Lactobacillus plantarum 17-1 Ameliorates DSS-Induced Colitis by Modulating the Colonic Microbiota Composition and Metabolome in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Treatments
2.3. Evaluation of the Disease Activity Index and Histopathology
2.4. Measurement of Colon Cytokines
2.5. 16S rRNA Gene Sequencing and Microbial Analysis
2.5.1. DNA Extraction and PCR Amplification
2.5.2. Illumina Sequencing and Data Processing
2.6. Untargeted Metabolome Profiling Analysis
2.6.1. Metabolite Extraction
2.6.2. UHPLC-MS/MS Analysis
2.6.3. Data Analysis
2.7. Statistical Analysis
3. Results
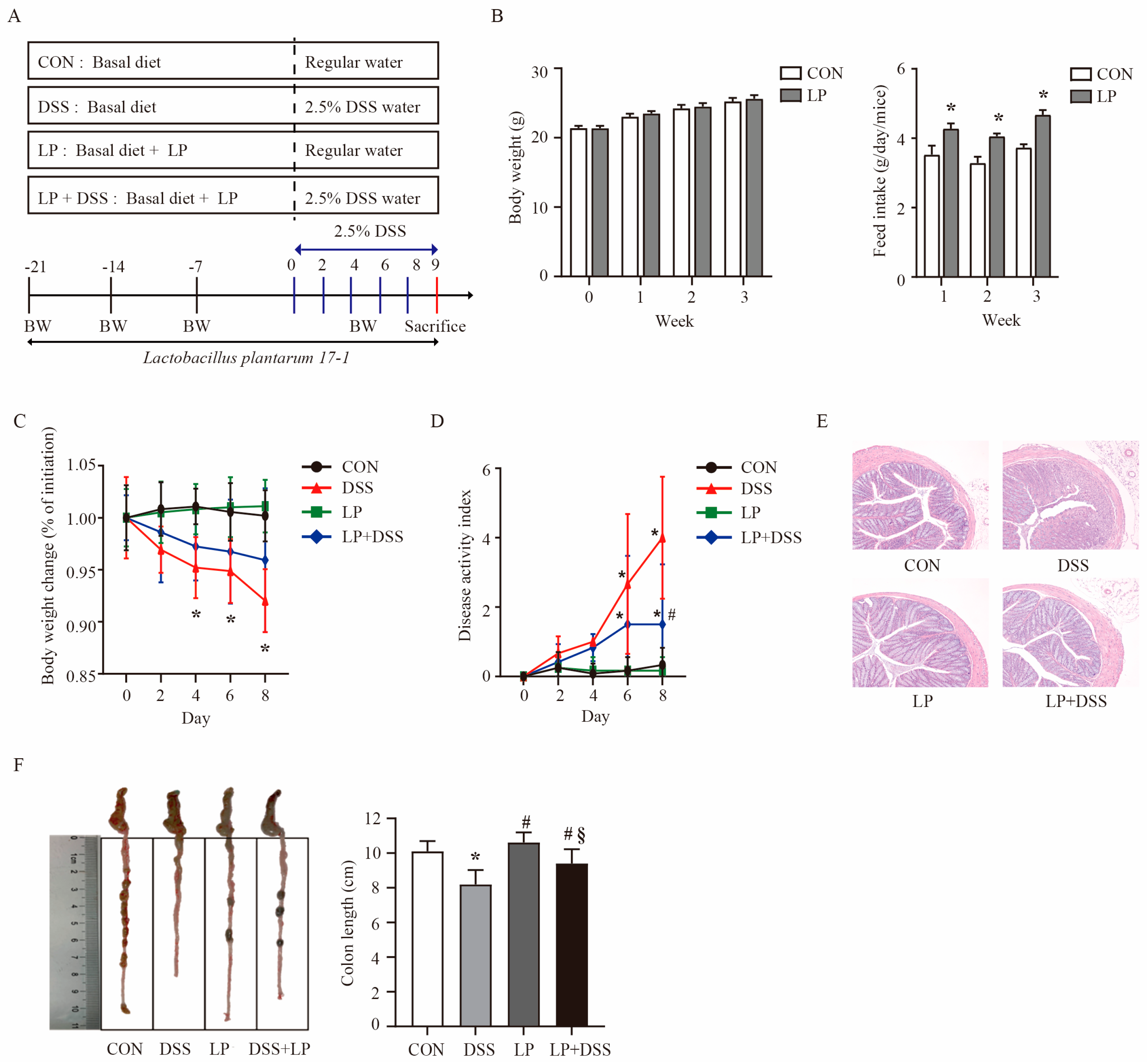
3.1. L. plantarum 17-1 Ameliorated Clinical Features and Intestinal Injury in DSS-Induced-Colitis Mice
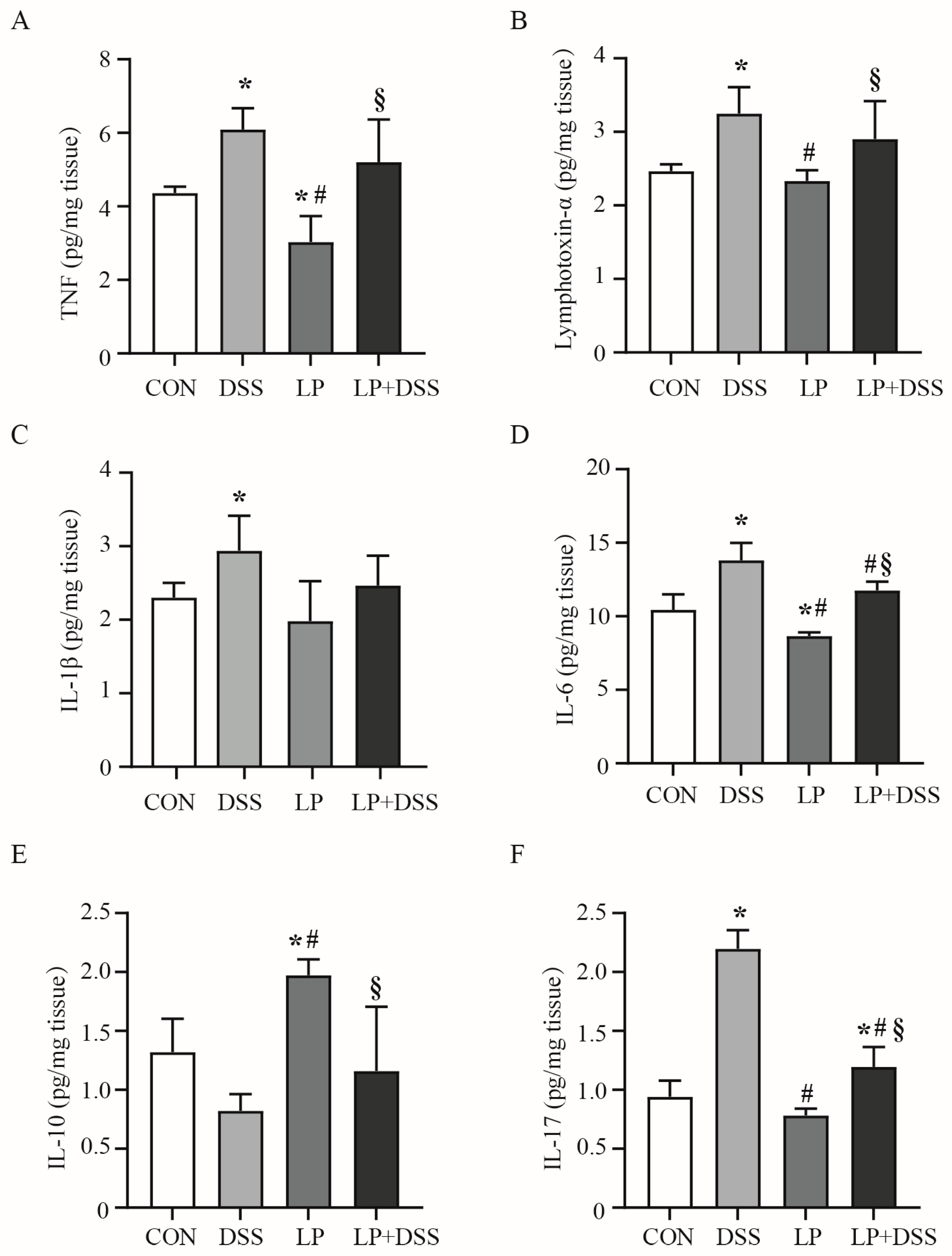
3.2. L. plantarum 17-1 Regulated the Production of Cytokines in the Colon of DSS-Induced-Colitis Mice
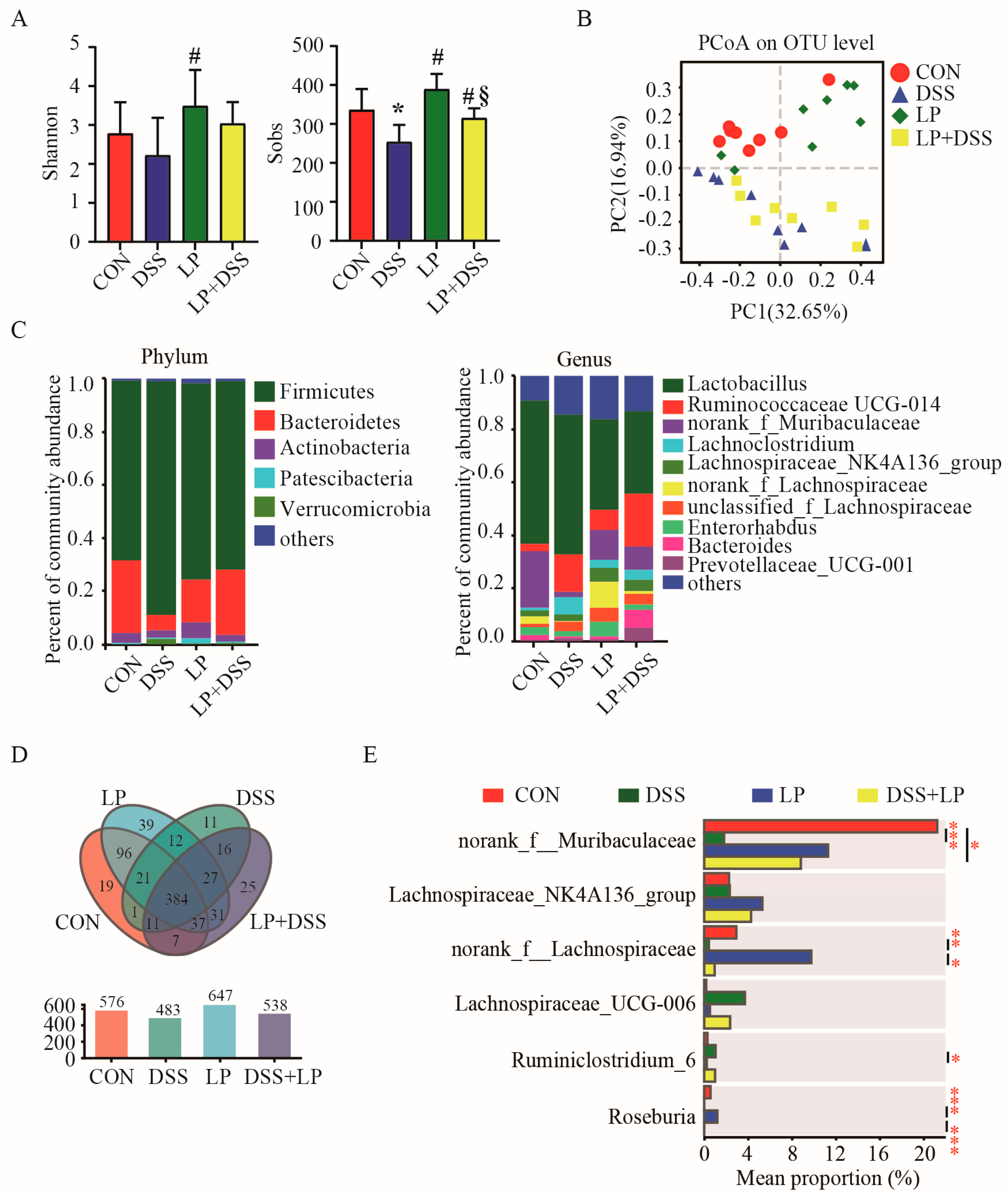
3.3. L. plantarum 17-1 Changed Microbiota Diversity in the Colon Digesta of DSS-Induced-Colitis Mice
3.4. L. plantarum 17-1 Induced Shift in Gut Microbiota Composition in DSS-Induced-Colitis Mice
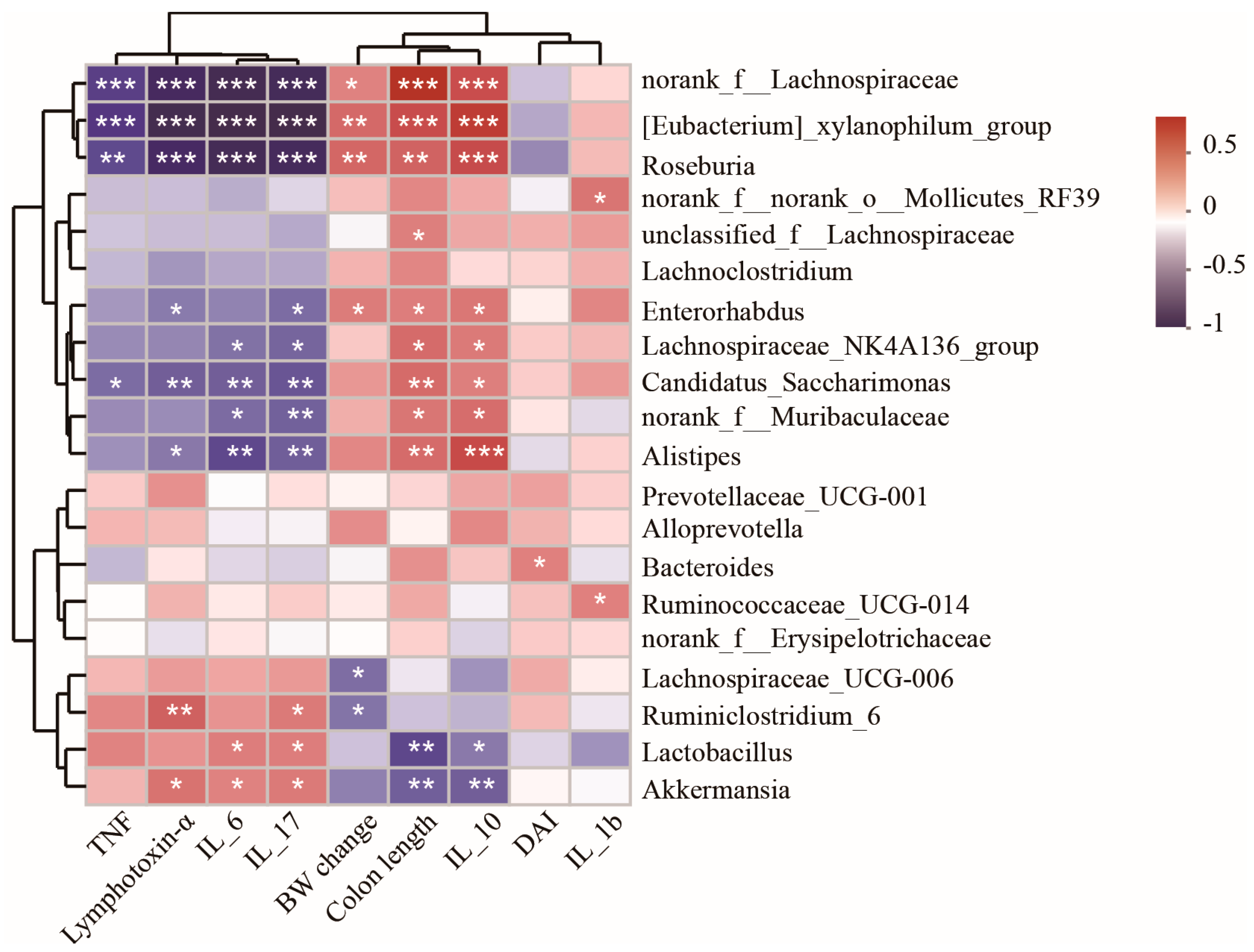
3.5. Spearman Correlation Analysis of Differentially Abundant Genera and Colitis Injury Indices
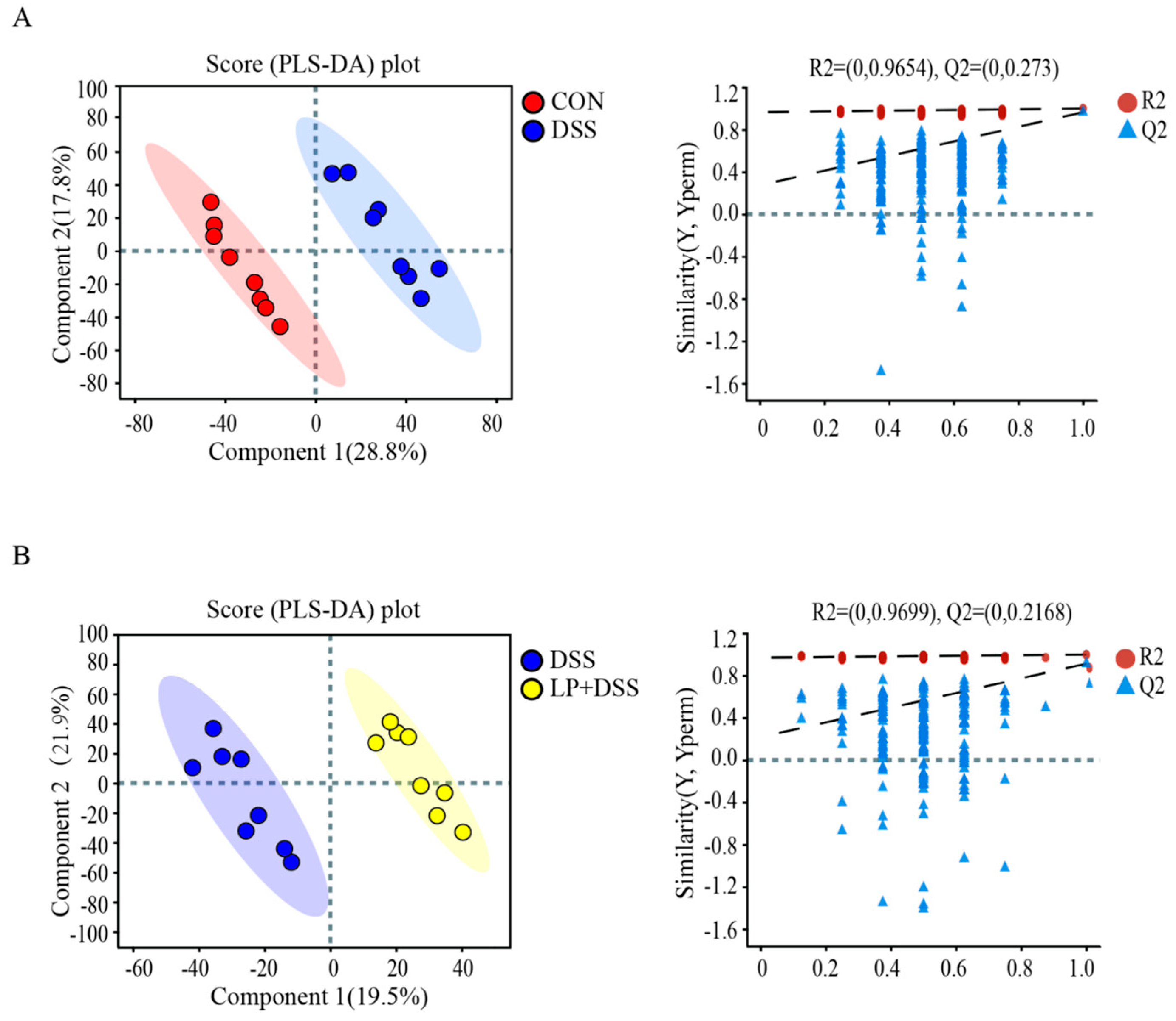
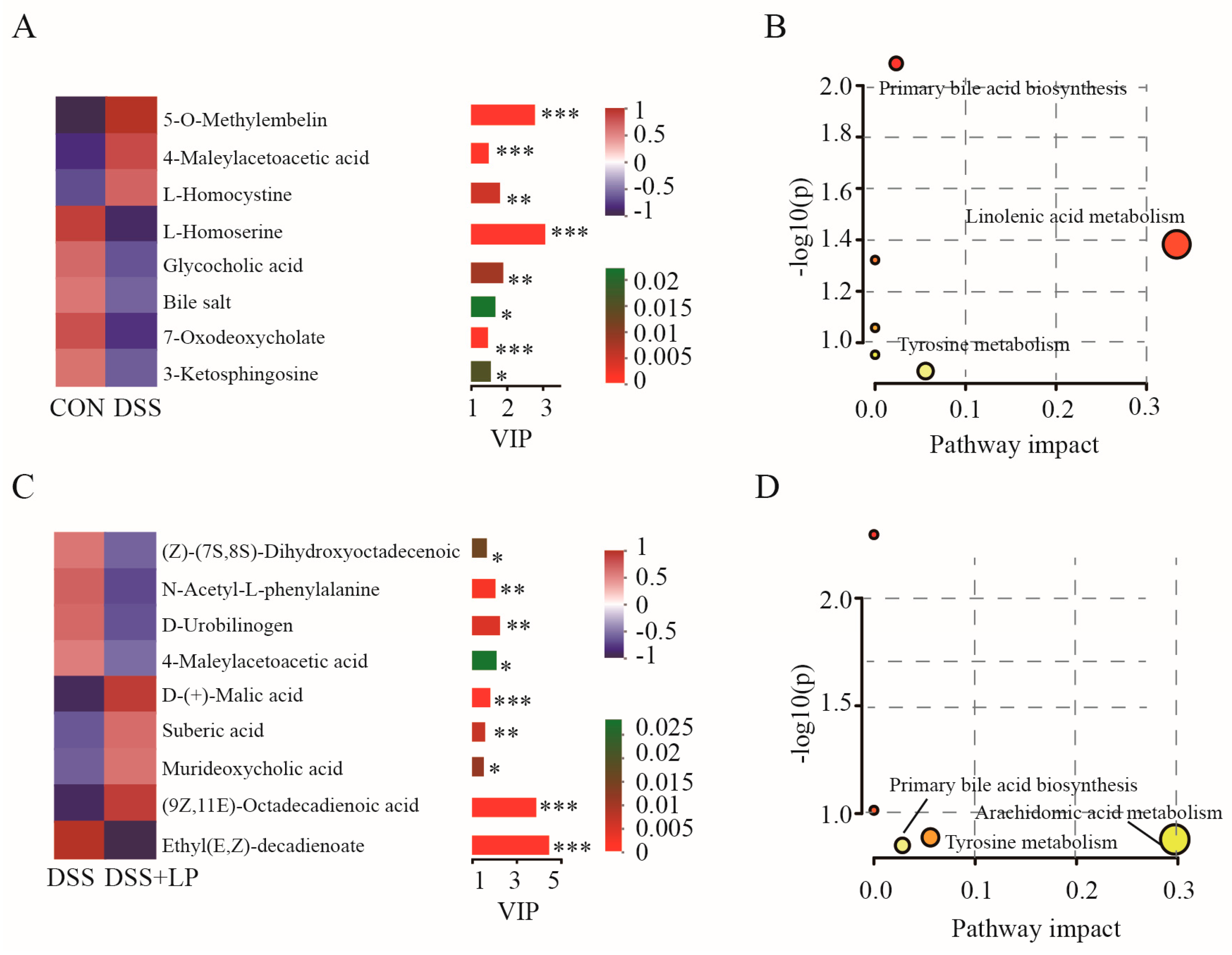
3.6. L. plantarum 17-1 Altered Gut Metabolic Profiles in DSS-Induced-Colitis Mice
3.7. Spearman Correlation Analysis of Differentially Abundant Genera and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BW | body weight |
| DSS | dextran sulfate sodium |
| DAI | disease activity index |
| ELISA | enzyme-linked immunosorbent assay |
| ESI | electrospray ionization |
| LEfSe | linear discriminant analysis effect size |
| IBD | inflammatory bowel disease |
| IL | interleukin |
| OTU | operational taxonomic unit |
| SD | standard deviation |
| TNF | tumor necrosis factor α |
| UC | ulcerative colitis |
| UHPLC-MS | ultra-high-performance liquid chromatography–mass spectrometry |
| PCA | principal component analysis |
| PLS-DA | partial least-squares discrimination analysis |
| QC | quality control |
| VIP | variable importance in the projection |
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Bromke, M.A.; Krzystek-Korpacka, M. Bile acid signaling in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 9096. [Google Scholar] [CrossRef]
- Kim, J.E.; Suh, D.H.; Park, Y.J.; Oh, C.H.; Oh, S.J.; Kang, H.; Ji, Y.; Kim, Y.J.; Kim, W.; Jung, E.S.; et al. Identifying robust biomarkers for the diagnosis and subtype distinction of inflammatory bowel disease through comprehensive serum metabolomic profiling. Sci. Rep. 2025, 15, 5661. [Google Scholar] [CrossRef]
- Zou, J.; Liu, C.; Jiang, S.; Qian, D.; Duan, J. Cross talk between gut microbiota and intestinal mucosal immunity in the development of ulcerative colitis. Infect. Immun. 2021, 89, e0001421. [Google Scholar] [CrossRef]
- Wilson, D.C.; Thomas, A.G.; Croft, N.M.; Newby, E.; Akobeng, A.K.; Sawczenko, A.; Fell, J.M.E.; Murphy, M.S.; Beattie, R.M.; Sandhu, B.K.; et al. Systematic review of the evidence base for the medical treatment of paediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2010, 50 (Suppl. 1), S14–S34. [Google Scholar] [CrossRef]
- Troncone, E.; Monteleone, G. The safety of non-biological treatments in ulcerative colitis. Expert. Opin. Drug Saf. 2017, 16, 779–789. [Google Scholar] [CrossRef]
- Bian, X.; Yang, L.; Wu, W.; Lv, L.; Jiang, X.; Wang, Q.; Wu, J.J.; Li, Y.T.; Ye, J.Z.; Fang, D.; et al. Pediococcus pentosaceus LI05 alleviates DSS-induced colitis by modulating immunological profiles, the gut microbiota and short-chain fatty acid levels in a mouse model. Microb. Biotechnol. 2020, 13, 1228–1244. [Google Scholar] [CrossRef]
- Chen, P.; Xu, H.; Tang, H.; Zhao, F.; Yang, C.; Kwok, L.; Cong, C.; Wu, Y.; Zhang, W.; Zhou, X.; et al. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb. Biotechnol. 2020, 13, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Song, Y.; Gao, Y.; Zhao, F.; Luo, Y.; Fang, Q.; Mu, G.Q.; Tuo, Y.F. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020, 11, 5205–5222. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, P.; Huang, Y.; Zhang, W.; Nie, Y.; Gao, C. Metabolic engineering of Lactobacilli spp. for disease treatment. Microb. Cell Fact. 2025, 24, 53. [Google Scholar] [CrossRef]
- Qi, W.; Liang, X.; Yun, T.; Guo, W. Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J. Food Sci. Technol. 2019, 56, 1398–1404. [Google Scholar] [CrossRef]
- Wen, Y.; Tan, L.; Chen, S.; Wu, N.; Yao, Y.; Xu, L.; Xu, M.; Zhao, Y.; Tu, Y. Egg yolk phosphatidylcholine alleviates DSS-induced colitis in BALB/c mice. Food Funct. 2023, 14, 9309–9323. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Y.; Wang, G.; Yang, Y.; Ai, L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 2020, 67, 103854. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, H.; Liu, H.; Wang, S.; Wang, J.; Wang, Y. Changes in the diversity and composition of gut microbiota of weaned piglets after oral administration of Lactobacillus or an antibiotic. Appl. Microbiol. Biotechnol. 2016, 100, 10081–10093. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lu, H.; Wang, Z.; Wang, T.; Fang, F.; Wang, J.; Yu, J.; Gao, R. A sensitive method for the determination of the gender difference of neuroactive metabolites in tryptophan and dopamine pathways in mouse serum and brain by UHPLC-MS/MS. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2018, 1093–1094, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Fang, J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J. Nutr. Biochem. 2024, 124, 109505. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hao, W.; Wang, X.; Zhou, R.; Lin, Q. Probiotics for the treatment of ulcerative colitis: A review of experimental research from 2018 to 2022. Front. Microbiol. 2023, 14, 1211271. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Forssten, S.; Hibberd, A.A.; Lyra, A.; Stahl, B. Probiotic approach to prevent antibiotic resistance. Ann. Med. 2016, 48, 246–255. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, L. Inflammatory bowel diseases and gut microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef]
- Keubler, L.M.; Buettner, M.; Häger, C.; Bleich, A.A. A multihit model: Colitis lessons from the interleukin-10-deficient mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, N.; Fu, M.; Ye, M. Identification of critical modules and biomarkers of ulcerative colitis by using WGCNA. J. Inflamm. Res. 2023, 16, 1611–1628. [Google Scholar] [CrossRef]
- Papoutsopoulou, S.; Pollock, L.; Walker, C.; Tench, W.; Samad, S.S.; Bergey, F.; Lenzi, L.; Sheibani-Tezerji, R.; Rosenstiel, P.; Alam, M.T.; et al. Impact of interleukin 10 deficiency on intestinal epithelium responses to inflammatory signals. Front. Immunol. 2021, 12, 690817. [Google Scholar] [CrossRef]
- Yang, B.; Yue, Y.; Chen, Y.; Ding, M.; Li, B.; Wang, L.; Wang, Q.; Stanton, C.; Ross, R.P.; Zhao, J.; et al. Lactobacillus plantarum CCFM1143 alleviates chronic diarrhea via inflammation regulation and gut microbiota modulation: A double-blind, randomized, placebo-controlled study. Front. Immunol. 2021, 12, 746585. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Guarner, F.; Pérez-Arribas, L.V.; Sánchez, B.; Borruel, N.; Manichanh, C. Probiotics as anti-inflammatory agents in IBD: Mechanisms of action and clinical evidence. Front. Microbiol. 2019, 10, 2832. [Google Scholar]
- Liu, Y.; Liu, Q.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Strain-specific regulative effects of Lactobacillus plantarum on intestinal barrier dysfunction are associated with their capsular polysaccharides. Int. J. Biol. Macromol. 2022, 222, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Lv, Y.; Chen, Q.; Feng, J.; Zhao, X. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Sci. Rep. 2018, 8, 2229. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shao, J.; Xia, Y.; Wang, G.; Xiong, Z.Q.; Yang, Y.J.; Xin, S.; Yu, W.; Ai, L.Z. The bsh1 gene of Lactobacillus plantarum AR113 ameliorates liver injury in colitis mice. npj Sci. Food 2025, 9, 22. [Google Scholar] [CrossRef]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef]
- Chingwaru, W.; Vidmar, J. Potential of Zimbabwean commercial probiotic products and strains of Lactobacillus plantarum as prophylaxis and therapy against diarrhoea caused by Escherichia coli in children. Asian Pac. J. Trop. Med. 2017, 10, 57–63. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Zhang, Y.; Li, J. Staphylococcus aureus enterotoxins: A trigger for gut inflammation and barrier dysfunction. Front. Cell Infect. Microbiol. 2023, 13, 1234567. [Google Scholar]
- Han, Y.; Teng, T.M.; Han, J.; Kim, H.S. Antibiotic-associated changes in Akkermansia muciniphila alter its effects on host metabolic health. Microbiome 2025, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol. Nutr. Food Res. 2021, 65, e2000745. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Li, B.; Xie, Y.; Shi, Y.; Le, G. Dietary methionine restriction improves gut microbiota composition and prevents cognitive impairment in D-galactose-induced aging mice. Food Funct. 2022, 13, 12896–12914. [Google Scholar] [CrossRef]
- Tian, B.; Liu, R.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Yang, K.; Zeng, X.; Peilong, S. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J. Sci. Food Agric. 2023, 103, 3050–3064. [Google Scholar] [CrossRef]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Rep. 2021, 171, 105767. [Google Scholar] [CrossRef]
- Cai, Y.; Li, S.; Zhang, X.; Cao, X.; Liu, D.; Zhu, Y.; Ye, S.; Xu, Z.; Liao, Q.; Hong, Y.; et al. Integrated microbiome-metabolomics analysis reveals the potential therapeutic mechanism of zuo-jin-wan in ulcerative colitis. Phytomedicine 2022, 98, 153914. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Alves, J.L.; Lemos, L.; Rodrigues, N.M.; Pereira, V.B.; Barros, P.A.V.; Canesso, M.C.C.; Guimarães, M.A.F.; Cara, D.C.; Miyoshi, A.; Azevedo, V.A.; et al. Immunomodulatory effects of different strains of Lactococcus lactis in DSS-induced colitis. Braz. J. Microbiol. 2023, 54, 1203–1215. [Google Scholar] [CrossRef]
- Wong, C.C.M.; Zhang, L.; Wu, W.K.K.; Shen, J.; Chan, R.L.Y.; Lu, L.; Hu, W.; Li, M.X.; Li, L.F.; Ren, S.X.; et al. Cathelicidin-encoding Lactococcus lactis promotes mucosal repair in murine experimental colitis. J. Gastroenterol. Hepatol. 2017, 32, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Zheng, Y.; Zhang, Y. Weissella paramesenteroides NRIC1542 inhibits dextran sodium sulfate-induced colitis in mice through regulating gut microbiota and SIRT1/NF-κB signaling pathway. FASEB J. 2024, 38, e23791. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chung, K.S.; Shin, J.S.; Jung, S.H.; Lee, S.; Lee, M.K.; Hong, H.D.; Rhee, Y.K.; Lee, K.T. Anti-colitic effect of an exopolysaccharide fraction from Pediococcus pentosaceus KFT-18 on sextran sulfate sodium-induced colitis through suppression of inflammatory mediators. Polymers 2022, 14, 3594. [Google Scholar] [CrossRef] [PubMed]
- Awad, K.; Newhart, S.L.; Brotto, L.; Brotto, M. Lipidomics profiling of the linoleic acid metabolites after whole-body vibration in humans. Methods Mol. Biol. 2024, 2816, 241–252. [Google Scholar]
- Li, X.; Duan, W.; Zhu, Y.; Ji, R.; Feng, K.; Kathuria, Y.; Xiao, H.; Yu, Y.; Cao, Y. Transcriptomics and metabolomics reveal the alleviation effect of pectic polysaccharide on dextran sodium sulfate-induced colitis mice. Int. J. Biol. Macromol. 2025, 288, 138755. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, B.L.; Cheng, W.; Wang, T.; Wang, Z.; Liang, Y.N. Mechanism of tryptanthrin in treatment of ulcerative colitis in mice based on serum metabolomics. China J. Chin. Mater. Med. 2023, 48, 2193–2202. [Google Scholar]
- Sun, Q.; Li, B.R.; Li, D.H.; Wang, X.Y.; Wang, Q.Y.; Jiang, Z.M.; Ning, B.; Sun, T. WKB ameliorates DSS-induced colitis through inhibiting enteric glial cells activation and altering the intestinal microbiota. J. Transl. Med. 2025, 23, 93. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, M.; Choi, Y.; Kang, T.; Kim, J.; Lee, N.H.; Kim, G.O.; Shin, T. Alpha-linolenic acid alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Inflammation 2020, 43, 1876–1883. [Google Scholar] [CrossRef]
- Yang, S.; Shang, J.; Liu, L.; Tang, Z.; Meng, X. Strains producing different short-chain fatty acids alleviate DSS-induced ulcerative colitis by regulating intestinal microecology. Food Funct. 2022, 13, 12156–12169. [Google Scholar] [CrossRef]
- Liu, T.; Fan, S.; Meng, P.; Ma, M.; Wang, Y.; Han, J.; Wu, Y.; Li, X.; Su, X.; Lu, C. Dietary dihydroquercetin alleviated colitis via the short-chain fatty acids/miR-10a-5p/PI3K-Akt signaling pathway. J. Agric. Food Chem. 2024, 72, 23211–23223. [Google Scholar] [CrossRef]
- Chen, L.; Ye, Z.; Li, J.; Wang, L.; Chen, Y.; Yu, M.; Han, J.; Huang, J.; Li, D.; Lv, Y.; et al. Gut bacteria Prevotellaceae related lithocholic acid metabolism promotes colonic inflammation. J. Transl. Med. 2025, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, Y.; Li, J.; Ran, X.; Gu, Z.; Song, L.; Zhang, L.; Wen, L.; Ji, G.; Wang, R. Bile acid-gut microbiota imbalance in cholestasis and its long-term effect in mice. mSystems 2024, 9, e0012724. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, W.; Yu, Y.; Yin, S.; Ye, B.C.; Zhou, Y. The role of the gut microbial metabolism of sterols and bile acids in human health. Biochimie 2024, 13, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, M.Y.; Liu, Y.X.; Yu, J.Y.; Wang, Y.B.; Zhang, X.J.; Sun, H.P. Branched-chain amino acids promote hepatic Cyp7a1 expression and bile acid synthesis via suppressing FGF21-ERK pathway. Acta Pharmacol. Sin. 2024, 46, 662–671. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Duan, T.; Hu, D.; Chen, L.; Qiao, L.; Song, D.; Wang, L.; Fan, S.; Teng, K.; Wang, W.; et al. Lactobacillus plantarum 17-1 Ameliorates DSS-Induced Colitis by Modulating the Colonic Microbiota Composition and Metabolome in Mice. Nutrients 2025, 17, 1348. https://doi.org/10.3390/nu17081348
He B, Duan T, Hu D, Chen L, Qiao L, Song D, Wang L, Fan S, Teng K, Wang W, et al. Lactobacillus plantarum 17-1 Ameliorates DSS-Induced Colitis by Modulating the Colonic Microbiota Composition and Metabolome in Mice. Nutrients. 2025; 17(8):1348. https://doi.org/10.3390/nu17081348
Chicago/Turabian StyleHe, Beibei, Tao Duan, Dandan Hu, Lixian Chen, Lin Qiao, Dan Song, Li Wang, Shijie Fan, Kunru Teng, Weiwei Wang, and et al. 2025. "Lactobacillus plantarum 17-1 Ameliorates DSS-Induced Colitis by Modulating the Colonic Microbiota Composition and Metabolome in Mice" Nutrients 17, no. 8: 1348. https://doi.org/10.3390/nu17081348
APA StyleHe, B., Duan, T., Hu, D., Chen, L., Qiao, L., Song, D., Wang, L., Fan, S., Teng, K., Wang, W., & Li, A. (2025). Lactobacillus plantarum 17-1 Ameliorates DSS-Induced Colitis by Modulating the Colonic Microbiota Composition and Metabolome in Mice. Nutrients, 17(8), 1348. https://doi.org/10.3390/nu17081348




