The Physiological and Performance Effects of Actovegin during Maximal Cardiopulmonary Exercise Testing: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Procedures
2.2.1. Body Composition
2.2.2. Cardiopulmonary Exercise Test
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machicao, F.; Muresanu, D.F.; Hundsberger, H.; Pflüger, M.; Guekht, A. Pleiotropic neuroprotective and metabolic effects of Actovegin’s mode of action. J. Neurol. Sci. 2012, 322, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, E.; Sotonyi, P.; Nemes, A. The effects of a deproteinized blood extract on the myocardial changes developing during experimentally induced intermittent hypoxia. Arzneimittelforschung 1979, 29, 1376–1381. [Google Scholar] [PubMed]
- Buchmayer, F.; Pleiner, J.; Elmlinger, M.W.; Lauer, G.; Nell, G.; Sitte, H.H. Actovegin®: A biological drug for more than 5 decades. Wien. Med. Wochenschr. 2011, 161, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Movsesyan, L.; Mankovsky, B.; Gurieva, I.; Abylaiuly, Z.; Strokov, I. Treatment of symptomatic polyneuropathy with actovegin in type 2 diabetic patients. Diabetes Care 2009, 32, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.; Golding, D.; Smith, P.M.; Nokes, L.; Kwan, A.; Lee, P.Y. Update on the role of Actovegin in musculoskeletal medicine: A review of the past 10 years. Clin. J. Sport Med. 2020, 30, 83–90. [Google Scholar] [CrossRef]
- Kosik, B.; Larsen, S.; Bergdahl, A. Actovegin improves skeletal muscle mitochondrial respiration and functional aerobic capacity in a type 1 diabetic male murine model. Appl. Physiol. Nutr. Metab. 2023, 49, 265–272. [Google Scholar] [CrossRef]
- Reichl, F.-X.; Holdt, L.M.; Teupser, D.; Schütze, G.; Metcalfe, A.J.; Hickel, R.; Högg, C.; Bloch, W. Comprehensive analytics of Actovegin® and its effect on muscle cells. Int. J. Sports Med. 2017, 38, 809–818. [Google Scholar] [CrossRef]
- Lee, P.; Rattenberry, A.; Connelly, S.; Nokes, L. Our experience on Actovegin, is it cutting edge? Int. J. Sports Med. 2011, 32, 237–241. [Google Scholar] [CrossRef]
- Milovanović, D.; Stojanović, E. The implications of Actovegin® in sport: A brief review. Exp. Appl. Biomed. Res. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Maillo, L. Anaphylactic shock with multiorgan failure in a cyclist after intravenous administration of Actovegin. Ann. Intern. Med. 2008, 148, 407. [Google Scholar] [CrossRef]
- United States Anti-Doping Agency. Available online: https://www.usada.org/wpcontent/uploads/Vaughters-Jonathan-Affidavit.pdf (accessed on 25 February 2024).
- Court of Arbitration for Sport. Available online: https://jurisprudence.tas-cas.org/Shared%20Documents/5045.pdf (accessed on 25 February 2024).
- Court of Arbitration for Sport. Available online: https://www.sennferrero.com/descargaspdf/tas-cas/201704/4708.pdf (accessed on 25 February 2024).
- McArdle, D. Doping and Human Rights in Pariah States. In Yearbook of International Sports Arbitration 2017: Doping and Human Rights in Pariah States; Duval, A., Rigozzi, A., Eds.; Asser Press: Hague, The Netherlands, 2021; pp. 29–49. [Google Scholar]
- World Anti-Doping Agency. Available online: https://www.wada-ama.org/sites/default/files/resources/files/WADA_Investigations_Guidelines_May2011_EN.pdf (accessed on 25 February 2024).
- Curtis, A.; Gerrard, D.; Burt, P.; Osborne, H. Drug misuse in sport: A New Zealand perspective. N. Z. Med. J. 2015, 128, 62–68. [Google Scholar]
- Søndergård, S.D.; Dela, F.; Helge, J.W.; Larsen, S. Actovegin, a non-prohibited drug increases oxidative capacity in human skeletal muscle. Eur. J. Sport Sci. 2016, 16, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Nokes, L.; Smith, P. No effect of intravenous Actovegin® on peak aerobic capacity. Int. J. Sports Med. 2012, 33, 305–309. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform 2021, 17, 317–331. [Google Scholar] [CrossRef]
- Dumke, C. Health-related Physical Fitness Testing and Interpretation. In ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J., Liguori, G., Magal, M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Talevi, A.; Quiroga, A. Introduction. Biopharmaceutics and Pharmacokinetics. In ADME Processes in Pharmaceutical Sciences: Dosage, Design, and Pharmacotherapy Success; Talevi, A., Quiroga, P., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Soulliard, Z.A.; Kauffman, A.A.; Fitterman-Harris, H.F.; Perry, J.E.; Ross, M.J. Examining positive body image, sport confidence, flow state, and subjective performance among student athletes and non-athletes. Body Image 2019, 28, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Simms, M.; Arnold, R.; Turner, J.E.; Hays, K. A repeated-measures examination of organizational stressors, perceived psychological and physical health, and perceived performance in semi-elite athletes. J. Sports Sci. 2021, 39, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Almagro, B.J.; Sáenz-López, P.; Fierro-Suero, S.; Conde, C. Perceived performance, intrinsic motivation and adherence in athletes. Int. J. Environ. Res. Public Health 2020, 17, 9441. [Google Scholar] [CrossRef]
- Nicholls, A.R.; Polman, R.; Levy, A.R. Coping self-efficacy, pre-competitive anxiety, and subjective performance among athletes. Eur. J. Sport Sci. 2010, 10, 97–102. [Google Scholar] [CrossRef]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and agreement of various InBody body composition analyzers as compared to dual-energy X-ray absorptiometry in healthy men and women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- Silva, S.C.; Monteiro, W.D.; Cunha, F.A.; Farinatti, P. Influence of Different Treadmill Inclinations on Vo2max and Ventilatory Thresholds During Maximal Ramp Protocols. J. Strength Cond. Res. 2021, 35, 233–239. [Google Scholar] [CrossRef]
- Beltz, N.M.; Gibson, A.L.; Janot, J.M.; Kravitz, L.; Mermier, C.M.; Dalleck, L.C. Graded exercise testing protocols for the determination of VO 2 max: Historical perspectives, progress, and future considerations. J. Sports Med. 2016, 2016, 3968393. [Google Scholar] [CrossRef]
- Kinnear, W.; Blakely, J. A Practical Guide to Cardiopulmonary Exercise Testing; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Allard, N.A.; Schirris, T.J.; Verheggen, R.J.; Russel, F.G.; Rodenburg, R.J.; Smeitink, J.A.; Thompson, P.D.; Hopman, M.T.; Timmers, S. Statins affect skeletal muscle performance: Evidence for disturbances in energy metabolism. J. Clin. Endocrinol. Metab. 2018, 103, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Cavigli, L.; Pagliaro, A.; Valente, S.; Valentini, F.; Cameli, M.; Focardi, M.; Mochi, N.; Dendale, P.; Hansen, D. The importance of ventilatory thresholds to define aerobic exercise intensity in cardiac patients and healthy subjects. Scand. J. Med. Sci. Sports 2021, 31, 1796–1808. [Google Scholar] [CrossRef]
- Schneider, D.A.; Phillips, S.E.; Stoffolano, S. The simplified V-slope method of detecting the gas exchange threshold. Med. Sci. Sports Exerc. 1993, 25, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J. An effect size primer: A guide for clinicians and researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Beedie, C.J. Placebo effects in competitive sport: Qualitative data. J. Sports Sci. Med. 2007, 6, 21. [Google Scholar] [PubMed]
- Hurst, P.; Schipof-Godart, L.; Szabo, A.; Raglin, J.; Hettinga, F.; Roelands, B.; Lane, A.; Foad, A.; Coleman, D.; Beedie, C. The placebo and nocebo effect on sports performance: A systematic review. Eur. J. Sport Sci. 2020, 20, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Mychka, V.; Mamyrbaeva, K.; Sergienko, V.; Masenko, V.; Chazova, I. Cerebrovascular events primary prevention in metabolic syndrome patients. Cardiovasc. Ther. Prev. 2006, 5, 33–39. [Google Scholar]
- Cardoso, C.G., Jr.; Gomides, R.S.; Queiroz, A.C.C.; Pinto, L.G.; da Silveira Lobo, F.; Tinucci, T.; Mion Jr, D.; de Moraes Forjaz, C.L. Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics 2010, 65, 317–325. [Google Scholar] [CrossRef]
- Tari, S.H.; Sohouli, M.H.; Lari, A.; Fatahi, S.; Rahideh, S.T. The effect of inositol supplementation on blood pressure: A systematic review and meta-analysis of randomized-controlled trials. Clin. Nutr. ESPEN 2021, 44, 78–84. [Google Scholar] [CrossRef]
- Lee, P.; Kwan, A.; Nokes, L. Actovegin®-Cutting-edge Sports Medicine or” Voodoo” Remedy? Curr. Sports Med. Rep. 2011, 10, 186–190. [Google Scholar] [CrossRef]

| Characteristic | Placebo Group (n = 8) | Actovegin Group (n = 8) | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Weekly training frequency (n) | 4.5 ± 1.2 | 4.4 ± 1.1 | 0.1 (−1.1 to 1.3) | 0.828 |
| Sex, males/females (n) | 5/3 | 5/3 | 1.000 | |
| Age (years) | 19.0 ± 0.0 | 19.3 ± 0.7 | −0.3 (−0.8 to 0.3) | 0.351 |
| Body height (cm) | 180.7 ± 10.1 | 176.8 ± 11.2 | 3.9 (−7.6 to 15.4) | 0.477 |
| Body mass at baseline (kg) | 76.3 ± 15.6 | 72.9 ± 16.9 | 3.3 (−14.1 to 20.8) | 0.688 |
| Body mass after 7 days (kg) | 76.5 ± 15.7 | 73.0 ± 16.1 | 3.5 (−13.5 to 20.6) | 0.664 |
| Body fat at baseline (%) | 19.2 ± 9.3 | 15.6 ± 6.2 | 3.7 (5.0 to 12.3) | 0.373 |
| Body fat after 7 days (%) | 19.0 ± 8.6 | 15.6 ± 6.7 | 3.4 (5.0 to 11.7) | 0.401 |
| Muscle mass at baseline (%) | 45.6 ± 5.9 | 47.6 ± 4.7 | −2.0 (−7.7 to 3.7) | 0.462 |
| Muscle mass after 7 days (%) | 45.8 ± 5.6 | 47.7 ± 5.0 | −1.9 (−7.6 to 3.8) | 0.493 |
| Body mass index at baseline (kg·m−2) | 23.3 ± 3.9 | 23.0 ± 2.9 | 0.3 (−3.4 to 4.0) | 0.864 |
| Body mass index after 7 days (kg·m−2) | 23.4 ± 3.9 | 23.1 ± 2.8 | 0.3 (−3.5 to 4.0) | 0.887 |
| Variable | Placebo Group (n = 8) | Actovegin Group (n = 8) | Interaction | Time | Group | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Administration | Baseline | Post-Administration | p | p | p | ||||
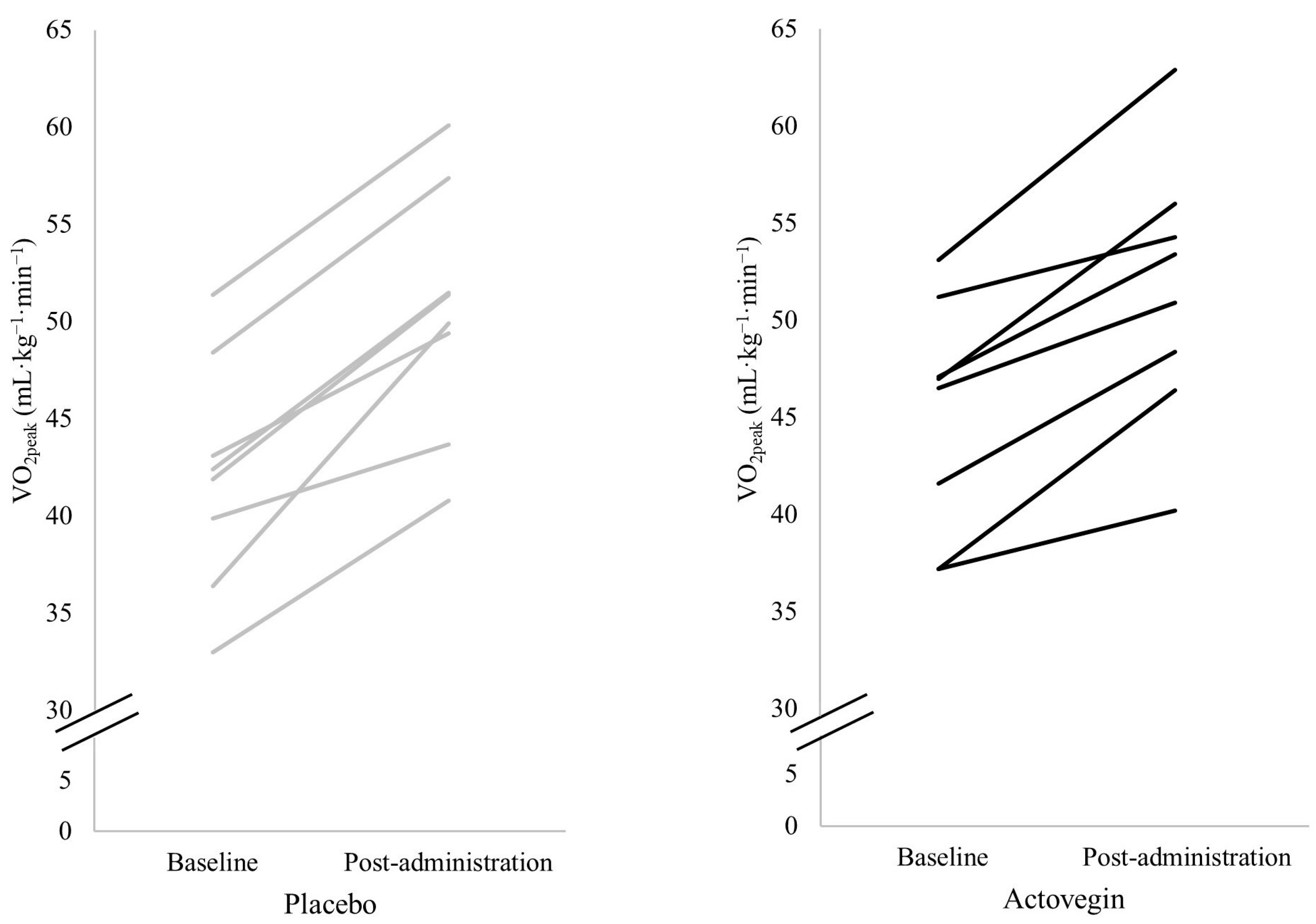
| VO2peak (mL·kg−1·min−1) | 42.1 ± 5.9 | 50.3 ± 6.4 | 45.1 ± 6.0 | 51.6 ± 6.8 | 0.167 | 0.132 | <0.001 | 0.893 | 0.515 | 0.031 |
| RERpeak | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 0.344 | 0.064 | <0.001 | 0.695 | 0.439 | 0.043 |
| Tpeak (s) | 603.6 ± 100.0 | 607.3 ± 97.2 | 606.6 ± 82.5 | 656.1 ± 88.9 | 0.212 | 0.109 | 0.152 | 0.141 | 0.554 | 0.026 |
| HRpeak (beats·min−1) | 193.3 ± 2.8 | 193.5 ± 3.9 | 192.9 ± 9.4 | 191.4 ± 7.0 | 0.381 | 0.055 | 0.529 | 0.029 | 0.686 | 0.012 |
| HR 1st minute (beats·min−1) | 168.9 ± 11.8 | 171.8 ± 3.9 | 170.8 ± 9.4 | 171.6 ± 7.9 | 0.648 | 0.015 | 0.397 | 0.052 | 0.822 | 0.004 |
| HR 2nd minute (beats·min−1) | 149.5 ± 12.7 | 149.1 ± 8.0 | 148.4 ± 10.5 | 147.1 ± 12.0 | 0.902 | 0.001 | 0.819 | 0.004 | 0.718 | 0.010 |
| HR 3rd minute (beats·min−1) | 135.6 ± 12.9 | 137.9 ± 7.5 | 133.4 ± 11.4 | 132.9 ± 11.7 | 0.672 | 0.013 | 0.787 | 0.005 | 0.436 | 0.044 |
| Systolic pressure (mmHg) | 151.3 ± 21.8 | 151.9 ± 18.3 | 161.9 ± 20.0 | 146.3 ± 22.6 | 0.036 | 0.278 | 0.050 | 0.247 | 0.802 | 0.005 |
| Diastolic pressure (mmHg) | 76.9 ± 7.5 | 76.9 ± 7.0 | 78.1 ± 5.9 | 73.1 ± 7.0 | 0.373 | 0.057 | 0.373 | 0.057 | 0.568 | 0.024 |
| VEpeak (L·min−1) | 120.0 ± 35.1 | 137.0 ± 30.9 | 122.1 ± 36.1 | 136.9 ± 42.6 | 0.826 | 0.004 | 0.004 | 0.456 | 0.957 | 0.000 |
| VEqVCO2 | 25.4 ± 3.9 | 28.0 ± 3.7 | 26.8 ± 5.1 | 28.7 ± 4.7 | 0.524 | 0.030 | 0.002 | 0.517 | 0.629 | 0.017 |
| VO2/HR (mL·beat−1) | 20.4 ± 7.6 | 22.5 ± 7.5 | 21.1 ± 6.6 | 22.9 ± 7.2 | 0.756 | 0.007 | 0.006 | 0.434 | 0.877 | 0.002 |
| HR at VT1 (beats·min−1) | 167.3 ± 19.2 | 173.1 ± 19.1 | 173.9 ± 10.2 | 172.8 ± 12.7 | 0.200 | 0.114 | 0.377 | 0.056 | 0.682 | 0.012 |
| Velocity at VT1 (km·h−1) | 8.8 ± 1.8 | 9.3 ± 2.0 | 9.9 ± 1.4 | 9.6 ± 1.2 | 0.165 | 0.133 | 0.811 | 0.004 | 0.353 | 0.062 |
| VO2 at VT1 (mL·kg−1·min−1) | 33.5 ± 6.8 | 38.5 ± 8.0 | 38.1 ± 6.5 | 41.7 ± 4.8 | 0.514 | 0.031 | 0.002 | 0.520 | 0.232 | 0.100 |
| HR at VT2 (beats·min−1) | 184.3 ± 11.4 | 188.8 ± 7.7 | 188.6 ± 9.3 | 185.4 ± 10.4 | 0.070 | 0.216 | 0.756 | 0.007 | 0.913 | 0.001 |
| Velocity at VT2 (km·h−1) | 10.9 ± 2.2 | 11.9 ± 1.7 | 12.3 ± 1.9 | 12.8 ± 1.4 | 0.492 | 0.034 | <0.001 | 0.997 | 0.362 | 0.060 |
| VO2 at VT2 (mL·kg−1·min−1) | 41.1 ± 7.9 | 49.7 ± 8.2 | 46.3 ± 8.1 | 51.4 ± 5.4 | 0.230 | 0.101 | <0.001 | 0.628 | 0.337 | 0.066 |
| Vpeak (km·h−1) | 12.4 ± 1.6 | 12.9 ± 1.7 | 13.0 ± 1.6 | 13.4 ± 1.5 | 0.678 | 0.013 | 0.010 | 0.386 | 0.491 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanović, D.; Radovanović, D.; Živković, V.; Srejović, I.; Glišić, M.; Jakovljević, V.; Scanlan, A.; Ponorac, N.; Stojanović, E. The Physiological and Performance Effects of Actovegin during Maximal Cardiopulmonary Exercise Testing: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 3332. https://doi.org/10.3390/nu16193332
Milovanović D, Radovanović D, Živković V, Srejović I, Glišić M, Jakovljević V, Scanlan A, Ponorac N, Stojanović E. The Physiological and Performance Effects of Actovegin during Maximal Cardiopulmonary Exercise Testing: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(19):3332. https://doi.org/10.3390/nu16193332
Chicago/Turabian StyleMilovanović, Dragana, Dragan Radovanović, Vladimir Živković, Ivan Srejović, Miloš Glišić, Vladimir Jakovljević, Aaron Scanlan, Nenad Ponorac, and Emilija Stojanović. 2024. "The Physiological and Performance Effects of Actovegin during Maximal Cardiopulmonary Exercise Testing: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 19: 3332. https://doi.org/10.3390/nu16193332
APA StyleMilovanović, D., Radovanović, D., Živković, V., Srejović, I., Glišić, M., Jakovljević, V., Scanlan, A., Ponorac, N., & Stojanović, E. (2024). The Physiological and Performance Effects of Actovegin during Maximal Cardiopulmonary Exercise Testing: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 16(19), 3332. https://doi.org/10.3390/nu16193332







