Association of Urinary Sodium, Potassium, and the Sodium-to-Potassium Ratio with Impaired Kidney Function Assessed with 24-H Urine Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
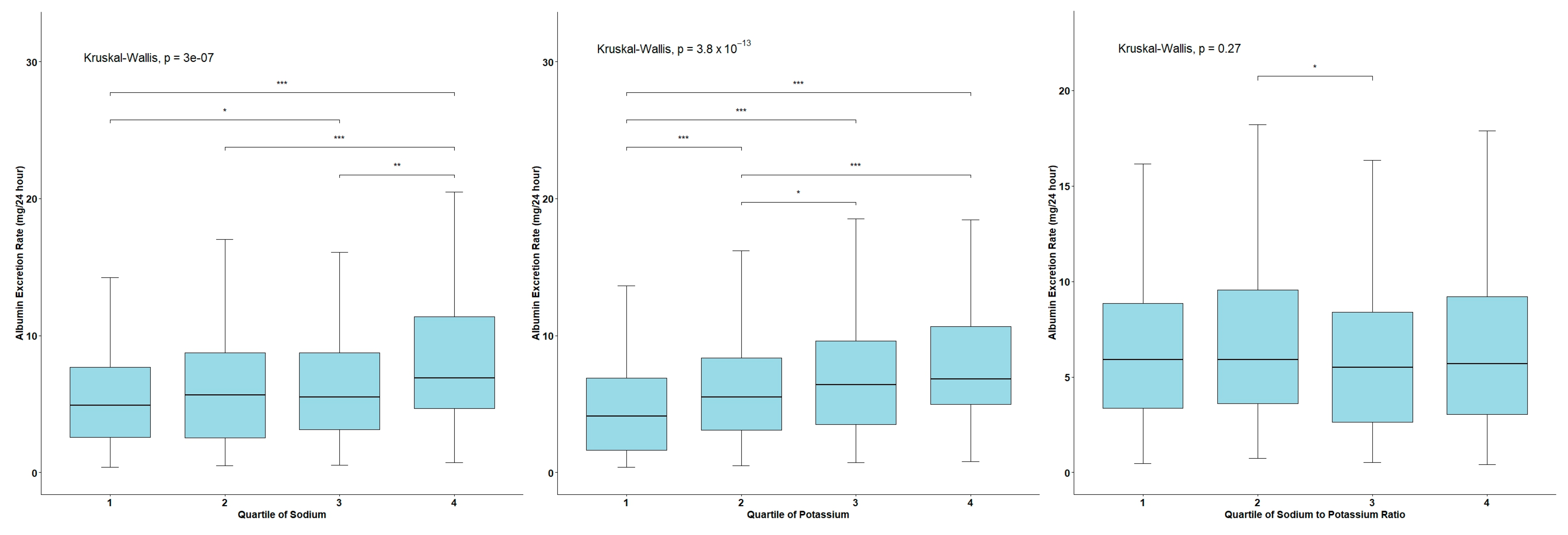
3.2. AER Levels across the Quartiles of Urinary Sodium and Potassium Excretion and the Na/K Ratio
3.3. Correlation Analysis between Urinary Sodium and Potassium Excretion, the Na/K Ratio, and AER
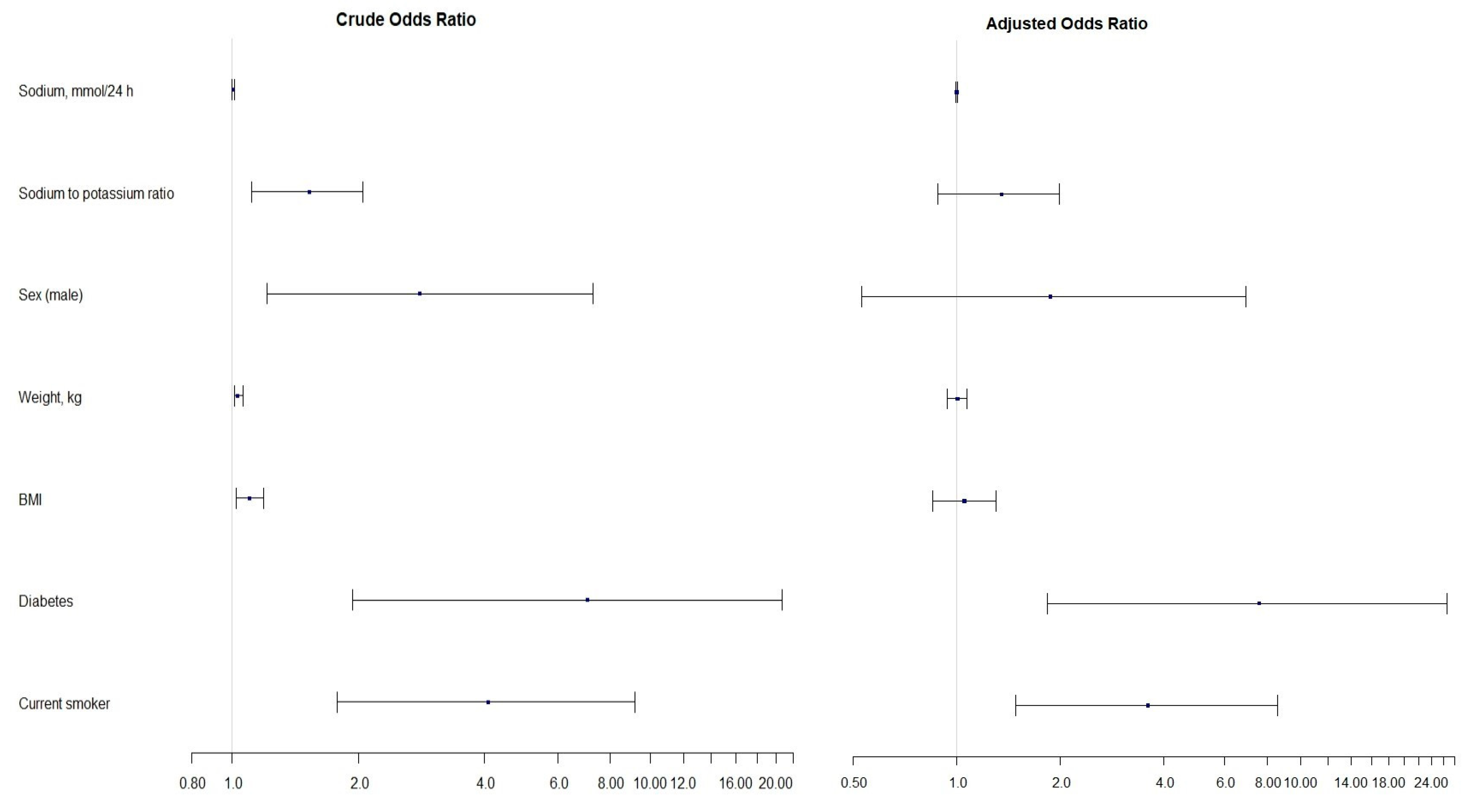
3.4. Estimates of Linear and Logistic Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Aitken, G.R.; Roderick, P.J.; Fraser, S.; Mindell, J.S.; O’Donoghue, D.; Day, J.; Moon, G. Change in prevalence of chronic kidney disease in England over time: Comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open 2014, 4, e005480. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Dember, L.M.; Ingelfinger, J.R.; Vinson, A.; Neugarten, J.; Sandberg, K.L.; Sullivan, J.C.; Maric-Bilkan, C.; Rankin, T.L.; Kimmel, P.L.; et al. Sex and the kidneys: Current understanding and research opportunities. Nat. Rev. Nephrol. 2019, 15, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Márquez, D.F.; Ruiz-Hurtado, G.; Segura, J.; Ruilope, L. Microalbuminuria and cardiorenal risk: Old and new evidence in different populations. F1000Research 2019, 8, 1659. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Li, Y.; He, F.J.; Wu, J.; Xu, J.; Zhang, X.; Li, X.; Song, J. Association of Sodium, Potassium and Sodium-to-Potassium Ratio with Urine Albumin Excretion among the General Chinese Population. Nutrients 2021, 13, 3456. [Google Scholar] [CrossRef]
- Hillege, H.L.; Janssen, W.M.; Bak, A.A.; Diercks, G.F.; Grobbee, D.E.; Crijns, H.J.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E.; Prevend Study Group. Microalbuminuria Is Common, Also in a Nondiabetic, Nonhypertensive Population, and an Independent Indicator of Cardiovascular Risk Factors and Cardiovascular Morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef]
- Mancia Chairperson, G.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Eae, A.; Azizi, M.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar]
- Ma, H.; Xue, Q.; Wang, X.; Li, X.; Franco, O.H.; Li, Y.; Heianza, Y.; Manson, J.E.; Qi, L. Adding salt to foods and hazard of premature mortality. Eur. Heart J. 2022, 43, 2878–2888. [Google Scholar] [CrossRef] [PubMed]
- Deriaz, D.; Guessous, I.; Vollenweider, P.; Devuyst, O.; Burnier, M.; Bochud, M.; Ponte, B. Estimated 24-h urinary sodium and sodium-to-potassium ratio are predictors of kidney function decline in a population-based study. J. Hypertens. 2019, 37, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Khaledifar, A.; Gharipour, M.; Bahonar, A.; Sarrafzadegan, N.; Khosravi, A. Association between Salt Intake and Albuminuria in Normotensive and Hypertensive Individuals. Int. J. Hypertens. 2013, 2013, 523682. [Google Scholar] [CrossRef] [PubMed]
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. Adv. Exp. Med. Biol. 2017, 956, 61–84. [Google Scholar]
- Elfassy, T.; Zhang, L.; Raij, L.; Bibbins-Domingo, K.; Lewis, C.E.; Allen, N.B.; Liu, K.J.; Peralta, C.A.; Odden, M.C.; Al Hazzouri, A.Z. Results of the CARDIA study suggest that higher dietary potassium may be kidney protective. Kidney Int. 2020, 98, 187–194. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, J.T.; Yoo, T.H.; Lee, J.; Chung, W.; Lee, K.B.; Chae, D.W.; Ahn, C.; Kang, S.W.; Choi, K.H.; et al. Urinary Potassium Excretion and Progression of CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 330–340. [Google Scholar] [CrossRef]
- Jardine, M.J.; Li, N.; Ninomiya, T.; Feng, X.; Zhang, J.; Shi, J.; Zhang, Y.; Zhang, R.; Zhang, J.; Hao, J.; et al. Dietary Sodium Reduction Reduces Albuminuria: A Cluster Randomized Trial. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2019, 29, 276–284. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2011, CD004022. [Google Scholar] [CrossRef]
- Kou, C.; Zhao, X.; Fan, X.; Lin, X.; Wang, Q.; Yu, J. Dietary sodium/potassium intake and cognitive impairment in older patients with hypertension: Data from NHANES 2011–2014. J. Clin. Hypertens. 2023, 25, 534–544. [Google Scholar] [CrossRef]
- Guideline: Sodium Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150483-6.
- Guideline: Potassium Intake for Adults and Children; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150482-9.
- Cogswell, M.E.; Loria, C.M.; Terry, A.L.; Zhao, L.; Wang, C.Y.; Chen, T.C.; Wright, J.D.; Pfeiffer, C.M.; Merritt, R.; Moy, C.S.; et al. Estimated 24-H Urinary Sodium and Potassium Excretion in US Adults. JAMA 2018, 319, 1209–1220. [Google Scholar] [CrossRef]
- Zakauskiene, U.; Macioniene, E.; Zabuliene, L.; Sukackiene, D.; Linkeviciute-Dumce, A.; Banys, V.; Bratcikoviene, N.; Karosiene, D.; Slekiene, V.; Kontrimas, V.; et al. Sodium, Potassium and Iodine Intake in an Adult Population of Lithuania. Nutrients 2022, 14, 3817. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A.P.; McNulty, B.A.; Flynn, A.; Walton, J. Sodium and Potassium Intakes and Their Ratio in Adults (18-90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Obreja, G.; Ciobanu, A.; Breda, J.; Jewell, J.; Cappuccio, F.P. Sodium, Potassium and Iodine Intake, in A National Adult Population Sample of the Republic of Moldova. Nutrients 2019, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- D’elia, L.; Brajović, M.; Klisic, A.; Breda, J.; Jewell, J.; Cadjenović, V.; Cappuccio, F.P. Sodium and Potassium Intake, Knowledge Attitudes and Behaviour Towards Salt Consumption Amongst Adults in Podgorica, Montenegro. Nutrients 2019, 11, 160. [Google Scholar] [CrossRef]
- Intersalt Cooperative Research Group. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 1988, 297, 319–328. [Google Scholar] [CrossRef]
- Joo, Y.S.; Kim, H.W.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Urinary Sodium-to-Potassium Ratio and Incident Chronic Kidney Disease: Results From the Korean Genome and Epidemiology Study. Mayo Clin. Proc. 2022, 97, 2259–2270. [Google Scholar] [CrossRef]
- Hattori, H.; Hirata, A.; Kubo, S.; Nishida, Y.; Nozawa, M.; Kawamura, K.; Hirata, T.; Kubota, Y.; Sata, M.; Kuwabara, K.; et al. Estimated 24 h Urinary Sodium-to-Potassium Ratio Is Related to Renal Function Decline: A 6-Year Cohort Study of Japanese Urban Residents. Int. J. Environ. Res. Public Health 2020, 17, 5811. [Google Scholar] [CrossRef]
- Koo, H.; Hwang, S.; Kim, T.H.; Kang, S.W.; Oh, K.H.; Ahn, C.; Kim, Y.H. The ratio of urinary sodium and potassium and chronic kidney disease progression: Results from the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). Medicine 2018, 97, e12820. [Google Scholar] [CrossRef]
- John, K.A.; Cogswell, M.E.; Campbell, N.R.; Nowson, C.A.; Legetic, B.; Hennis, A.J.M.; Patel, S.M. Accuracy and Usefulness of Select Methods for Assessing Complete Collection of 24-H Urine: A Systematic Review. J. Clin. Hypertens. 2016, 18, 456–467. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Brantsma, A.H.; de Zeeuw, D.; Bakker, S.J.; de Jong, P.E.; Gansevoort, R.T.; PREVEND Study Group. Albuminuria assessed from first-morning-void urine samples versus 24-h urine collections as a predictor of cardiovascular morbidity and mortality. Am. J. Epidemiol. 2008, 168, 897–905. [Google Scholar] [CrossRef]
- Mann, S.J.; Gerber, L.M. Addressing the problem of inaccuracy of measured 24-h urine collections due to incomplete collection. J. Clin. Hypertens. 2019, 21, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for the Eastern Mediterranean How to Obtain Measures of Population-Level Sodium Intake in 24-H Urine Samples; World Health Organization: Geneva, Switzerland; Regional Office for the Eastern Mediterranean: Cairo, India, 2018. [Google Scholar]
- Pan American Health Organization; World Health Organization. Salt-Smart Americas: A Guide for Country-Level Action; PAHO: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization; Regional Office for Europe. How to Obtain Measures of Population-Level Sodium Intake in 24-H Urine Samples: Protocol; World Health Organization: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults. Treatment Guidelines. In Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1998. Available online: https://www.ncbi.nlm.nih.gov/books/NBK2004/ (accessed on 24 July 2024).
- Lucko, A.M.; Doktorchik, C.; Woodward, M.; Cogswell, M.; Neal, B.; Rabi, D.; Anderson, C.; He, F.J.; MacGregor, G.A.; L’Abbe, M.; et al. Percentage of ingested sodium excreted in 24-h urine collections: A systematic review and meta-analysis. J. Clin. Hypertens. 2018, 20, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. INTERSALT study findings. Public health and medical care implications. Hypertension 1989, 14, 570–577. [Google Scholar] [CrossRef]
- Forman, J.P.; Scheven, L.; de Jong, P.E.; Bakker, S.J.L.; Curhan, G.C.; Gansevoort, R.T. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation 2012, 125, 3108–3116. [Google Scholar] [CrossRef]
- Aaron, K.J.; Campbell, R.C.; Judd, S.E.; Sanders, P.W.; Muntner, P. Association of dietary sodium and potassium intakes with albuminuria in normal-weight, overweight, and obese participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study1234. Am. J. Clin. Nutr. 2011, 94, 1071–1078. [Google Scholar] [CrossRef]
- Nam, G.E.; Han, K.; Park, Y.G.; Kim, Y.H.; Han, B.; Kim, S.M.; Choi, Y.S.; Cho, K.H.; Lee, K.S.; Kim, D.H. Prevalence and related risk factors of albuminuria in Korean adults: The 2011 Korea National Health and Nutrition Examination Survey. Nephron Clin. Pract. 2013, 124, 232–238. [Google Scholar] [CrossRef]
- Yan, L.; Guo, X.; Wang, H.; Zhang, J.; Tang, J.; Lu, Z.; Cai, X.; Liu, L.; Gracely, E.J.; Ma, J. Population-based association between urinary excretion of sodium, and potassium and its ratio with albuminuria in Chinese. Asia Pac. J. Clin. Nutr. 2016, 25, 785–797. [Google Scholar]
- Han, S.Y.; Hong, J.W.; Noh, J.H.; Kim, D.J. Association of the Estimated 24-H Urinary Sodium Excretion with Albuminuria in Adult Koreans: The 2011 Korea National Health and Nutrition Examination Survey. PLoS ONE 2014, 9, e109073. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Jia, Y.; Zou, M.; Zhen, Z.; Xue, Y. Effect of a sodium restriction diet on albuminuria and blood pressure in diabetic kidney disease patients: A meta-analysis. Int. Urol. Nephrol. 2022, 54, 1249–1260. [Google Scholar] [CrossRef]
- Xu, C.; Du, X.; Fang, L.; Zhong, J.; Lu, F. Association of 24-h urinary sodium excretion with microalbuminuria in a Chinese population. Sci. Rep. 2023, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Binia, A.; Jaeger, J.; Hu, Y.; Singh, A.; Zimmermann, D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 2015, 33, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wang, T.; Yue, W. The potassium puzzle: Exploring the intriguing connection to albuminuria. Front. Nutr. 2024, 11, 1375010. [Google Scholar] [CrossRef] [PubMed]
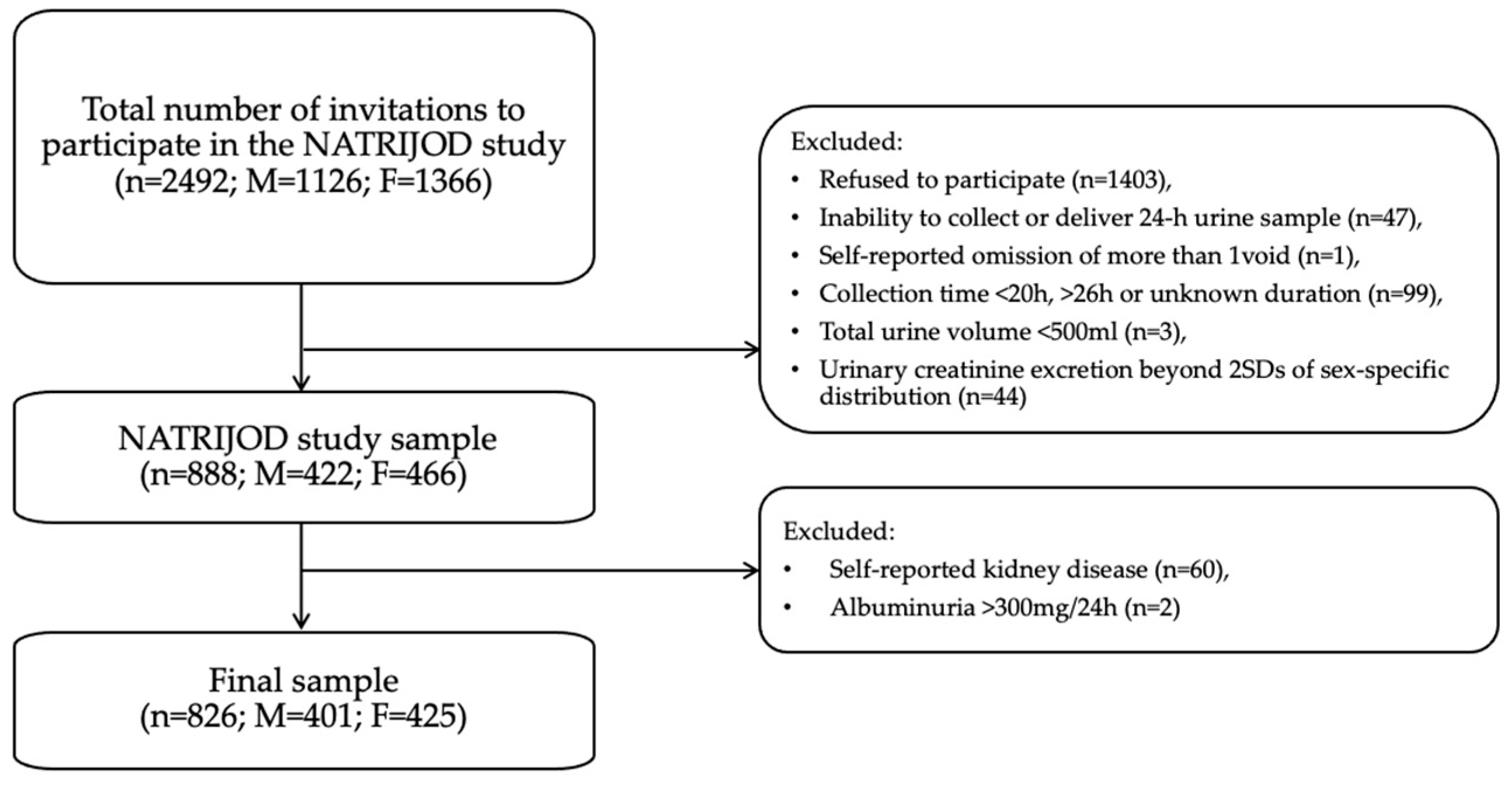

| Primary Exclusion Criteria | Secondary Exclusion Criteria | Laboratory Exclusion Criteria |
|---|---|---|
|
|
|
| Analyte (Urine) | Method | Traceability to Reference Material |
|---|---|---|
| Creatinine | Photometric, enzymatic | NIST SRM 967 |
| Sodium, potassium, chloride | Indirect potentiometry (indirect ion selective electrode (ISE)) | NIST SRM 918 and NIST SRM 919 |
| Urea | Photometric, enzymatic | NIST SRM 912 |
| Albumin | Immunoturbidimetric | CRM 470 |
| Variables | All Participants | Albuminuria (−) Group | Albuminuria (+) Group | p-Value |
|---|---|---|---|---|
| n | 826 | 801 | 25 | |
| Sex: male, n (%) | 401 (48.5) | 383 (47.8) | 18 (72.0) | <0.001 * |
| Age, year | 47.2 (12.1) | 47.1 (12.1) | 50.8 (12.3) | 0.138 |
| Height, cm | 173.4 (9.5) | 173.3 (9.5) | 176.3 (6.5) | 0.056 |
| Weight, kg | 79.8 (16.6) | 79.5 (16.5) | 89.5 (15.9) | 0.002 |
| BMI, kg/m2 | 26.5 (4.6) | 26.4 (4.6) | 28.7 (4.7) | 0.008 |
| BMI, n (%) | 0.01 ** | |||
| <18.5 kg/m2 | 13 (1.6) | 13 (1.7) | 0 (0.0) | |
| 18.5–24.9 kg/m2 | 335 (41.7) | 332 (42.6) | 3 (12.0) | |
| 25.0–29.9 kg/m2 | 278 (34.6) | 264 (33.9) | 14 (56.0) | |
| ≥30 kg/m2 | 178 (22.1) | 170 (21.8) | 8 (32.0) | |
| Systolic BP, mmHg | 124.0 (22.9) | 123.8 (23.1) | 132.3 (15.3) | 0.002 |
| Diastolic BP, mmHg | 77.9 (9.3) | 77.8 (9.3) | 81.4 (9.2) | 0.079 |
| Pulse rate, bpm | 72.7 (10.1) | 72.6 (10.2) | 74.2 (9.0) | 0.283 |
| Hypertension, n (%) | 284 (34.8) | 272 (34.3) | 12 (50.0) | 0.170 * |
| Antihypertensive medication, n (%) | 70 (8.5) | 68 (8.5) | 2 (8.0) | 1.000 * |
| Diabetes, n (%) | 25 (3.0) | 21 (2.6) | 4 (16.0) | 0.001 * |
| Current smokers, n (%) | 140 (16.9) | 129 (16.1) | 11 (44.0) | <0.001 * |
| Physical activity: active, n (%) | 543 (65.8) | 522 (65.2) | 21 (84.0) | 0.082 * |
| Variables | All Participants | Albuminuria (−) Group | Albuminuria (+) Group | p-Value |
|---|---|---|---|---|
| n | 826 | 801 | 25 | |
| Sodium, mmol/24 h | 163.3 (86.4) 146.3 (106.5–199.4) | 161.9 (85.1) 145.8 (106.4–197.5) | 208.2 (114.3) 196.6 (124.5–299.0) | 0.024 |
| Potassium, mmol/24 h | 73.8 (29.6) 69.9 (52.9–88.3) | 73.8 (29.6) 69.9 (52.8–88.4) | 72.1 (28.0) 72.3 (60.5–82.1) | 0.871 |
| Na/K ratio | 2.3 (1.1) 2.1 (1.6–2.9) | 2.3 (1.1) 2.1 (1.6–2.9) | 2.9 (1.3) 2.9 (1.8–4.0) | 0.016 |
| Salt intake, g/24 h | 10 (5.3) 9 (6.5–12.2) | 9.9 (5.2) 8.9 (6.5–12.1) | 12.8 (7.0) 12.1 (7.6–18.4) | 0.024 |
| Potassium intake, g/24 h | 3.3 (1.3) 3.1 (2.4–4.0) | 3.3 (1.3) 3.1 (2.4–4.0) | 3.2 (1.3) 3.2 (2.7–3.7) | 0.876 |
| AER, mg/24 h | 8.8 (16.1) 5.8 (3.1–9) | 6.7 (5.1) 5.7 (3.0–8.6) | 77.3 (55) 51.4 (38.5–102.8) | <0.001 |
| Factor | Univariate Model | Multivariable Model | ||
|---|---|---|---|---|
| Parameter β (95% CI) | p-Value | Parameter β (95% CI) | p-Value | |
| Sodium, mmol/24 h | 0.028 (0.015, 0.041) | <0.001 | 0.028 (0.016, 0.041) | <0.001 |
| Potassium, mmol/24 h | 0.040 (0.003, 0.077) | 0.035 | statistically insignificant | |
| Na/K ratio | 1.234 (0.210, 2.259) | 0.018 | statistically insignificant | |
| Sex (male) | 2.940 (0.747, 5.133) | 0.009 | statistically insignificant | |
| Weight, kg | 0.130 (0.062, 0.197) | <0.001 | statistically insignificant | |
| BMI, kg/m2 | 0.389 (0.147, 0.631) | 0.002 | statistically insignificant | |
| Systolic blood pressure, mmHg | 0.054 (0.007, 0.102) | 0.025 | statistically insignificant | |
| Diastolic blood pressure, mmHg | 0.153 (0.037, 0.270) | 0.010 | statistically insignificant | |
| Hypertension | 4.135 (1.867, 6.404) | <0.001 | statistically insignificant | |
| Diabetes | 18.383 (12.082, 24.684) | <0.001 | 18.475 (12.246, 24.703) | <0.001 |
| Factor | Crude | Adjusted | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Sodium, mmol/24 h | 1.00 (1.00, 1.01) | 0.010 | 1.00 (1.00, 1.00) | statistically insignificant |
| Na/K ratio | 1.53 (1.11, 2.05) | 0.006 | 1.35 (0.88, 1.99) | statistically insignificant |
| Sex (male) | 2.81 (1.21, 7.29) | 0.022 | 1.87 (0.53, 6.93) | statistically insignificant |
| Weight, kg | 1.03 (1.01, 1.06) | 0.003 | 1.00 (0.94, 1.07) | statistically insignificant |
| BMI, kg/m2 | 1.10 (1.02, 1.19) | 0.014 | 1.05 (0.85, 1.30) | statistically insignificant |
| Diabetes | 7.07 (1.94, 20.66) | <0.001 | 7.55 (1.83, 26.63) | 0.003 |
| Smoking | 4.09 (1.78, 9.20) | <0.001 | 3.59 (1.48, 8.56) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakauskiene, U.; Bratcikoviene, N.; Macioniene, E.; Zabuliene, L.; Sukackiene, D.; Linkeviciute-Dumce, A.; Karosiene, D.; Banys, V.; Migline, V.; Utkus, A.; et al. Association of Urinary Sodium, Potassium, and the Sodium-to-Potassium Ratio with Impaired Kidney Function Assessed with 24-H Urine Analysis. Nutrients 2024, 16, 3400. https://doi.org/10.3390/nu16193400
Zakauskiene U, Bratcikoviene N, Macioniene E, Zabuliene L, Sukackiene D, Linkeviciute-Dumce A, Karosiene D, Banys V, Migline V, Utkus A, et al. Association of Urinary Sodium, Potassium, and the Sodium-to-Potassium Ratio with Impaired Kidney Function Assessed with 24-H Urine Analysis. Nutrients. 2024; 16(19):3400. https://doi.org/10.3390/nu16193400
Chicago/Turabian StyleZakauskiene, Urte, Nomeda Bratcikoviene, Ernesta Macioniene, Lina Zabuliene, Diana Sukackiene, Ausra Linkeviciute-Dumce, Dovile Karosiene, Valdas Banys, Vilma Migline, Algirdas Utkus, and et al. 2024. "Association of Urinary Sodium, Potassium, and the Sodium-to-Potassium Ratio with Impaired Kidney Function Assessed with 24-H Urine Analysis" Nutrients 16, no. 19: 3400. https://doi.org/10.3390/nu16193400







