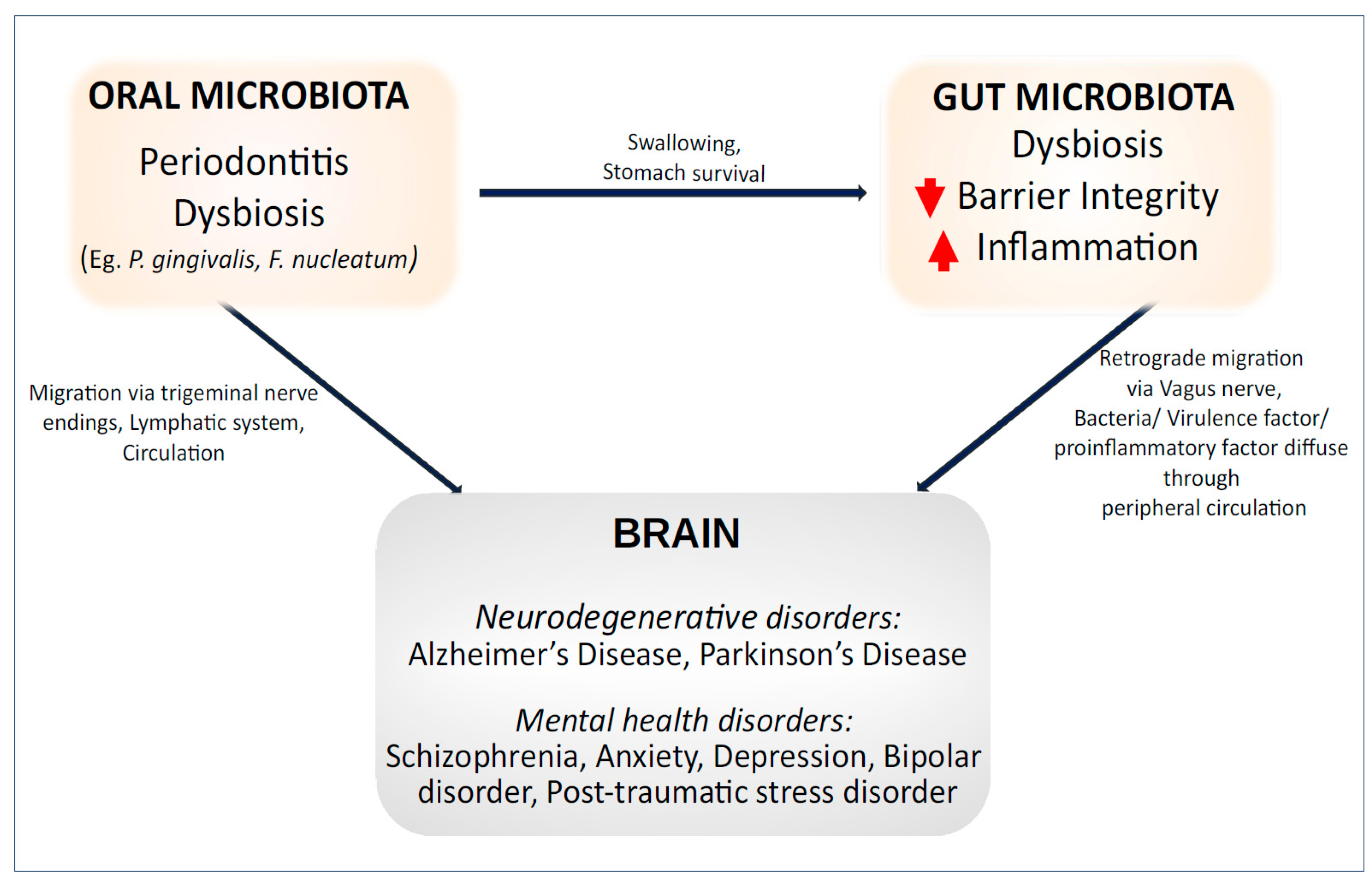
2. Role of the Oral Microbiota in Gut Health
3. The Oral–Gut–Brain Axis—Implications in Neurological and Mental Health
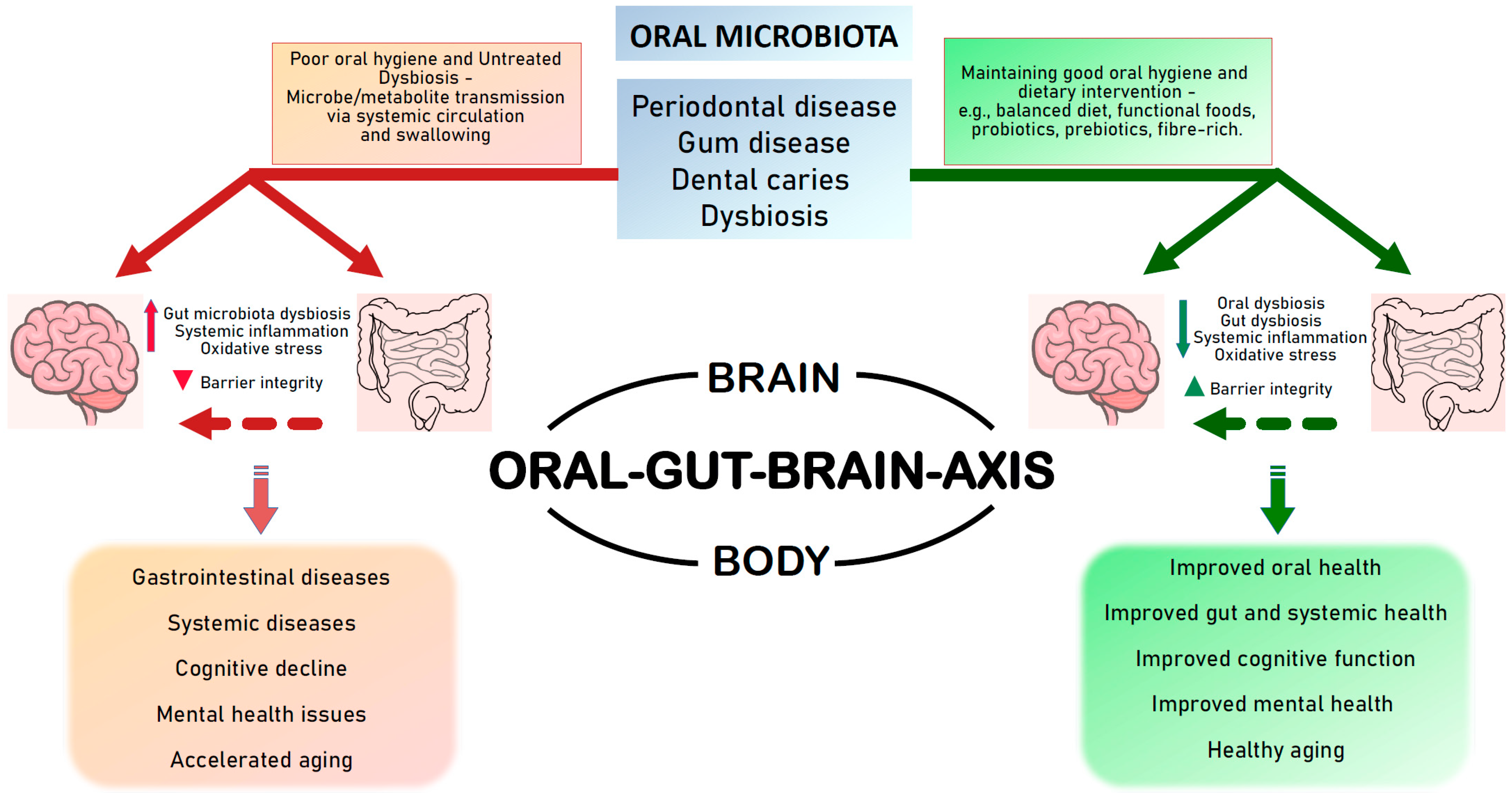
4. Evidence of Oral Health Interventions Supporting Improved Gut Health and Brain Health
5. Targeting Oral Health for a Healthy Oral–Gut–Brain Axis
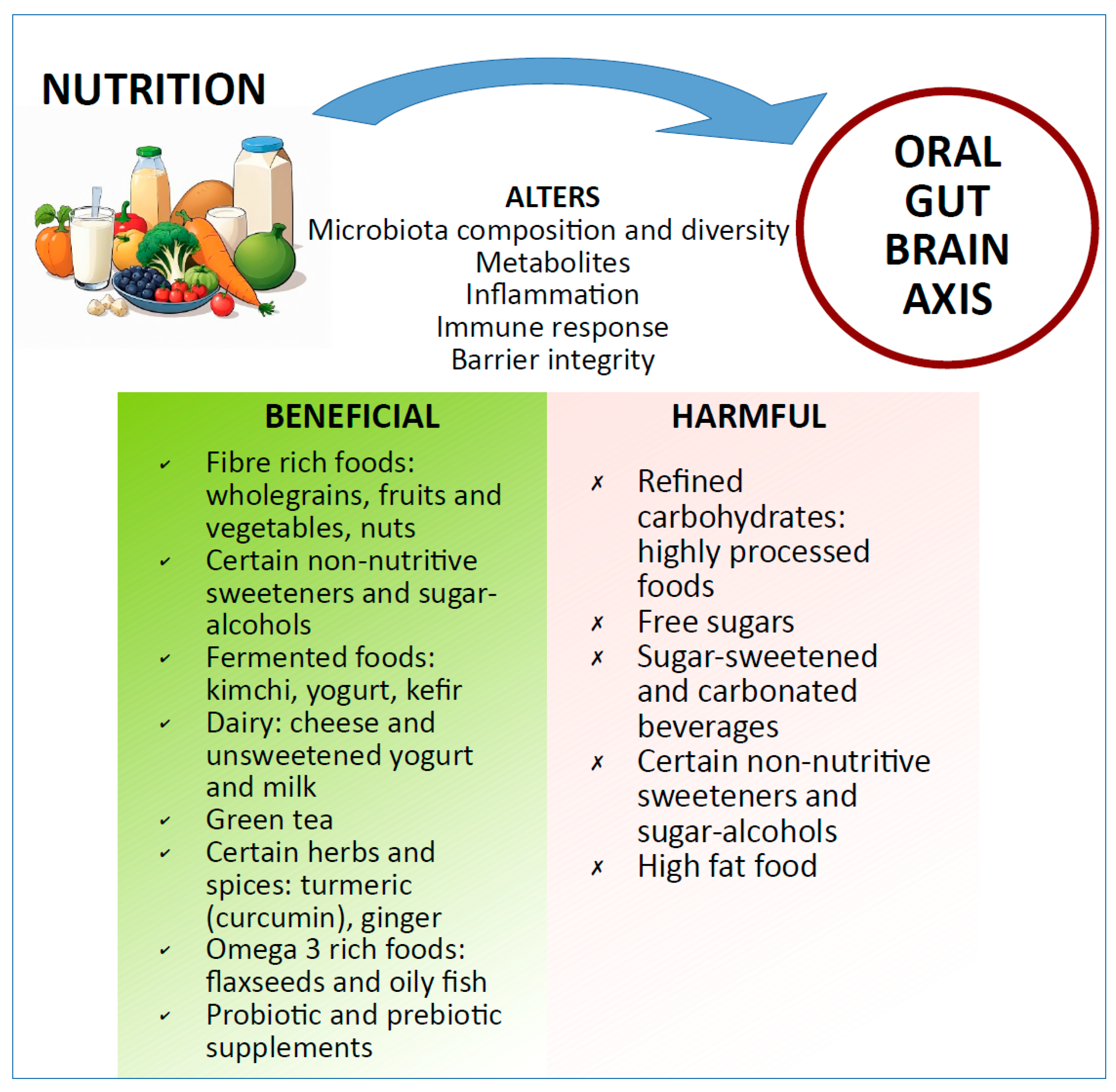
5.1. Through Diet/Functional Foods
5.2. Sugar Alcohols, Non-Nutritive Sweeteners, and Rare Sugars
5.3. Probiotics
5.4. Prebiotic Supplementation
5.5. Through Oral Care—Beyond Diet
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reza, M.M.; Finlay, B.B.; Pettersson, S. Gut microbes, ageing & organ function: A chameleon in modern biology? EMBO Mol. Med. 2019, 11, e9872. [Google Scholar]
- Hall, A.; Versalovic, J. Microbial Metabolism in the Mammalian Gut: Molecular Mechanisms and Clinical Implications. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S72–S79. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut microbiota and extreme longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; McCormick, B.A. Ageing, Frailty, and the Microbiome: How Dysbiosis Influences Human Ageing and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. Manipulation of epithelial integrity and mucosal immunity by host and microbiota-derived metabolites. Eur. J. Immunol. 2020, 50, 921–931. [Google Scholar] [CrossRef]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Hartland, E.L.; Phumoonna, T.; Lyras, D.; Rood, J.I.; Robins-Browne, R.M.; van Driel, I.R. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect. Immun. 2008, 76, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gui, W.; Koo, I.; Smith, P.B.; Allman, E.L.; Nichols, R.G.; Rimal, B.; Cai, J.; Liu, Q.; Patterson, A.D. The microbiome modulating activity of bile acids. Gut Microbes 2020, 11, 979–996. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive transmission of microbes along the gastrointestinal tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Lim, M.; Ok, S.H.; Lee, S.K.; Chun, K.S.; Park, K.K.; Hu, Y.; Chung, W.Y.; Song, N.Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Read, E.; Curtis, M.A.; Neves, J.F. The role of oral bacteria in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 731–742. [Google Scholar] [CrossRef]
- Frese, C.; Zenthöfer, A.; Aurin, K.; Schoilew, K.; Wohlrab, T.; Sekundo, C. Oral health of centenarians and supercentenarians. J. Oral Sci. 2020, 62, 9–12. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Deligios, M.; Milanesi, L.; Langille, M.G.I.; Zinellu, A.; Rubino, S.; Carru, C.; Kelvin, D.J. Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia. mSphere 2020, 5, e00558-19. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Michaud, D.S.; Kelsey, K.T.; Papathanasiou, E.; Genco, C.A.; Giovannucci, E. Periodontal disease and risk of all cancers among male never smokers: An updated analysis of the Health Professionals Follow-up Study. Ann. Oncol. 2016, 27, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Shinjo, T.; Nishimura, F. The bidirectional association between diabetes and periodontitis, from basic to clinical. Jpn. Dent. Sci. Rev. 2024, 60, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.; Gajagowni, S.; Qadeer, Y.; Wang, Z.; Virani, S.S.; Meurman, J.H.; Krittanawong, C. Oral Health and Cardiovascular Disease. Am. J. Med. 2024, 137, 304–307. [Google Scholar] [CrossRef]
- Sanz, M.; Del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Glob. Heart 2020, 15, 1. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Liu, Y.; Huang, J.; Lu, Z.; Xie, L.; Sun, W.; Ji, Y. Porphyromonas gingivalis infection reduces regulatory T cells in infected atherosclerosis patients. PLoS ONE 2014, 9, e86599. [Google Scholar] [CrossRef]
- Cai, Y.; Kobayashi, R.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch. Oral Biol. 2014, 59, 1183–1191. [Google Scholar] [CrossRef]
- Rusthen, S.; Kristoffersen, A.K.; Young, A.; Galtung, H.K.; Petrovski, B.É.; Palm, Ø.; Enersen, M.; Jensen, J.L. Dysbiotic salivary microbiota in dry mouth and primary Sjögren’s syndrome patients. PLoS ONE 2019, 14, e0218319. [Google Scholar] [CrossRef]
- van der Meulen, T.A.; Harmsen, H.J.M.; Bootsma, H.; Liefers, S.C.; Vich Vila, A.; Zhernakova, A.; Weersma, R.K.; Spijkervet, F.K.L.; Kroese, F.G.M.; Vissink, A. Reduced salivary secretion contributes more to changes in the oral microbiome of patients with primary Sjögren’s syndrome than underlying disease. Ann. Rheum. Dis. 2018, 77, 1542–1544. [Google Scholar] [CrossRef]
- Saúco, C.; Rus, M.J.; Nieto, M.R.; Barros, C.; Cantiga-Silva, C.; Lendines-Cordero, D.; Calderer-Ortiz, M.; Zurita-García, M.; Arias-Herrera, S.; Monsalve-Guil, L.; et al. Hyposalivation but not Sjögren’s syndrome associated with microbial dysbiosis in women. Front. Microbiol. 2023, 14, 1240891. [Google Scholar] [CrossRef]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Wu, Y.T.; Chang, Y.C. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: A population-based retrospective matched-cohort study. PeerJ. 2017, 5, e3647. [Google Scholar] [CrossRef]
- Scassellati, C.; Marizzoni, M.; Cattane, N.; Lopizzo, N.; Mombelli, E.; Riva, M.A.; Cattaneo, A. The Complex Molecular Picture of Gut and Oral Microbiota-Brain-Depression System: What We Know and What We Need to Know. Front. Psychiatry 2021, 12, 722335. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [PubMed]
- Melby, M.K.; Tharmabalan, R.T.; Sällberg Chen, M.; Pettersson, S.; Jayaraman, A. Gut Microbes: Gateway to Reshaping Biological Aging. In Handbook of Aging, Health and Public Policy; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: Probiotics, prebiotics and the low FODMAP diet. Proc. Nutr. Soc. 2016, 75, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- Luo, S.C.; Wei, S.M.; Luo, X.T.; Yang, Q.Q.; Wong, K.H.; Cheung, P.C.K.; Zhang, B.B. How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: An oral microbiota perspective. npj Biofilms Microbiomes 2024, 10, 14. [Google Scholar] [CrossRef]
- Pang, L.; Zhi, Q.; Jian, W.; Liu, Z.; Lin, H. The Oral Microbiome Impacts the Link between Sugar Consumption and Caries: A Preliminary Study. Nutrients 2022, 14, 3693. [Google Scholar] [CrossRef]
- Rungsri, P.; Akkarachaneeyakorn, N.; Wongsuwanlert, M.; Piwat, S.; Nantarakchaikul, P.; Teanpaisan, R. Effect of fermented milk containing Lactobacillus rhamnosus SD11 on oral microbiota of healthy volunteers: A randomized clinical trial. J. Dairy Sci. 2017, 100, 7780–7787. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.; Hoyles, L.; McCartney, A.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- Hartemink, R.; Quataert, M.C.; van Laere, K.M.; Nout, M.J.; Rombouts, F.M. Degradation and fermentation of fructo-oligosaccharides by oral streptococci. J. Appl. Bacteriol. 1995, 79, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Ebadi, M.; Weisdorf, D.J.; Costalonga, M.; Staley, C. No evidence for colonization of oral bacteria in the distal gut in healthy adults. Proc. Natl. Acad. Sci. USA 2021, 118, e2114152118. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Li, L.; Zhang, Y.; Wang, M.; Chen, F.; Ge, S.; Chen, B.; Yan, F. Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral Sci. 2022, 14, 32. [Google Scholar] [CrossRef]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; ohno, H.; Yamazaki, K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ogawa, Y.; Hashizume-Takizawa, T.; Kurita-Ochiai, T. Oral bacteria affect the gut microbiome and intestinal immunity. Pathog. Dis. 2020, 78, ftaa024. [Google Scholar] [CrossRef] [PubMed]
- Simas, A.M.; Kramer, C.D.; Weinberg, E.O.; Genco, C.A. Oral infection with a periodontal pathogen alters oral and gut microbiomes. Anaerobe 2021, 71, 102399. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef]
- Komazaki, R.; Katagiri, S.; Takahashi, H.; Maekawa, S.; Shiba, T.; Takeuchi, Y.; Kitajima, Y.; Ohtsu, A.; Udagawa, S.; Sasaki, N.; et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 2017, 7, 13950. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, W.; Dai, K.; Liu, N.; Wang, J.; Lu, X.; Ma, J.; Zhang, M.; Xu, M.; Long, X.; et al. Inflammatory response of gut, spleen, and liver in mice induced by orally administered Porphyromonas gingivalis. J. Oral Microbiol. 2022, 14, 2088936. [Google Scholar] [CrossRef]
- Burns, G.L.; Wark, J.A.; Hoedt, E.C.; Minahan, K.; Sherwin, S.; Bruce, J.K.; Lim, Y.; Teh, J.J.; Jamaluddin, M.F.B.; Soh, W.S.; et al. The novel duodenal isolate streptococcus salivarius AGIRA0003 promotes barrier dysfunction and IgG responses in functional dyspepsia. medRxiv 2024. [Google Scholar] [CrossRef]
- Imai, J.; Ichikawa, H.; Kitamoto, S.; Golob, J.L.; Kaneko, M.; Nagata, J.; Takahashi, M.; Gillilland, M.G.; Tanaka, R.; Nagao-Kitamoto, H.; et al. A potential pathogenic association between periodontal disease and Crohn’s disease. JCI Insight 2021, 6, e148543. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, M.M.H.; Hatting, M.; Bott, A.; Dahlhausen, A.; Keller, D.; Trautwein, C.; Conrads, G. The oral-gut axis: Salivary and fecal microbiome dysbiosis in patients with inflammatory bowel disease. Front. Cell. Infect. Microbiol. 2022, 12, 1010853. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Png, E.; Gowans, M.; Ong, D.E.H.; de Sessions, R.F.; Song, J.; Nagarajan, N. Ectopic gut colonization: A metagenomic study of the oral and gut microbiome in Crohn’s disease. Gut Pathog. 2021, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Bertl, K.; Burisch, J.; Pandis, N.; Bruckmann, C.; Klinge, B.; Stavropoulos, A. Periodontitis prevalence in patients with ulcerative colitis and Crohn’s disease—PPCC: A case-control study. J. Clin. Periodontol. 2022, 49, 1262–1274. [Google Scholar] [CrossRef]
- Said, H.S.; Suda, W.; Nakagome, S.; Chinen, H.; Oshima, K.; Kim, S.; Kimura, R.; Iraha, A.; Ishida, H.; Fujita, J.; et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014, 21, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kayani, M.U.R.; Hong, L.; Zhang, C.; Zhong, J.; Wang, Z.; Chen, L. Dynamics of the Salivary Microbiome during Different Phases of Crohn’s Disease. Front. Cell. Infect. Microbiol. 2020, 10, 544704. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Forner, L.; Larsen, T.; Kilian, M.; Holmstrup, P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 2006, 33, 401–407. [Google Scholar] [CrossRef]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgard, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate from the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tanernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Neuberg, D.; Huang, K.; Guevara, F.; Nelson, T.; et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Yohei, M.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, N.; Tommasina, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Miguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 28, 424–432. [Google Scholar] [CrossRef]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontol. 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.F.J.; De Vos, P.; Dekker, J.; et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci. Rep. 2019, 9, 1437. [Google Scholar] [CrossRef]
- Fan, C.; Guo, L.; Gu, H.; Huo, Y.; Lin, H. Alterations in Oral-Nasal-Pharyngeal Microbiota and Salivary Proteins in Mouth-Breathing Children. Front. Microbiol. 2020, 11, 575550. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Gangula, P.; Ravella, K.; Chukkapalli, S.; Rivera, M.; Srinivasan, S.; Hale, A.; Channon, K.; Southerland, J.; Kesavalu, L. Polybacterial Periodontal Pathogens Alter Vascular and Gut BH4/nNOS/NRF2-Phase II Enzyme Expression. PLoS ONE 2015, 10, e0129885. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, M.S.; Kliger, D.; MacLellan, A.D.; Yu, A.C.; Langlois, J.; Fan, M.; Boroomand, S.; Kharazyan, F.; Hsiung, R.G.Y.; MacVicar, B.A.; et al. The Oral and Fecal Microbiota in a Canadian Cohort of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 87, 247–258. [Google Scholar] [CrossRef]
- Wu, Y.F.; Lee, W.F.; Salamanca, E.; Yao, W.L.; Su, J.N.; Wang, S.Y.; Hu, C.J.; Chang, W.J. Oral Microbiota Changes in Elderly Patients, an Indicator of Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2023, 29, 1341–1355. [Google Scholar] [CrossRef]
- Issilbayeva, A.; Kaiyrlykyzy, A.; Vinogradova, E.; Jarmukhanov, Z.; Kozhakhmetov, S.; Kassenova, A.; Nurgaziyev, M.; Mukhanbetzhanov, N.; Alzhanova, D.; Zholdasbekova, G.; et al. Oral Microbiome Stamp in Alzheimer’s Disease. Pathogens 2024, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Taati Moghadam, M.; Amirmozafari, N.; Mojtahedi, A.; Bakhshayesh, B.; Shariati, A.; Masjedian Jazi, F. Association of perturbation of oral bacterial with incident of Alzheimer’s disease: A pilot study. J. Clin. Lab. Anal. 2022, 36, e24483. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, J.; Bai, H.; Ren, L.; Yang, J.; Ding, Y.; Liu, C.; Chen, X. Periodontal Status and Saliva Metabolic Signature in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 95, 603–613. [Google Scholar] [CrossRef]
- Liu, X.X.; Jiao, B.; Liao, X.X.; Guo, L.N.; Yuan, Z.H.; Wang, X.; Xiao, X.W.; Zhang, X.Y.; Tang, B.S.; Shen, L. Analysis of Salivary Microbiome in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 72, 633–640. [Google Scholar] [CrossRef]
- Rozas, N.S.; Tribble, G.D.; Jeter, C.B. Oral Factors That Impact the Oral Microbiota in Parkinson’s Disease. Microorganisms 2021, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Fleury, V.; Zekeridou, A.; Lazarevic, V.; Gaïa, N.; Giannopoulou, C.; Genton, L.; Cancela, J.; Girard, M.; Goldstein, R.; Bally, J.F.; et al. Oral Dysbiosis and Inflammation in Parkinson’s Disease. J. Parkinsons Dis. 2021, 11, 619–631. [Google Scholar] [CrossRef]
- Zapała, B.; Stefura, T.; Milewicz, T.; Wątor, J.; Piwowar, M.; Wójcik-Pędziwiatr, M.; Doręgowska, M.; Dudek, A.; Jania, Z.; Rudzińska-Bar, M. The Role of the Western Diet and Oral Microbiota in Parkinson’s Disease. Nutrients 2022, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ren, T.; Li, H.; Huang, M.; Chen, J.; He, Q.; Lv, W.; Liu, H.; Xu, R.; Zhang, X. Oral Microbiota and Porphyromonas gingivalis Kgp Genotypes Altered in Parkinson’s Disease with Mild Cognitive Impairment. Mol. Neurobiol. 2024. [CrossRef] [PubMed]
- Chen, L.; Xu, X.; Wu, X.; Cao., H.; Li, X.; Hou, Z.; Wang, B.; Liu, J.; Ji, X.; Zhang, P.; et al. A comparison of the composition and functions of the oral and gut microbiotas in Alzheimer’s patients. Front. Cell. Infect. Microbiol. 2022, 12, 942460. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Troci, A.; Philippen, S.; Rausch, P.; Rave, J.; Weyland, G.; Niemann, K.; Jessen, K.; Schmill, L.P.; Aludin, S.; Franke, A.; et al. Disease- and stage-specific alterations of the oral and fecal microbiota in Alzheimer’s disease. PNAS Nexus 2023, 3, pgad427. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimer’s Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, S.; Huang, Y.; Qian, J.; Tan, B.; Qian, X.; Zhuang, J.; Zou, X.; Li, Y.; Yan, F. Periodontitis-related salivary microbiota aggravates Alzheimer’s disease via gut-brain axis crosstalk. Gut Microbes 2022, 14, 2126272. [Google Scholar] [CrossRef]
- Yan, C.; Diao, Q.; Zhao, Y.; Zhang, C.; He, X.; Huang, R.; Li, Y. Fusobacterium nucleatum infection-induced neurodegeneration and abnormal gut microbiota composition in Alzheimer’s disease-like rats. Front. Neurosci. 2022, 16, 884543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Kuraji, R.; Ye, C.; Gao, L.; Radaic, A.; Kamarajan, P.; Taketani, Y.; Kapila, Y.L. Nisin a probiotic bacteriocin mitigates brain microbiome dysbiosis and Alzheimer’s disease-like neuroinflammation triggered by periodontal disease. J. Neuroinflamm. 2023, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chau, S.W.H.; Liu, Y.; Chan, J.W.Y.; Wang, J.; Ma, S.L.; Zhang, J.; Chan, P.K.S.; Yeoh, Y.K.; Chen, Z.; et al. Gut microbiome dysbiosis across early Parkinson’s disease, REM sleep behavior disorder and their first-degree relatives. Nat. Commun. 2023, 14, 2501. [Google Scholar] [CrossRef]
- Adams, B.; Nunes, J.M.; Page, M.J.; Roberts, T.; Carr, J.; Nell, T.A.; Kell, D.B.; Pretorius, E. Parkinson’s Disease: A Systemic Inflammatory Disease Accompanied by Bacterial Inflammagens. Front. Aging Neurosci. 2019, 11, 210. [Google Scholar] [CrossRef]
- Bai, X.B.; Xu, S.; Zhou, L.J.; Meng, X.Q.; Li, Y.L.; Chen, Y.L.; Jiang, Y.H.; Lin, W.Z.; Chen, B.Y.; Du, L.J.; et al. Oral pathogens exacerbate Parkinson’s disease by promoting Th1 cell infiltration in mice. Microbiome 2023, 11, 254. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Li, H.; Malhotra, R.; Leite, F.R.M.; Peres, K.G.; Chan, A.; Peres, M.A. Chewing Disability Is Associated with Cognitive Impairment among Older Adults: A Population-Based Cohort Study. J. Gerontol. 2024, 79, glae074. [Google Scholar] [CrossRef]
- Kiuchi, S.; Cooray, U.; Aida, J.; Osaka, K.; Chan, A.; Malhotra, R.; Peres, M.A. Effect of Tooth Loss on Cognitive Function among Older Adults in Singapore. J. Dent. Res. 2023, 102, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Peres, K.G.; Chan, A.; Wu, B.; Mittinty, M. Investigating the causal effect of cognition on the self-reported loss of functional dentition using marginal structural models: The Panel on Health and Ageing of Singaporean Elderly study. J. Clin. Periodontol. 2023, 50, 408–417. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Liu, X.; Yan, X.; Wu, L.; Shao, L.; Gao, J.; Lei, W.; Song, Q.; Zhao, L.; et al. Altered oral microbiota and immune dysfunction in Chinese elderly patients with schizophrenia: A cross-sectional study. Transl. Psychiatry 2023, 13, 383. [Google Scholar] [CrossRef]
- Choi, J.; Price, J.; Ryder, S.; Siskind, D.; Solmi, M.; Kisely, S. Prevalence of dental disorders among people with mental illness: An umbrella review. Aust. N. Z. J. Psychiatry 2022, 56, 949–963. [Google Scholar] [CrossRef]
- Kisely, S.; Baghaie, H.; Lalloo, R.; Siskind, D.; Johnson, N.W. A systematic review and meta-analysis of the association between poor oral health and severe mental illness. Psychosom. Med. 2015, 77, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, N.; Leskelä, J.; Manzoor, M.; Holmer, J.; Paju, S.; Hiltunen, K.; Roitto, H.M.; Saarela, R.K.; Pitkälä, K.; Eriksdotter, M.; et al. Subgingival microbiome at different levels of cognition. J. Oral Microbiol. 2023, 15, 2178765. [Google Scholar] [CrossRef]
- Kisely, S.; Sawyer, E.; Siskind, D.; Lalloo, R. The oral health of people with anxiety and depressive disorders—A systematic review and meta-analysis. J. Affect. Disord. 2016, 200, 119–132. [Google Scholar] [CrossRef]
- Urien, L.; Jauregizar, N.; Lertxundi, U.; Fernández, U.; Morera-Herreras, T. Medication impact on oral health in schizophrenia. Med. Oral Patol. Oral Cir. Bucal 2024, 29, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Postolache, T.; García-Bueno, B.; Leza, J.; Figuero, E.; Lowry, C.; Malan-Müller, S. The Role of the Oral Microbiota Related to Periodontal Diseases in Anxiety, Mood and Trauma- and Stress-Related Disorders. Front. Psychiatry 2022, 12, 814177. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Wu, F.; Dai, N.; Chen, M.; Yu, J.; Guan, J.; Li, F. Variations of Oral Microbiome in Chronic Insomnia Patients with Different Tongue Features. Am. J. Chin. Med. 2020, 48, 923–944. [Google Scholar] [CrossRef]
- Cui, G.; Qing, Y.; Li, M.; Sun, L.; Zhang, J.; Feng, L.; Li, J.; Chen, T.; Wang, J.; Wan, C. Salivary Metabolomics Reveals that Metabolic Alterations Precede the Onset of Schizophrenia. J. Proteome Res. 2021, 20, 5010–5023. [Google Scholar] [CrossRef]
- Qing, Y.; Xu, L.; Cui, G.; Sun, L.; Hu, X.; Yang, X.; Jiang, J.; Zhang, J.; Zhang, T.; Wang, T.; et al. Salivary microbiome profiling reveals a dysbiotic schizophrenia-associated microbiota. npj Schizophr. 2021, 7, 51. [Google Scholar] [CrossRef]
- Gluszek-Osuch, M.; Ciesla, E.; Suliga, E. Relationship between the number of lost teeth and the occurrence of depressive symptoms in middle-aged adults: A cross-sectional study. BMC Oral Health 2024, 24, 559. [Google Scholar] [CrossRef]
- Wingfield, B.; Lapsley, C.; McDowell, A.; Miliotis, G.; McLafferty, M.; O’Neill, S.M.; Coleman, S.; McGinnity, T.M.; Bjourson, A.J.; Murray, E.K. Variations in the oral microbiome are associated with depression in young adults. Sci. Rep. 2021, 11, 15009. [Google Scholar] [CrossRef]
- Simpson, C.A.; Adler, C.; du Plessis, M.R.; Landau, E.R.; Dashper, S.G.; Reynolds, E.C.; Schwartz, O.S.; Simmons, J.G. Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol. Behav. 2020, 226, 113126. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Wen, Y.; Jia, Y.; Cheng, S.; Liu, L.; Zhang, H.; Pan, C.; Zhang, J.; Zhang, Z.; et al. A genetic association study reveals the relationship between the oral microbiome and anxiety and depression symptoms. Front. Psychiatry 2022, 13, 960756. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.A.; Cota, L.O.M.; Cortelli, S.C.; Miranda, T.B.; Neves, F.S.; Cortelli, J.R.; Costa, F.O. Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: A case-control study. J. Periodontal Res. 2019, 54, 63–72. [Google Scholar] [CrossRef]
- Levert-Levitt, E.; Shapira, G.; Sragovich, S.; Shomron, N.; Lam, J.C.K.; Li, V.O.K.; Heimesaat, M.M.; Bereswill, S.; Yehuda, A.B.; Sagi-Schwartz, A.; et al. Oral microbiota signatures in post-traumatic stress disorder (PTSD) veterans. Mol. Psychiatry 2022, 27, 4590–4598. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Sikaroodi, M.; Fagan, A.; Heuman, D.; Gilles, H.; Gavis, E.A.; Fuchs, M.; Gonzalez-Maeso, J.; Nizam, S.; Gillevet, P.M.; et al. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G661–G669. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Dashash, M.; Latifeh, Y. A short-term approach for promoting oral health of internally displaced children with PTSD: The key is improving mental health—Results from a quasi-randomized trial. BMC Oral Health 2021, 21, 58. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Hsu, C.W.; Lu, M.C.; Koo, M. Increased risks of psychiatric disorders in patients with primary Sjögren’s syndrome-a secondary cohort analysis of nationwide, population-based health claim data. Clin. Rheumatol. 2019, 38, 3195–3203. [Google Scholar] [CrossRef]
- Delalande, S.; de Seze, J.; Fauchais, A.L.; Hachulla, E.; Stojkovic, T.; Ferriby, D.; Dubucquoi, S.; Pruvo, J.P.; Vermersch, P.; Hatron, P.Y. Neurologic manifestations in primary Sjögren syndrome: A study of 82 patients. Medicine 2004, 83, 280–291. [Google Scholar] [CrossRef]
- Osborn, D.P. The poor physical health of people with mental illness. West. J. Med. 2001, 175, 329–332. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M.J. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Y.; Gong, T. The interplay between oral microbiota, gut microbiota and systematic diseases. J. Oral Microbiol. 2023, 15, 2213112. [Google Scholar] [CrossRef] [PubMed]
- Sansores-España, L.D.; Melgar-Rodríguez, S.; Olivares-Sagredo, K.; Cafferata, E.A.; Martínez-Aguilar, V.M.; Vernal, R.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Oral-Gut-Brain Axis in Experimental Models of Periodontitis: Associating Gut Dysbiosis with Neurodegenerative Diseases. Front. Aging 2021, 2, 781582. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Cheng, X.; Lin, L.; Yang, T.; Sun, J.; Feng, Y.; Liang, F.; Pei, Z.; Teng, W. Porphyromonas gingivalis-Induced Cognitive Impairment Is Associated with Gut Dysbiosis, Neuroinflammation, and Glymphatic Dysfunction. Front. Cell. Infect. Microbiol. 2021, 11, 755925. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Wu, X.; Xu, X.; Ji, X.; Wang, B.; Zhang, P.; Li, H. Effects of oral health intervention strategies on cognition and microbiota alterations in patients with mild Alzheimer’s disease: A randomized controlled trial. Geriatr. Nurs. 2022, 48, 103–110. [Google Scholar] [CrossRef]
- Jung, E.S.; Choi, Y.Y.; Lee, K.H. Effects of Integrative Cognitive Function Improvement Program on Cognitive Function, Oral Health, and Mental Health in Older People: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 14339. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, C.; Shirobe, M.; Furuya, J.; Watanabe, Y.; Motokawa, K.; Edahiro, A.; Ohara, Y.; Awata, S.; Kim, H.; Fujiwara, Y.; et al. Effect of oral health intervention on cognitive decline in community-dwelling older adults: A randomized controlled trial. Arch. Gerontol. Geriatr. 2021, 92, 104267. [Google Scholar] [CrossRef]
- Kok, C.R.; Rose, D.; Hutkins, R. Predicting Personalized Responses to Dietary Fiber Interventions: Opportunities for Modulation of the Gut Microbiome to Improve Health. Annu. Rev. Food Sci. Technol. 2023, 14, 157–182. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; da Silveira, T.M.; Bezerra, B.; Pirih, F.Q.; Pola, N.M. Association between different dietary patterns and eating disorders and periodontal diseases. Front. Oral Health 2023, 4, 1152031. [Google Scholar] [CrossRef]
- Hansen, T.H.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.; Pedersen, O. Impact of a vegan diet on the human salivary microbiota. Sci. Rep. 2018, 8, 5847. [Google Scholar] [CrossRef]
- Tennert, C.; Reinmuth, A.C.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. MicrobiologyOpen 2020, 9, e1056. [Google Scholar] [CrossRef] [PubMed]
- Lula, E.; Ribeiro, C.; Hugo, F.; Alves, C.; Silva, A. Added sugars and periodontal disease in young adults: An analysis of NHANES III data. Am. J. Clin. Nutr. 2014, 100, 1182–1187. [Google Scholar] [CrossRef]
- Kondo, K.; Ishikado, A.; Morino, K.; Nishio, Y.; Ugi, S.; Kajiwara, S.; Kurihara, M.; Iwakawa, H.; Nakao, K.; Uesaki, S.; et al. A high-fiber, low-fat diet improves periodontal disease markers in high-risk subjects: A pilot study. Nutr. Res. 2014, 34, 491–498. [Google Scholar] [CrossRef]
- Altun, E.; Walther, C.; Borof, K.; Petersen, E.; Lieske, B.; Kasapoudis, D.; Jalilvand, N.; Beikler, T.; Jagemann, B.; Zyriax, B.-C.; et al. Association between Dietary Pattern and Periodontitis—A Cross-Sectional Study. Nutrients 2021, 13, 4167. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.C.; Fernandez, K.J.; Suresh, S.; Sudhakar, U.; Ramesh, S.; Balakrishnan, A. Garcinia mangostana as an adjunct to Non-Surgical Therapy on Chronic Schizophrenic patients with Periodontitis—A Miracle fruit. J. Pharm. Sci. Res. 2020, 12, 864–868. [Google Scholar]
- Nielsen, S.J.; Trak-Fellermeier, M.A.; Joshipura, K.; Dye, B.A. Dietary Fiber Intake Is Inversely Associated with Periodontal Disease among US Adults. J. Nutr. 2016, 146, 2530–2536. [Google Scholar] [CrossRef]
- Papathanasiou, E.; Alreshaid, R.; Araujo de Godoi, M. Anti-Inflammatory Benefits of Food Ingredients in Periodontal Diseases. Pathogens 2023, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2017, 17, 28. [Google Scholar] [CrossRef]
- Azzola, L.G.; Fankhauser, N.; Srinivasan, M. Influence of the vegan, vegetarian and omnivore diet on the oral health status in adults: A systematic review and meta-analysis. Evid.-Based Dent. 2023, 24, 43–44. [Google Scholar] [CrossRef]
- Pandya, V.S.; Fiorillo, L.; Kalpe, S.; Mehta, V.; Meto, A.; Di Certo, A.; Russo, D.; Gorassini, F.; Mancini, M.; Mancini, A.; et al. Veganism and Oral Health-An Overview through the Perspective. Eur. J. Gen. Dent. 2023, 12, 067–071. [Google Scholar] [CrossRef]
- Pärnänen, P.; Lomu, S.; Räisänen, I.T.; Tervahartiala, T.; Sorsa, T. Effects of Fermented Lingonberry Juice Mouthwash on Salivary Parameters-A One-Year Prospective Human Intervention Study. Dent. J. 2022, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, P.; Lomu, S.; Räisänen, I.T.; Tervahartiala, T.; Sorsa, T. Antimicrobial and Anti-Inflammatory Oral Effects of Fermented Lingonberry Juice-A One-Year Prospective Human Intervention Study. Eur. J. Dent. 2023, 17, 1235–1240. [Google Scholar] [CrossRef]
- Janket, S.-J.; Benwait, J.; Isaac, P.; Ackerson, L.K.; Meurman, J.H. Oral and Systemic Effects of Xylitol Consumption. Caries Res. 2019, 53, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Conz, A.; Salmona, M.; Diomede, L. Effect of Non-Nutritive Sweeteners on the Gut Microbiota. Nutrients 2023, 15, 1869. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Onyango, S.O.; De Clercq, N.; Beerens, K.; Van Camp, J.; Desmet, T.; Van de Wiele, T. Oral Microbiota Display Profound Differential Metabolic Kinetics and Community Shifts upon Incubation with Sucrose, Trehalose, Kojibiose, and Xylitol. Appl. Environ. Microbiol. 2020, 86, e01170-20. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.; Merenstein, D.; Pot, B.; Morelli, L.; Canani, R.; Flint, H.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e282–e288. [Google Scholar] [CrossRef]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on gingival health, dental plaque, and periodontopathogens in adolescents: A randomised placebo-controlled clinical trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef]
- Ferrer, M.D.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topical application of the probiotic Streptococcus dentisani improves clinical and microbiological parameters associated with oral health. Front. Cell. Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Kim, M.; Nam, S.-H.; Kang, M.-S.; Lee, S.-A. Effects of oral probiotics on subjective halitosis, oral health, and psychosocial health of college students: A randomized, double-blind, placebo-controlled study. Int. J. Environ. Res. Public Health 2021, 18, 1143. [Google Scholar] [CrossRef] [PubMed]
- Mughal, R. PRO-biotics? Are pre- and probiotics a valuable adjunct to fluoridated toothpaste in the battle against dental decay? Evid.-Based Dent. 2024, 25, 39–40. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, Y.T.; Ho, H.H.; Hsieh, P.S.; Kuo, Y.W.; Lin, J.H.; Liu, C.R.; Huang, Y.F.; Chen, C.W.; Hsu, C.H.; et al. Lozenges with probiotic strains enhance oral immune response and health. Oral Dis. 2021, 28, 1723–1732. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, Y.T.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Chen, J.F.; Lin, J.H.; Liu, C.R.; Lin, C.H.; Yeh, Y.T.; et al. Impact of the food grade heat-killed probiotic and postbiotic oral lozenges in oral hygiene. Aging 2022, 14, 2221–2238. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, P.; Magno, M.B.; Masterson, D.; Gomes da Cruz, A.; Maia, L.C. Are dairy products containing probiotics beneficial for oral health? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2763–2785. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Slomka, V.; Hernandez-Sanabria, E.; Herrero, E.R.; Zaidel, L.; Bernaerts, K.; Boon, N.; Quirynen, M.; Teughels, W. Nutritional stimulation of commensal oral bacteria suppresses pathogens: The prebiotic concept. J. Clin. Periodontol. 2017, 44, 344–352. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, B.; Huang, K.; Li, S.; Cao, H.; Guan, X. Bound polyphenols of oat bran released by gut microbiota mitigate high-fat diet-induced oxidative stress and strengthen the gut barrier via the colonic ROS/Akt/Nrf2 pathway. J. Agric. Food Chem. 2024, 72, 13099–13110. [Google Scholar] [CrossRef]
- Ayakdaş, G.; Ağagündüz, D. Microbiota-accessible carbohydrates (MACs) as novel gut microbiome modulators in noncommunicable diseases. Heliyon 2023, 9, e19888. [Google Scholar] [CrossRef] [PubMed]
- Touger-Decker, R.; van Loveren, C. Sugars and dental caries. Am. J. Clin. Nutr. 2003, 78, 881S–892S. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Lif Holgerson, P. Milk and oral health. In Milk and Milk Products in Human Nutrition; Nestlé Nutrition Institute Workshop Series. Pediatric Program; S. Karger AG: Basel, Switzerland, 2011; Volume 67, pp. 55–66. [Google Scholar] [CrossRef]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casa-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of chlorhexidine mouthwash on the oral microbiota. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Brookes, Z.; Teoh, L.; Cieplik, F.; Kumar, P. Mouthwash effects on the oral microbiota: Are they good, bad, or balanced? Int. Dent. J. 2023, 73 (Suppl S2), S74–S81. [Google Scholar] [CrossRef]
- Carvalho, L.R.R.A.; Boeder, A.M.; Shimari, M.; Kleschyov, A.L.; Esberg, A.; Johansson, I.; Weitzberg, E.; Lundberg, J.O.; Carlstrom, M. Antibacterial mouthwash alters gut microbiota, reducing nutrient absorption and fat accumulation in Western diet-fed mice. Sci. Rep. 2024, 14, 4025. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Xiao, H.; Zhang, G. Triclosan, a common antimicrobial ingredient, on gut microbiota and gut health. Gut Microbes 2019, 10, 434–437. [Google Scholar] [CrossRef]
- Shang, Q.; Gao, Y.; Qin, T.; Wang, S.; Shi, Y.; Chen, T. Interaction of oral and toothbrush microbiota affects oral cavity health. Front. Cell. Infect. Microbiol. 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Lyra, P.; Machado, V.; Proença, L.; Domingos, J.; Godinho, C.; Mendes, J.J.; Botelho, J. Parkinson’s Disease, Periodontitis and Patient-Related Outcomes: A Cross-Sectional Study. Medicina 2020, 56, 383. [Google Scholar] [CrossRef]
- Delwel, S.; Binnekade, T.; Perez, R.; Hertogh, C.; Scherder, E.; Lobbezoo, F. Oral hygiene and oral health in older people with dementia: A comprehensive review with focus on oral soft tissues. Clin. Oral Investig. 2018, 22, 93–108. [Google Scholar] [CrossRef]
- García-Ríos, P.; Pecci-Lloret, M.P.; Oñate-Sánchez, R.E. Oral Manifestations of Systemic Lupus Erythematosus: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11910. [Google Scholar] [CrossRef]
- Molania, T.; Alimohammadi, M.; Akha, O.; Mousavi, J.; Razvini, R.; Salehi, M. The effect of xerostomia and hyposalivation on the quality of life of patients with type II diabetes mellitus. Electron. Physician 2017, 9, 5814–5819. [Google Scholar] [CrossRef] [PubMed]
- Samnieng, P.; Ueno, M.; Shinada, K.; Zaitsu, T.; Wright, F.A.; Kawaguchi, Y. Association of hyposalivation with oral function, nutrition and oral health in community-dwelling elderly Thai. Community Dent. Health 2012, 29, 117–123. [Google Scholar] [PubMed]
- Buranarom, N.; Komin, O.; Matangkasombut, O. Hyposalivation, oral health, and Candida colonization in independent dentate elders. PLoS ONE 2020, 15, e0242832. [Google Scholar] [CrossRef] [PubMed]
- Khovidhunkit, S.O.; Suwantuntula, T.; Thaweboon, S.; Mitrirattanakul, S.; Chomkhakhai, U.; Khovidhunkit, W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J. Med. Assoc. Thai. 2009, 92, 1220–1228. [Google Scholar]
- Aga, O.O.; Bolstad, A.I.; Lie, S.A.; Svanes Fevang, B.T. Periodontitis in patients with primary Sjögren’s syndrome: A nation-wide register study. Eur. J. Oral Sci. 2023, 131, e12950. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Sun, P.; Liu, Y.; Hua, W. Periodontitis and Sjogren’s syndrome: A bidirectional two-sample mendelian randomization study. BMC Oral Health 2024, 24, 380. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Northridge, M.E.; Kumar, A.; Kaur, R. Disparities in Access to Oral Health Care. Annu. Rev. Public Health 2020, 41, 513–535. [Google Scholar] [CrossRef]
| Reference | Study Design | Participant Characteristics | Mental/Cognitive Health | Intervention | Impact/s of Intervention |
|---|---|---|---|---|---|
| Chen et al. 2022 [125] | RCT | Intervention N = 33 (10 M, 23 F, average age 82.70). Control N = 33 (9 M, 24 F, average age 83). | AD | Structured visits (three times/week), oral self-care (three times/week), and self-management training (45 min session, once a week) for 24 weeks, facilitated by educators from medical schools/teaching hospitals. | The intervention group had improvements across Kayser-Jones BOHSE, NPI, MMSE, NHAS, and ADCS-ADL scores. The overall oral microbiota composition of the intervention group was improved, and pathogenic bacteria were reduced. |
| Hamid et al. 2021 [117] | quasi-RCT | Intervention N = 60 (23 M, 27 F, average age 10.9). Control N = 58 (20 M, 38 F, average age 11.1). | PTSD | A combination of psychosocial support (eight sessions) with social workers and oral health education (four sessions) with a pediatric dentist over a six-week period. | Oral health measures: The intervention group had significantly lower PI (1.52 ± 0.55) and GI (1.48 ± 0.56) compared to the control group (PI = 1.89 ± 0.39, GI = 2.14 ± 0.32). Mental health measures: CPQ11–14 scores were significantly lower in the intervention group (47.16 ± 12.24) compared to the control group (72.65 ± 14.47). CPTSD-RI was significantly decreased in the intervention group (34.41 ± 12.23) compared to the control group (47.91 ± 14.24). |
| Jung et al. 2022 [126] | RCT | Intervention groups: CN, N = 18 (3 M, 15 F). MCI, N = 17 (2 M, 15 F). Control Group N = 17 (1 M, 16 F). Participants ranged in age from 65–85+ years. | MCI | Two 90-min group-learning sessions per week, for six weeks. Activities included 30 min each of:
| Cognitive/Mental health measures: AL, indicative of the level of resistance to extrinsic disease and stress; PT, indicative of muscular or mental tension; MD, indicative of anxiety, tension, or excitation. CN and MCI groups had significant improvements in AL and PT post-intervention. There were no significant MD differences in any group. Happiness in old age scores increased by 6.94 and 7.30 points in the CN and MCI groups, respectively. Oral health measures: The mean O’Leary index score (dental plaque formation) decreased by 0.42 and 0.40 points in the CN and MCI groups, respectively, and the Löe and Silness index score (gingivitis) decreased by 0.47 and 0.48 in the CN and MCI groups, respectively. Saliva flow rate increased by 0.13 g/min and 0.15 g/min in the CN and MCI groups, respectively. |
| Matsubara et al. 2021 [127] | Single-blind RCT | Intervention N = 25 (6 M, 19 F, average age 76). Control N = 25 (M = 5, F = 20, average age 74). | MCI | The intervention group received a monthly one-on-one oral health intervention for eight months, which included oral hygiene instructions and oral function exercises administered by dental hygienists. To reduce bias (due to positive effects of social interaction on cognitive health), the control group received a group health promotion intervention for 60 min twice a month, consisting of lectures regarding physical activity, cognitive function, and nutrition (but not oral health) delivered by a clinical psychologist, a registered dietitian, and a physical therapist. | The KCL (a comprehensive measure of total health, such as a decline in physical, cognitive and oral function, malnutrition, and depression) scores significantly improved in the intervention group, but not the control group. TMT-A and TMT-B assess attention function, speed of cognitive processing, and executive function, with the TMT-B considered more difficult as it requires a more complex cognitive function. Both groups showed improvements in TMT-A, however, only the intervention group showed improvements in TMT-B. The intervention group also showed significant improvements in periodontal disease scores and oral function. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerstens, R.; Ng, Y.Z.; Pettersson, S.; Jayaraman, A. Balancing the Oral–Gut–Brain Axis with Diet. Nutrients 2024, 16, 3206. https://doi.org/10.3390/nu16183206
Kerstens R, Ng YZ, Pettersson S, Jayaraman A. Balancing the Oral–Gut–Brain Axis with Diet. Nutrients. 2024; 16(18):3206. https://doi.org/10.3390/nu16183206
Chicago/Turabian StyleKerstens, Rebecca, Yong Zhi Ng, Sven Pettersson, and Anusha Jayaraman. 2024. "Balancing the Oral–Gut–Brain Axis with Diet" Nutrients 16, no. 18: 3206. https://doi.org/10.3390/nu16183206
APA StyleKerstens, R., Ng, Y. Z., Pettersson, S., & Jayaraman, A. (2024). Balancing the Oral–Gut–Brain Axis with Diet. Nutrients, 16(18), 3206. https://doi.org/10.3390/nu16183206






