Gut Microbiota and Oral Contraceptive Use in Women with Polycystic Ovary Syndrome: A Systematic Review
Abstract
1. Introduction
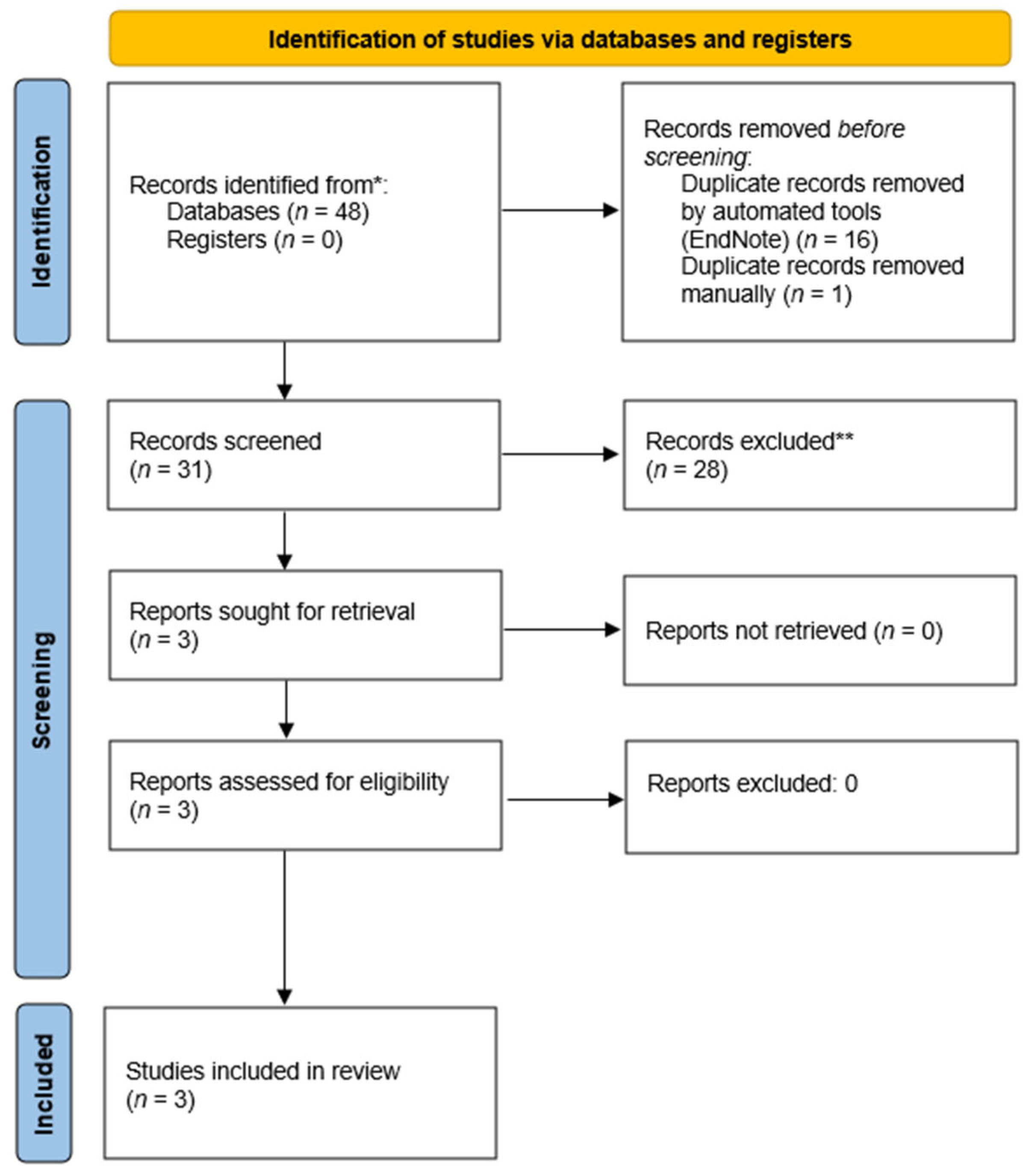
2. Material and Methods
- -
- Population: women with polycystic ovary syndrome;
- -
- Intervention: treatment with oral contraceptives (OCs);
- -
- Comparison: women with PCOS before treatment with OCs;
- -
- Outcomes: comparison of gut microbiota composition in women with PCOS before and after treatment with OCs.
3. Data Extraction
4. Risk of Bias
5. Results
6. Synthesis of Results and Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Hayek, S.; Bitar, L.; Hamdar, L.H.; Mirza, F.G.; Daoud, G. Poly Cystic Ovarian Syndrome: An Updated Overview. Front. Physiol. 2016, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Lanzone, A.; Fulghesu, A.M.; Fortini, A.; Cutillo, G.; Cucinelli, F.; Di Simone, N.; Caruso, A.; Mancuso, S. Effect of opiate receptor blockade on the insulin response to oral glucose load in polycystic ovarian disease. Hum. Reprod. 1991, 6, 1043–1049. [Google Scholar] [CrossRef]
- Lujan, M.E.; Chizen, D.R.; Pierson, R.A. Diagnostic criteria for polycystic ovary syndrome: Pitfalls and controversies. J. Obstet. Gynaecol. Can. 2008, 30, 671–679. [Google Scholar] [CrossRef]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302. [Google Scholar] [CrossRef]
- Guo, J.; Shao, J.; Yang, Y.; Niu, X.; Liao, J.; Zhao, Q.; Wang, D.; Li, S.; Hu, J. Gut Microbiota in Patients with Polycystic Ovary Syndrome: A Systematic Review. Reprod. Sci. 2022, 29, 69–83. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Z.; Chen, M.; Chen, G.; Huang, Q.; Yang, X.; Yin, H.; Chen, L.; Zhang, W.; Lin, H.; et al. Reduced stress-associated FKBP5 DNA methylation together with gut microbiota dysbiosis is linked with the progression of obese PCOS patients. NPJ Biofilms Microbiomes 2021, 7, 60. [Google Scholar] [CrossRef]
- Dong, S.; Jiao, J.; Jia, S.; Li, G.; Zhang, W.; Yang, K.; Wang, Z.; Liu, C.; Li, D.; Wang, X. 16S rDNA Full-Length Assembly Sequencing Technology Analysis of Intestinal Microbiome in Polycystic Ovary Syndrome. Front. Cell Infect. Microbiol. 2021, 11, 634981. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Kaakinen, M.A.; Draisma, H.; Zudina, L.; Ganie, M.A.; Rashid, A.; Balkhiyarova, Z.; Kiran, G.S.; Vogazianos, P.; Shammas, C.; et al. Bifidobacterium Is Enriched in Gut Microbiome of Kashmiri Women with Polycystic Ovary Syndrome. Genes 2022, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, Y. The gut microbial composition in polycystic ovary syndrome with insulin resistance: Findings from a normal-weight population. J. Ovarian Res. 2021, 14, 50. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, Z.; Cheng, W.; Yu, J.; Sun, S.; Zhai, D.; Yu, C.; Cai, Z. Characteristic gut microbiota and predicted metabolic functions in women with PCOS. Endocr. Connect. 2020, 9, 63–73. [Google Scholar] [CrossRef]
- Zeng, B.; Lai, Z.; Sun, L.; Zhang, Z.; Yang, J.; Li, Z.; Lin, J.; Zhang, Z. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): A pilot study. Res. Microbiol. 2019, 170, 43–52. [Google Scholar] [CrossRef]
- Yu, Z.; Qin, E.; Cheng, S.; Yang, H.; Liu, R.; Xu, T.; Liu, Y.; Yuan, J.; Yu, S.; Yang, J.; et al. Gut microbiome in PCOS associates to serum metabolomics: A cross-sectional study. Sci. Rep. 2022, 12, 22184. [Google Scholar] [CrossRef]
- Yin, G.; Chen, F.; Chen, G.; Yang, X.; Huang, Q.; Chen, L.; Chen, M.; Zhang, W.; Ou, M.; Cao, M.; et al. Alterations of bacteriome, mycobiome and metabolome characteristics in PCOS patients with normal/overweight individuals. J. Ovarian Res. 2022, 15, 117. [Google Scholar] [CrossRef]
- Mammadova, G.; Ozkul, C.; Yilmaz Isikhan, S.; Acikgoz, A.; Yildiz, B.O. Characterization of gut microbiota in polycystic ovary syndrome: Findings from a lean population. Eur. J. Clin. Investig. 2021, 51, e13417. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- Liang, Y.; Ming, Q.; Liang, J.; Zhang, Y.; Zhang, H.; Shen, T. Gut microbiota dysbiosis in polycystic ovary syndrome: Association with obesity—A preliminary report. Can. J. Physiol. Pharmacol. 2020, 98, 803–809. [Google Scholar] [CrossRef]
- Liang, Z.; Di, N.; Li, L.; Yang, D. Gut microbiota alterations reveal potential gut-brain axis changes in polycystic ovary syndrome. J. Endocrinol. Investig. 2021, 44, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, Z.; Ren, F.; Shi, H.; Zhao, Q.; Song, Y.; Fan, X.; Ma, X.; Qin, G. Alterations of Gut Microbiome and Fecal Fatty Acids in Patients With Polycystic Ovary Syndrome in Central China. Front. Microbiol. 2022, 13, 911992. [Google Scholar] [CrossRef]
- Jobira, B.; Frank, D.N.; Pyle, L.; Silveira, L.J.; Kelsey, M.M.; Garcia-Reyes, Y.; Robertson, C.E.; Ir, D.; Nadeau, K.J.; Cree-Green, M. Obese Adolescents With PCOS Have Altered Biodiversity and Relative Abundance in Gastrointestinal Microbiota. J. Clin. Endocrinol. Metab 2020, 105, e2134–e2144. [Google Scholar] [CrossRef] [PubMed]
- Insenser, M.; Murri, M.; Del Campo, R.; Martinez-Garcia, M.A.; Fernandez-Duran, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Toor, D.; Wsson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Unluhizarci, K.; Karaca, Z.; Kelestimur, F. Role of insulin and insulin resistance in androgen excess disorders. World J. Diabetes 2021, 12, 616–629. [Google Scholar] [CrossRef]
- Baptiste, C.G.; Battista, M.C.; Trottier, A.; Baillargeon, J.P. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010, 122, 42–52. [Google Scholar] [CrossRef]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 9240569. [Google Scholar] [CrossRef]
- Vrbikova, J.; Cibula, D. Combined oral contraceptives in the treatment of polycystic ovary syndrome. Hum. Reprod. Update 2005, 11, 277–291. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, S.F. Risks, benefits size and clinical implications of combined oral contraceptive use in women with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Angoorani, P.; Ejtahed, H.S.; Ettehad Marvasti, F.; Taghavi, M.; Mohammadpour Ahranjani, B.; Hasani-Ranjbar, S.; Larijani, B. The effects of probiotics, prebiotics, and synbiotics on polycystic ovarian syndrome: An overview of systematic reviews. Front. Med. 2023, 10, 1141355. [Google Scholar] [CrossRef]
- Talebi, S.; Zeraattalab-Motlagh, S.; Jalilpiran, Y.; Payandeh, N.; Ansari, S.; Mohammadi, H.; Djafarian, K.; Ranjbar, M.; Sadeghi, S.; Taghizadeh, M.; et al. The effects of pro-, pre-, and synbiotics supplementation on polycystic ovary syndrome: An umbrella review of meta-analyses of randomized controlled trials. Front. Nutr. 2023, 10, 1178842. [Google Scholar] [CrossRef] [PubMed]
- Pavlo, P.; Kamyshna, I.; Kamyshnyi, A. Effects of metformin on the gut microbiota: A systematic review. Mol. Metab 2023, 77, 101805. [Google Scholar] [CrossRef]
- Mihajlovic, J.; Leutner, M.; Hausmann, B.; Kohl, G.; Schwarz, J.; Rover, H.; Stimakovits, N.; Wolf, P.; Maruszczak, K.; Bastian, M.; et al. Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women. Environ. Microbiol. 2021, 23, 3037–3047. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, C.; Malpique, R.; Carbonetto, B.; Gonzalez-Torres, P.; Henares, D.; Brotons, P.; Munoz-Almagro, C.; Lopez-Bermejo, A.; de Zegher, F.; Ibanez, L. Gut microbiota in adolescent girls with polycystic ovary syndrome: Effects of randomized treatments. Pediatr. Obes. 2021, 16, e12734. [Google Scholar] [CrossRef]
- Tayachew, B.; Vanden Brink, H.; Garcia-Reyes, Y.; Rahat, H.; D’Alessandro, A.; Frank, D.N.; Robertson, C.E.; Silveira, L.; Kelsey, M.; Pyle, L.; et al. Combined Oral Contraceptive Treatment Does Not Alter the Gut Microbiome but Affects Amino Acid Metabolism in Sera of Obese Girls With Polycystic Ovary Syndrome. Front. Physiol. 2022, 13, 887077. [Google Scholar] [CrossRef]
- Eyupoglu, N.D.; Ergunay, K.; Acikgoz, A.; Akyon, Y.; Yilmaz, E.; Yildiz, B.O. Gut Microbiota and Oral Contraceptive Use in Overweight and Obese Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab 2020, 105, e4792–e4800. [Google Scholar] [CrossRef]
- Zou, Y.; Liao, R.; Cheng, R.; Chung, H.; Zhu, H.; Huang, Y. Alterations of gut microbiota biodiversity and relative abundance in women with PCOS: A systematic review and meta-analysis. Microb. Pathog. 2023, 184, 106370. [Google Scholar] [CrossRef] [PubMed]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab 2019, 30, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women With Polycystic Ovary Syndrome Correlates With Hyperandrogenism. J. Clin. Endocrinol. Metab 2018, 103, 1502–1511. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Sanchez-Garrido, M.A.; Martin-Nunez, G.M.; Perez-Jimenez, F.; Tena-Sempere, M.; Tinahones, F.J.; Queipo-Ortuno, M.I. Neonatal Androgen Exposure Causes Persistent Gut Microbiota Dysbiosis Related to Metabolic Disease in Adult Female Rats. Endocrinology 2016, 157, 4888–4898. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, Y.J.; Shin, M.J.; Yi, H. Difference in the Gut Microbiome between Ovariectomy-Induced Obesity and Diet-Induced Obesity. J. Microbiol. Biotechnol. 2017, 27, 2228–2236. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, Z.; Yu, J.; Cheng, W.; Cai, Z.; Yu, C. Correlation Between Fecal Metabolomics and Gut Microbiota in Obesity and Polycystic Ovary Syndrome. Front. Endocrinol. 2020, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, M.; Kan, Z. Causal relationship between gut microbiota and polycystic ovary syndrome: A literature review and Mendelian randomization study. Front. Endocrinol. 2024, 15, 1280983. [Google Scholar] [CrossRef]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut microbiota predicts body fat change following a low-energy diet: A PREVIEW intervention study. Genome Med. 2022, 14, 54. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, I.L.; Zhu, X.Y.; Chiu, W.C.; Chiu, Y.S. Red Clover Isoflavones Influence Estradiol Concentration, Exercise Performance, and Gut Microbiota in Female Mice. Front. Nutr. 2021, 8, 623698. [Google Scholar] [CrossRef]
- Yoon, K.; Kim, N. Roles of Sex Hormones and Gender in the Gut Microbiota. J. Neurogastroenterol. Motil. 2021, 27, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Dotsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Boytar, A.N.; Skinner, T.L.; Wallen, R.E.; Jenkins, D.G.; Dekker Nitert, M. The Effect of Exercise Prescription on the Human Gut Microbiota and Comparison between Clinical and Apparently Healthy Populations: A Systematic Review. Nutrients 2023, 15, 1534. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Alvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Munzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Harada, N.; Hanaoka, R.; Hanada, K.; Izawa, T.; Inui, H.; Yamaji, R. Hypogonadism alters cecal and fecal microbiota in male mice. Gut Microbes 2016, 7, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Paris, V.; Wong, X.Y.D.; Solon-Biet, S.M.; Edwards, M.C.; Aflatounian, A.; Gilchrist, R.B.; Simpson, S.J.; Handelsman, D.J.; Kaakoush, N.O.; Walters, K.A. The interplay between PCOS pathology and diet on gut microbiota in a mouse model. Gut Microbes 2022, 14, 2085961. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, N.D.; Caliskan Guzelce, E.; Acikgoz, A.; Uyanik, E.; Bjørndal, B.; Berge, R.K.; Svardal, A.; Yildiz, B.O. Circulating gut microbiota metabolite trimethylamine N-oxide and oral contraceptive use in polycystic ovary syndrome. Clin. Endocrinol. 2019, 91, 810–815. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, Y.H.; Sim, M.; Kim, S.A.; Joung, H.; Shin, D.M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, P.; Ho, B.S.; Sau, L.; Kelley, S.T.; Thackray, V.G. Letrozole treatment of pubertal female mice results in activational effects on reproduction, metabolism and the gut microbiome. PLoS ONE 2019, 14, e0223274. [Google Scholar] [CrossRef]
- Hsiao, T.H.; Chou, C.H.; Chen, Y.L.; Wang, P.H.; Brandon-Mong, G.J.; Lee, T.H.; Wu, T.Y.; Li, P.T.; Li, C.W.; Lai, Y.L.; et al. Circulating androgen regulation by androgen-catabolizing gut bacteria in male mouse gut. Gut Microbes 2023, 15, 2183685. [Google Scholar] [CrossRef]
| Database | Number of Results | Search Strategy |
|---|---|---|
| PubMed | 9 | (“Microbiota”[Mesh] OR microbiot* OR microbiom* OR microfilm* OR flora OR microflora OR microorganism* OR “high-throughput nucleotide sequencing”[Mesh] OR NGS OR (next AND generation AND sequencing) OR (shotgun AND metagenomic*) OR “RNA, ribosomal, 16S”[Mesh] OR (16S AND rrna)) AND (“polycystic ovary syndrome”[Mesh] OR polycystic OR pco OR pcos) AND (“contraceptives, oral, hormonal”[Mesh] OR “contraceptives, oral, combined”[Mesh] OR (oral AND contracept*) OR (combined AND contracept*)) |
| Scopus | 27 | TITLE-ABS-KEY(microbiot* OR microbiom* OR microfilm* OR flora OR microflora OR microorganism* OR “high-throughput nucleotide sequencing” OR NGS OR (next AND generation AND sequencing) OR (shotgun AND metagenomic*) OR “RNA, ribosomal, 16S” OR (16S AND rrna)) AND TITLE-ABS-KEY(“polycystic ovary syndrome” OR polycystic OR pco OR pcos) AND TITLE-ABS-KEY((oral AND contracept*) OR (combined AND contracept*)) |
| Web of Science | 12 | TS = (microbiot* OR microbiom* OR microfilm* OR flora OR microflora OR microorganism* OR “high-throughput nucleotide sequencing” OR NGS OR (next AND generation AND sequencing) OR (shotgun AND metagenomic*) OR “RNA, ribosomal, 16S” OR (16S AND rrna)) AND TS = (“polycystic ovary syndrome” OR polycystic OR pco OR pcos) AND TS = ((oral AND contracept*) OR (combined AND contracept*)) |
| Google Scholar | 139 | (microbiot* OR microbiom* OR microfilm* OR flora OR microflora OR microorganism* OR “high-throughput nucleotide sequencing” OR NGS OR (next AND generation AND sequencing) OR (shotgun AND metagenomic*) OR “RNA, ribosomal, 16S” OR (16S AND rrna)) AND (“polycystic ovary syndrome” OR polycystic OR pco OR pcos) AND ((oral AND contracept*) OR (combined AND contracept*)) |
| Authors, Year, Country of Origin | Study Design | Aim | Analyzed PCOS Population | Duration of Treatment | Treatment | Control Group | Effect of OC Treatment in PCOS Group | Diversity at Baseline | Species at Baseline | Effect of Treatment on Microbiota in PCOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Eyupoglu et al. 2020 Turkey [40] | Prospective observational study | To assess if gut mibrobiota is altered in PCOS and to determine the impact of OCs | n = 17 on OCs • median age 20 years • median BMI 29.6 kg/m2 (all between 25–40 kg/m2) • glucose-tolerant • diagnosis based on the Rotterdam criteria | 3 months between April and December 2018 | Dienogest and ethinyloestradiol (2 mg + 0.03 mg) + healthy diet + a minimum of 150min/week of moderate intensity physical activity | n = 15 age- and BMI-matched healthy controls | • lower BMI, WHR, TT, FAI • higher fasting insulin, HOMA-IR | • no difference in alpha and beta diversity, number of species between PCOS and controls | • Ruminococcaceae enriched in PCOS • OTU lower in obese PCOS than obese controls • OTU counts higher in overweight than obese irrespective of PCOS status | • no difference in OTU counts and alpha and beta diversity after treatment • abundance of Ruminococcaceae in PCOS did not change after OC • trend for a decrease in the relative abundance of Actinobacteria phylum after OC, significant in the obese PCOS subgroup |
| Garcia-Beltran et al. 2021 Spain [38] | Randomized controlled trial | To assess the gut microbiota composition of girls with PCOS without obesity and compare the effects of OCs or SPIOMET | n = 12 on OCs, n = 11 on SPIOMET • median age 15 years • mean BMI 25 kg/m2 (all without obesity) • glucose-tolerant • diagnosis based on hirsutism >8 mFG score, oligomenorrhea >45 days, gynecological age > 2.0 years | 1 year between December 2015 and October 2019 | • levonorgestrel and ethinyloestradiol (100 mg + 0.02 mg) OR • SPIOMET = spironolactone 50 mg/d, pioglitazone 7.5 mg/d, and metformin 850 mg/d | n = 31 age-matched healthy controls | • higher BMI, Z-score, SHBG, us-CRP, fat mass, abdominal fat measured by DXA, subcutaneous fat measured by MRI • lower TT, FAI | • lower alpha diversity in PCOS • differences in community structure regarding beta-diversity • baseline alpha diversity measures correlated with SHBG and FAI and ALT | • in PCOS abundant Family XI and depleted Prevotellaceae • Prevotella and Senegalimassilia depleted in PCOS | • no change in alpha and beta diversity, Family XI, Prevotellaceae, and Senegalimassilia after OC • OC reduced the abundance of genus Prevotella |
| Tayachew et al. 2022 USA [39] | Secondary analysis of 3 separate cross-sectional studies | To assess gut microbiome profiles, serum metabolomics, hormone levels and metabolism in adolescents with PCOS and obesity with and without OC treatment | n = 8 on OC • mean age 15.5, • mean BMI 32.5 kg/m2 (>90th centile)• without diabetes, • diagnosis of PCOS based on National Institute of Health criteria—adolescent adaptation | >6 months median 8 months (range 6–24) | COC | n = 21 age, race, ethnicity, age of menarche and BMI-matched untreated with PCOS | • lower mFG score, free testosterone and FAI • higher SHBG and platelets | unavailable | unavailable | • no difference in alpha and beta diversity • no differences at the phylum or family level between groups • at the genus level the %RA of Pseudobutyrivibrio higher in OC group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wydra, J.; Szlendak-Sauer, K.; Zgliczyńska, M.; Żeber-Lubecka, N.; Ciebiera, M. Gut Microbiota and Oral Contraceptive Use in Women with Polycystic Ovary Syndrome: A Systematic Review. Nutrients 2024, 16, 3382. https://doi.org/10.3390/nu16193382
Wydra J, Szlendak-Sauer K, Zgliczyńska M, Żeber-Lubecka N, Ciebiera M. Gut Microbiota and Oral Contraceptive Use in Women with Polycystic Ovary Syndrome: A Systematic Review. Nutrients. 2024; 16(19):3382. https://doi.org/10.3390/nu16193382
Chicago/Turabian StyleWydra, Jakub, Katarzyna Szlendak-Sauer, Magdalena Zgliczyńska, Natalia Żeber-Lubecka, and Michał Ciebiera. 2024. "Gut Microbiota and Oral Contraceptive Use in Women with Polycystic Ovary Syndrome: A Systematic Review" Nutrients 16, no. 19: 3382. https://doi.org/10.3390/nu16193382
APA StyleWydra, J., Szlendak-Sauer, K., Zgliczyńska, M., Żeber-Lubecka, N., & Ciebiera, M. (2024). Gut Microbiota and Oral Contraceptive Use in Women with Polycystic Ovary Syndrome: A Systematic Review. Nutrients, 16(19), 3382. https://doi.org/10.3390/nu16193382






