Comparative Effectiveness of Ascorbic Acid vs. Calcium Ascorbate Ingestion on Pharmacokinetic Profiles and Immune Biomarkers in Healthy Adults: A Preliminary Study
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Participants
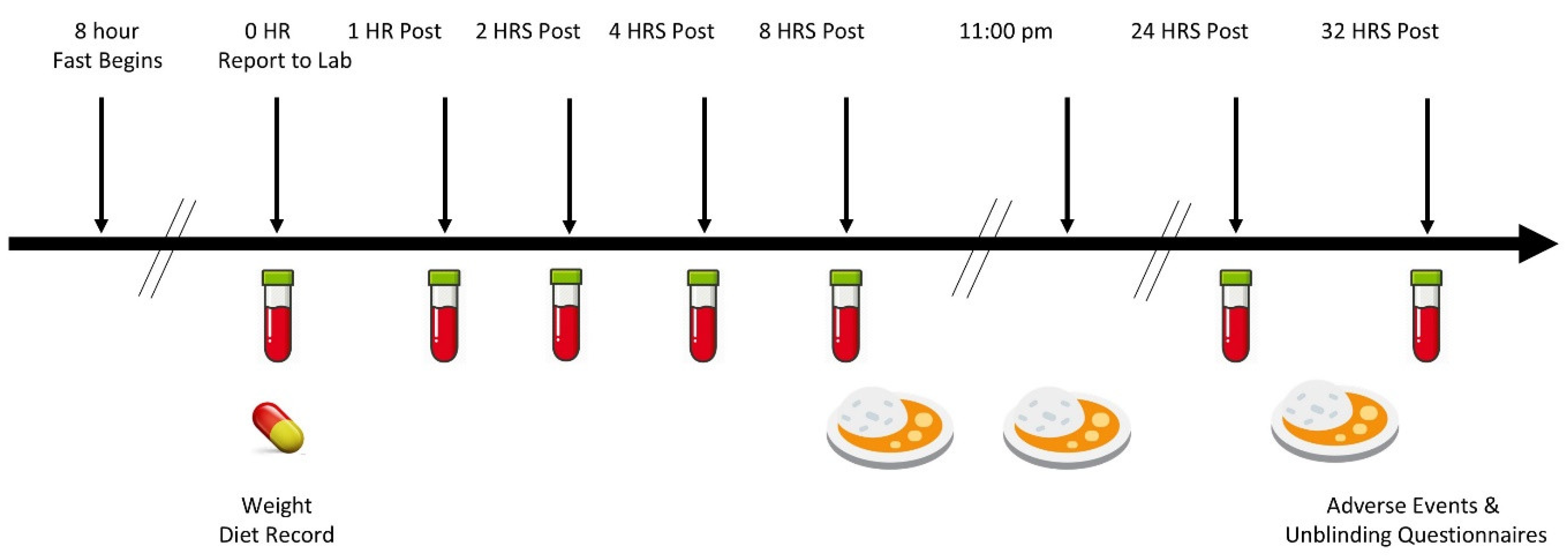
2.3. Experimental Timeline
2.4. Supplementation Procedure
3. Procedures
3.1. Descriptives
3.2. Blood Collection
3.3. Whole Blood and Serum Analysis
3.4. Vitamin C Analysis
3.5. Pharmacokinetic Analysis
3.6. Neutrophil Phagocytosis and Cell Differentiation Analysis
3.7. Statistical Analysis
4. Results
4.1. Descriptive Data
4.2. Baseline Serum Health Markers
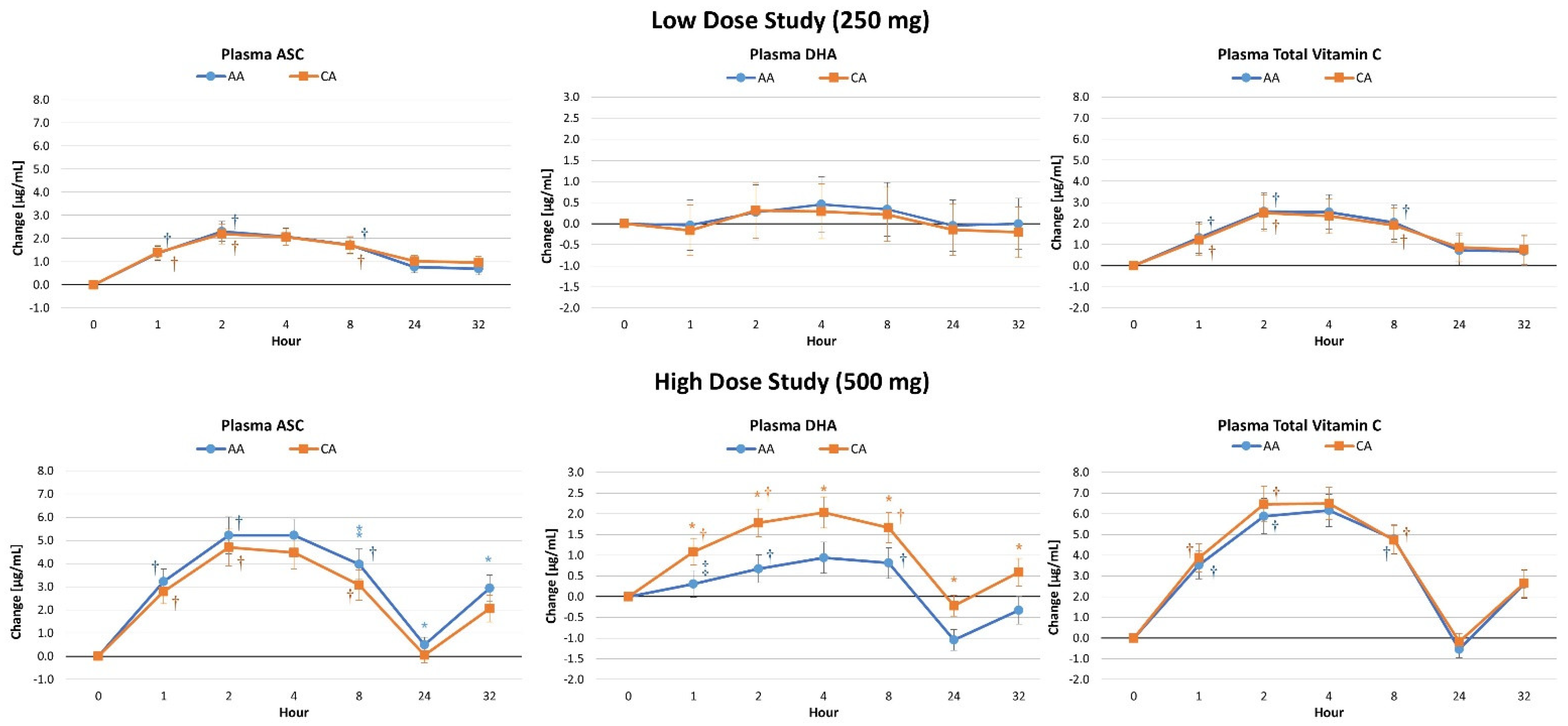
4.3. Plasma Vitamin C Concentrations
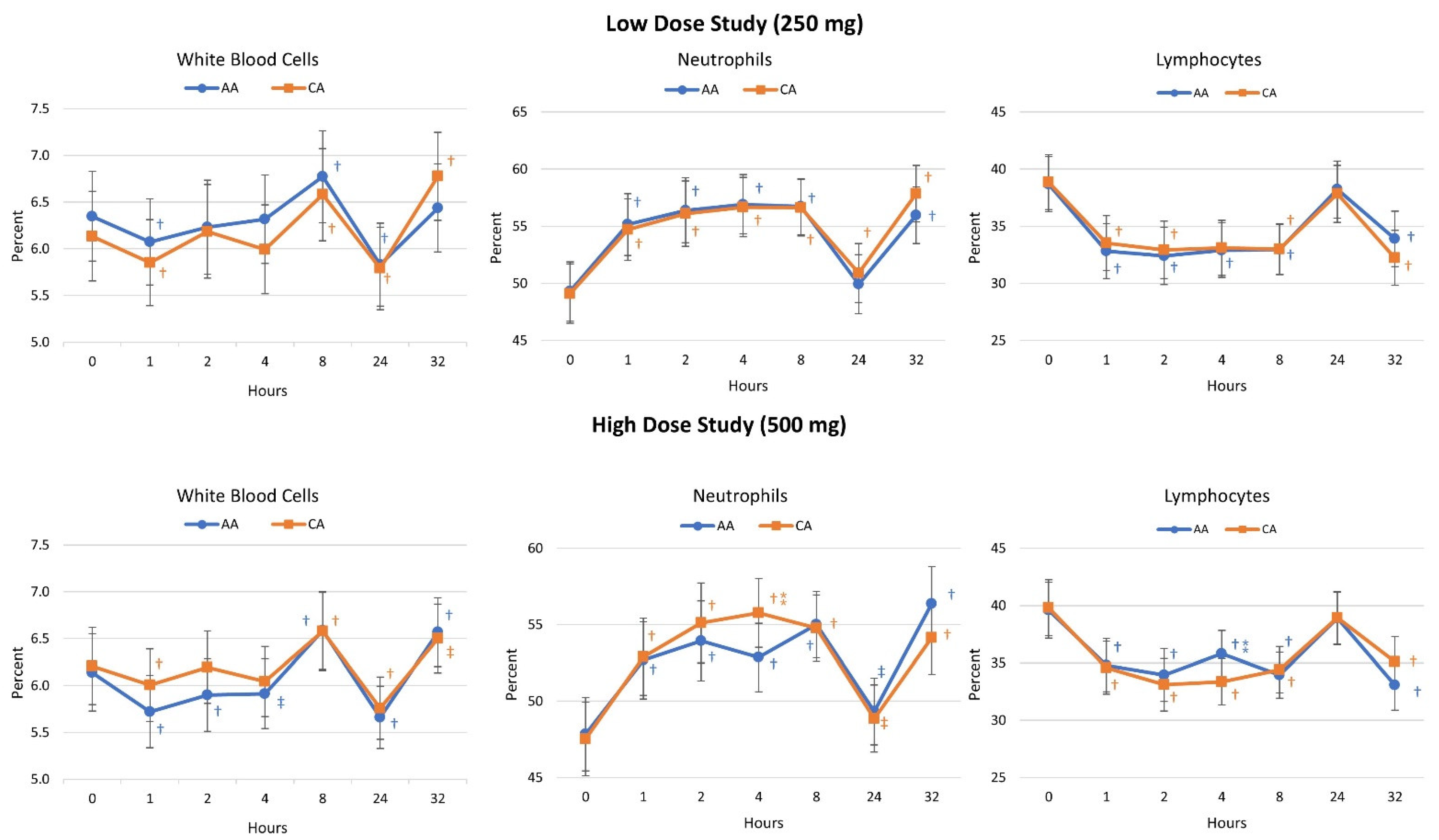
4.4. Lymphocyte Concentrations
4.5. Cell Blood Count Analysis
4.6. Pharmacokinetic Analysis
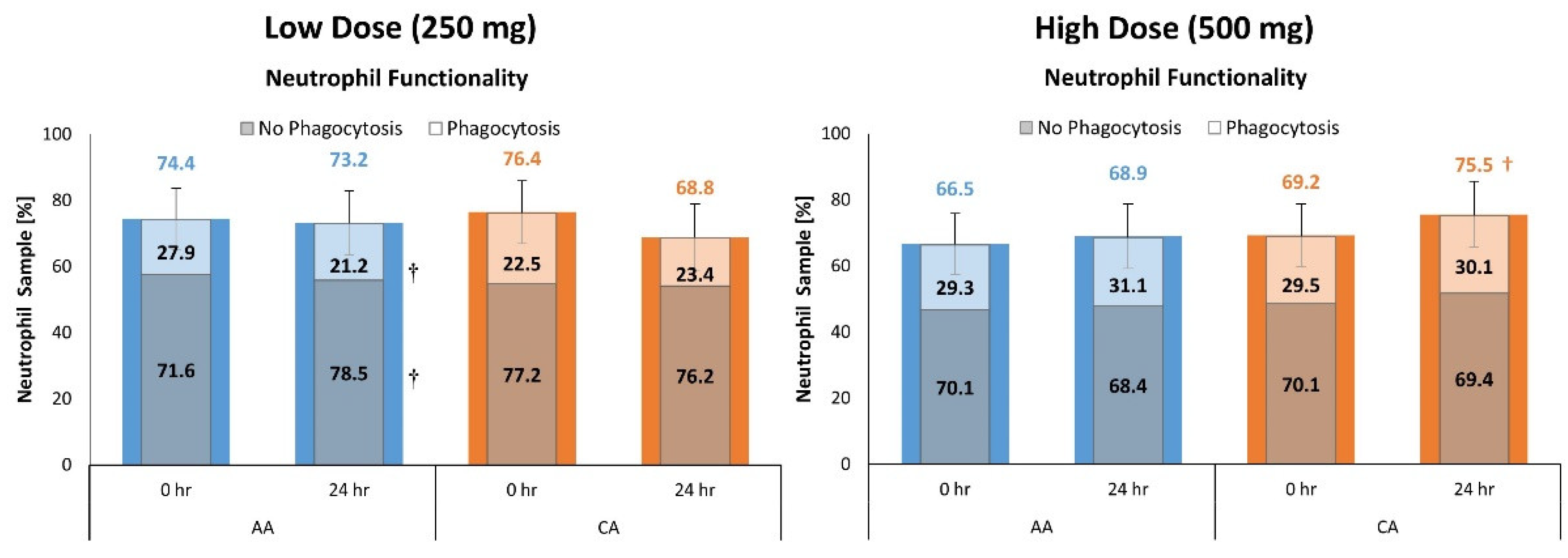
4.7. Neutrophil Functionality
4.8. Lymphocyte Cell Differentiation
4.9. Side Effects
5. Discussion
5.1. Primary Outcomes
5.2. Secondary Outcomes
5.3. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitmesser, S.H.; Ye, Q.; Evans, M.; Combs, M. Determination of plasma and leukocyte vitamin C concentrations in a randomized, double-blind, placebo-controlled trial with Ester-C(®). Springerplus 2016, 5, 1161. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Combs, M.A.; Vrablic, A.S.; Velasquez, J.; Turner, B.; Bernal, S. Vitamin C metabolites, independent of smoking status, significantly enhance leukocyte, but not plasma ascorbate concentrations. Adv. Ther. 2008, 25, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Mitmesser, S.; Combs, M.; Evans, M. Determination of plasma and leukocyte ascorbate concentrations in a randomized, double-blind trial (830.3). FASEB J. 2014, 28, 830.3. [Google Scholar] [CrossRef]
- Ye, Q.; Combs, M.; Mitmesser, S. Effects of Ester-C® and Ascorbic Acid on Gastrointestinal Outcomes: A Randomized, Double-Blind Trial. FASEB J. 2015, 29, 920.8. [Google Scholar] [CrossRef]
- Van Straten, M.; Josling, P. Preventing the common cold with a vitamin C supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2002, 19, 151–159. [Google Scholar] [CrossRef]
- Gruenwald, J.; Graubaum, H.J.; Busch, R.; Bentley, C. Safety and tolerance of ester-C compared with regular ascorbic acid. Adv. Ther. 2006, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Combs, M.A.; Crowley, D.C.; Baisley, J.E.; Sharma, P.; Vrablic, A.S.; Evans, M. Vitamin C with metabolites reduce oxalate levels compared to ascorbic acid: A preliminary and novel clinical urologic finding. Urol. Nurs. 2009, 29, 95–102. [Google Scholar]
- Purpura, M.; Jager, R.; Godavarthi, A.; Bhaskarachar, D.; Tinsley, G.M. Liposomal delivery enhances absorption of vitamin C into plasma and leukocytes: A double-blind, placebo-controlled, randomized trial. Eur. J. Nutr. 2024. [Google Scholar] [CrossRef]
- Ko, J.; Yoo, C.; Xing, D.; Gonzalez, D.E.; Jenkins, V.; Dickerson, B.; Leonard, M.; Nottingham, K.; Kendra, J.; Sowinski, R.; et al. Pharmacokinetic Analyses of Liposomal and Non-Liposomal Multivitamin/Mineral Formulations. Nutrients 2023, 15, 3073. [Google Scholar] [CrossRef]
- Wen, C.J.; Chiang, C.F.; Lee, C.S.; Lin, Y.H.; Tsai, J.S. Double Nutri (Liposomal Encapsulation) Enhances Bioavailability of Vitamin C and Extends Its Half-Life in Plasma. J. Biomed. Nanotechnol. 2022, 18, 922–927. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J. Liposome Res. 2021, 31, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Hickey, S.; Roberts, H.J.; Miller, N.J. Pharmacokinetics of oral vitamin C. J. Nutr. Environ. Med. 2008, 17, 169–177. [Google Scholar] [CrossRef]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.; Preut, R.; Schommer, K.; Schürmeyer, T.H. A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 2002, 159, 319–324. [Google Scholar] [CrossRef]
- Møller, P.; Viscovich, M.; Lykkesfeldt, J.; Loft, S.; Jensen, A.; Poulsen, H.E. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. Eur. J. Nutr. 2004, 43, 267–274. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef]
- Levy, R.; Shriker, O.; Porath, A.; Riesenberg, K.; Schlaeffer, F. Vitamin C for the treatment of recurrent furunculosis in patients with imparied neutrophil functions. J. Infect. Dis. 1996, 173, 1502–1505. [Google Scholar] [CrossRef]
- Jayachandran, M.; Rani, P.J.A.; Arivazhagan, P.; Panneerselvam, C. Neutrophil Phagocytic Function and Humoral Immune Response with Reference to Ascorbate Supplementation in Aging Humans. J. Anti-Aging Med. 2000, 3, 37–42. [Google Scholar] [CrossRef]
- Kim, B.; Stein, H. A spreadsheet program for making a balanced Latin Square design. Rev. Colomb. Cienc. Pecu. 2009, 22, 591–596. [Google Scholar] [CrossRef]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer | Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Smirnov, A.; Solga, M.D.; Lannigan, J.; Criss, A.K. Using Imaging Flow Cytometry to Quantify Neutrophil Phagocytosis. Methods Mol. Biol. 2020, 2087, 127–140. [Google Scholar] [CrossRef]
- Lamoreaux, L.; Roederer, M.; Koup, R. Intracellular cytokine optimization and standard operating procedure. Nat. Protoc. 2006, 1, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Mahnke, Y.D.; Beddall, M.H.; Roederer, M. OMIP-029: Human NK-cell phenotypization. Cytom. A 2015, 87, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A., 2nd; Seder, R.A. OMIP-067: 28-Color Flow Cytometry Panel to Evaluate Human T-Cell Phenotype and Function. Cytom. A 2020, 97, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. Bmj 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Drinkwater, E. Applications of confidence limits and effect sizes in sport research. Open Sports Sci. J. 2008, 1, 3–4. [Google Scholar] [CrossRef]
- Earnest, C.P.; Roberts, B.M.; Harnish, C.R.; Kutz, J.L.; Cholewa, J.M.; Johannsen, N.M. Reporting Characteristics in Sports Nutrition. Sports 2018, 6, 139. [Google Scholar] [CrossRef]
- Grabowski, B. “P < 0.05” Might Not Mean What You Think: American Statistical Association Clarifies P Values. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726–736. [Google Scholar]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J. Anaesth. 2021, 15, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Elste, V.; Troesch, B.; Eggersdorfer, M.; Weber, P. Emerging Evidence on Neutrophil Motility Supporting Its Usefulness to Define Vitamin C Intake Requirements. Nutrients 2017, 9, 503. [Google Scholar] [CrossRef]
- Douglas, R.M.; Hemila, H.; Chalker, E.; Treacy, B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2007, CD000980. [Google Scholar] [CrossRef]
- Constantini, N.W.; Dubnov-Raz, G.; Eyal, B.B.; Berry, E.M.; Cohen, A.H.; Hemila, H. The effect of vitamin C on upper respiratory infections in adolescent swimmers: A randomized trial. Eur. J. Pediatr. 2011, 170, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hemila, H.; Chalker, E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013, 2013, CD000980. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Barkyoumb, G.M.; Schumacher, S.S. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin C status: A randomized controlled trial. Nutrients 2014, 6, 2572–2583. [Google Scholar] [CrossRef]
- Cai, B.; Lv, X.; Lin, M.; Feng, C.; Chen, C. Clinical efficacy and safety of vitamin C in the treatment of septic shock patients: Systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 1369–1380. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Stephen, R.; Utecht, T. Scurvy identified in the emergency department: A case report. J. Emerg. Med. 2001, 21, 235–237. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C. The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. The physiological role of dehydroascorbic acid. FEBS Lett. 2002, 527, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef]
- Lee, J.K.; Jung, S.H.; Lee, S.E.; Han, J.H.; Jo, E.; Park, H.S.; Heo, K.S.; Kim, D.; Park, J.S.; Myung, C.S. Alleviation of ascorbic acid-induced gastric high acidity by calcium ascorbate in vitro and in vivo. Korean J. Physiol. Pharmacol. 2018, 22, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Boxer, L.A.; Vanderbilt, B.; Bonsib, S.; Jersild, R.; Yang, H.H.; Baehner, R.L. Enhancement of chemotactic response and microtubule assembly in human leukocytes by ascorbic acid. J. Cell Physiol. 1979, 100, 119–126. [Google Scholar] [CrossRef]
- Ella, K.; Csépányi-Kömi, R.; Káldi, K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 2016, 57, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Aroca-Crevillén, A.; Adrover, J.M.; Hidalgo, A. Circadian Features of Neutrophil Biology. Front. Immunol. 2020, 11, 576. [Google Scholar] [CrossRef]
- Adrover, J.M.; Del Fresno, C.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossío, I.; et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 2019, 50, 390–402.e310. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Namatalla, G. Enhancement of Human Natural Killer Cytotoxic Activity by Vitamin C in Pure and Augmented Formulations. J. Nutr. Environ. Med. 1997, 7, 187–196. [Google Scholar] [CrossRef]
- Heuser, G.; Vojdani, A. Enhancement of natural killer cell activity and T and B cell function by buffered vitamin C in patients exposed to toxic chemicals: The role of protein kinase-C. Immunopharmacol. Immunotoxicol. 1997, 19, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.A.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.M.G.; Tilanus, M.G.J.; Bos, G.M.J.; Wieten, L.; Germeraad, W.T.V. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, T.; Hirt, A.; Morell, A. Effect of ascorbic acid on human natural killer cells. Immunol. Lett. 1985, 10, 173–176. [Google Scholar] [CrossRef]
- Dutertre, C.-A.; Bonnin-Gélizé, E.; Pulford, K.; Bourel, D.; Fridman, W.-H.; Teillaud, J.-L. A novel subset of NK cells expressing high levels of inhibitory FcγRIIB modulating antibody-dependent function. J. Leukoc. Biol. 2008, 84, 1511–1520. [Google Scholar] [CrossRef]
- Cui, F.; Qu, D.; Sun, R.; Tao, H.; Si, J.; Xu, Y. The Role of Circulating CD16+CD56+ Natural Killer Cells in the Screening, Diagnosis, and Staging of Colorectal Cancer before Initial Treatment. Dis. Markers 2019, 2019, 7152183. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickerson, B.; Gonzalez, D.E.; Sowinski, R.; Xing, D.; Leonard, M.; Kendra, J.; Jenkins, V.; Gopalakrishnan, S.; Yoo, C.; Ko, J.; et al. Comparative Effectiveness of Ascorbic Acid vs. Calcium Ascorbate Ingestion on Pharmacokinetic Profiles and Immune Biomarkers in Healthy Adults: A Preliminary Study. Nutrients 2024, 16, 3358. https://doi.org/10.3390/nu16193358
Dickerson B, Gonzalez DE, Sowinski R, Xing D, Leonard M, Kendra J, Jenkins V, Gopalakrishnan S, Yoo C, Ko J, et al. Comparative Effectiveness of Ascorbic Acid vs. Calcium Ascorbate Ingestion on Pharmacokinetic Profiles and Immune Biomarkers in Healthy Adults: A Preliminary Study. Nutrients. 2024; 16(19):3358. https://doi.org/10.3390/nu16193358
Chicago/Turabian StyleDickerson, Broderick, Drew E. Gonzalez, Ryan Sowinski, Dante Xing, Megan Leonard, Jacob Kendra, Victoria Jenkins, Siddharth Gopalakrishnan, Choongsung Yoo, Joungbo Ko, and et al. 2024. "Comparative Effectiveness of Ascorbic Acid vs. Calcium Ascorbate Ingestion on Pharmacokinetic Profiles and Immune Biomarkers in Healthy Adults: A Preliminary Study" Nutrients 16, no. 19: 3358. https://doi.org/10.3390/nu16193358








