Fructose Consumption Affects Placental Production of H2S: Impact on Preeclampsia-Related Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Plasma Determinations
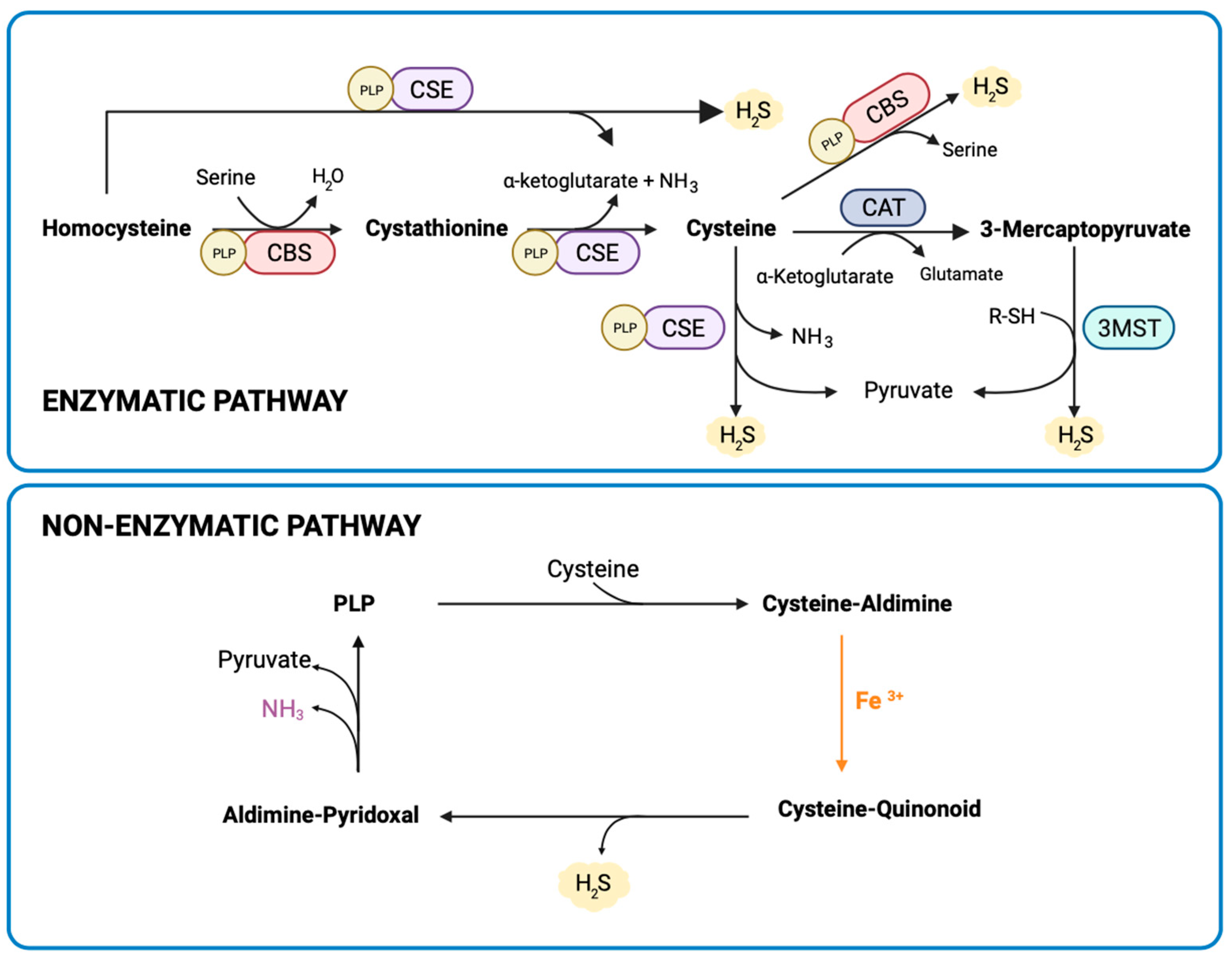
2.3. Measurement of Placental H2S Synthesis
2.4. RNA Extraction and Gene Expression Determination by qPCR
2.5. Statistical Analysis
3. Results
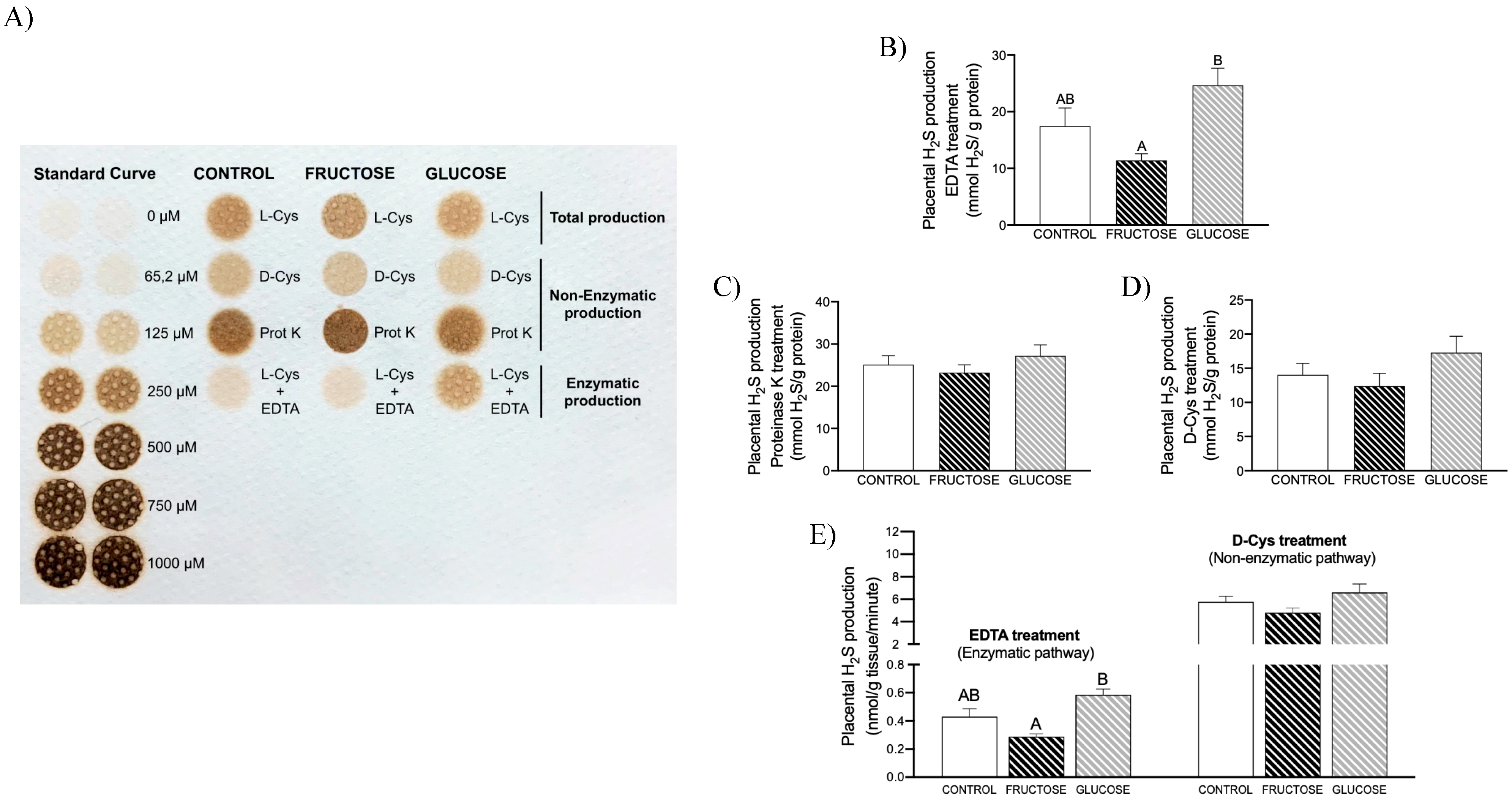
3.1. Placental H2S Is Mainly Non-Enzymatically Produced
3.2. Maternal Intake of Fructose Increases H2S Production in Placenta of Descendants
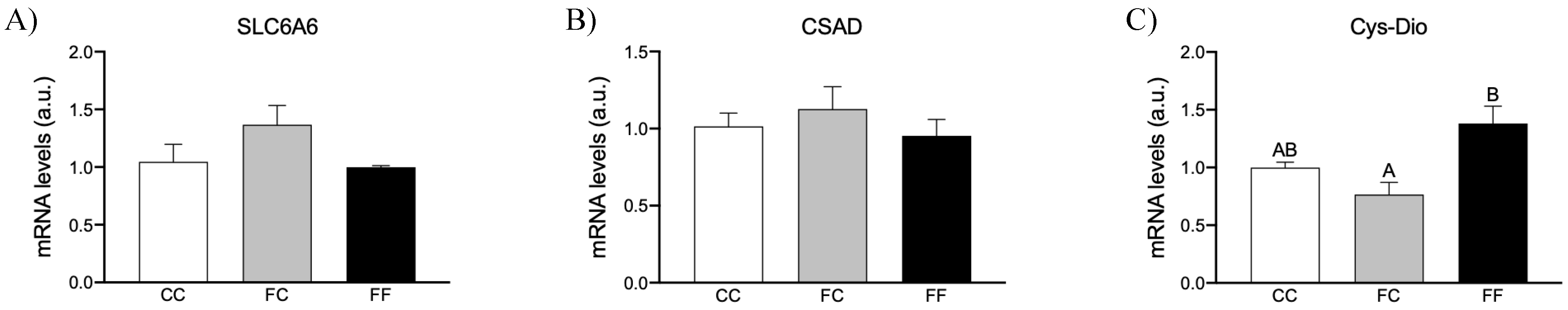
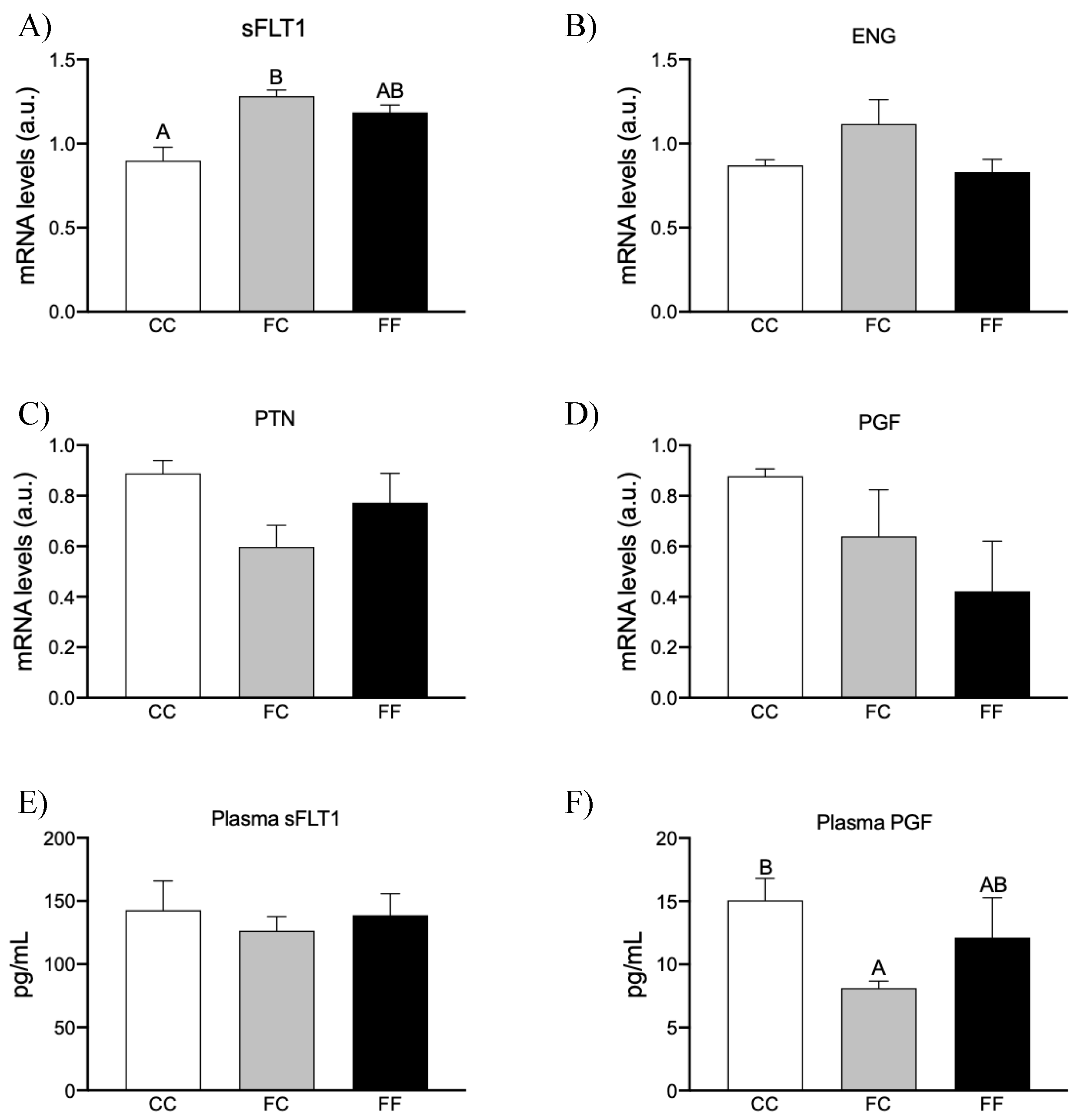
3.3. Maternal Fructose Intake and Fructose Consumption Impact on Preeclampsia-Related Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvallo, P.; Carvallo, E.; Barbosa-da-Silva, S.; Mandarim-de-Lacerda, C.A.; Hernández, A.; del-Sol, M. Efectos metabólicos del con-sumo excesivo de fructosa añadida. Int. J. Morphol. 2019, 37, 1058–1066. [Google Scholar] [CrossRef]
- Khitan, Z.; Kim, D.H. Fructose: A Key Factor in the Development of Metabolic Syndrome and Hypertension. J. Nutr. Metab. 2013, 2013, 682673. [Google Scholar] [CrossRef] [PubMed]
- Dupas, J.; Feray, A.; Goanvec, C.; Guernec, A.; Samson, N.; Bougaran, P.; Guerrero, F.; Mansourati, J. Metabolic Syndrome and Hypertension Resulting from Fructose Enriched Diet in Wistar Rats. BioMed Res. Int. 2017, 2017, 2494067. [Google Scholar] [CrossRef] [PubMed]
- Delbosc, S.; Paizanis, E.; Magous, R.; Araiz, C.; Dimo, T.; Cristol, J.P.; Cros, G.; Azay, J. Involvement of oxidative stress and NADPH oxidase ac-tivation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis 2005, 179, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Hocher, B. Fetal programming of cardiovascular diseases in later life–mechanisms beyond maternal undernutrition. J. Physiol. 2007, 579 Pt 2, 287–288. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; Picardi, A.; Buonocore, G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J. Clin. Pediatr. 2016, 5, 172–181. [Google Scholar] [CrossRef]
- Wallace, J.L.; Blackler, R.W.; Chan, M.V.; Da Silva, G.J.; Elsheikh, W.; Flannigan, K.L.; Gamaniek, I.; Manko, A.; Wang, L.; Motta, J.-P.; et al. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: Translation to therapeutics. Antioxid. Redox Signal. 2015, 22, 398–410. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Homocysteine. Int. J. Biochem. Cell Biol. 2000, 32, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, C.; You, X.; Olson, D.M.; Chemtob, S.; Gao, L.; Ni, X. Hydrogen Sulfide Delays LPS-Induced Preterm Birth in Mice via Anti-Inflammatory Pathways. PLoS ONE 2016, 11, e0152838. [Google Scholar] [CrossRef] [PubMed]
- You, X.-J.; Xu, C.; Lu, J.-Q.; Zhu, X.-Y.; Gao, L.; Cui, X.-R.; Li, Y.; Gu, H.; Ni, X. Expression of cystathionine β-synthase and cystathionine γ-lyase in human pregnant myometrium and their roles in the control of uterine contractility. PLoS ONE 2011, 6, e23788. [Google Scholar] [CrossRef]
- Holwerda, K.M.; Faas, M.M.; van Goor, H.; Lely, A.T. Gasotransmitters: A solution for the therapeutic dilemma in preeclampsia? Hypertension 2013, 62, 653–659. [Google Scholar] [CrossRef]
- Holwerda, K.M.; Bos, E.M.; Rajakumar, A.; Ris-Stalpers, C.; van Pampus, M.G.; Timmer, A.; Erwich, J.J.H.M.; Faas, M.M.; van Goor, H.; Lely, A.T. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta 2012, 33, 518–521. [Google Scholar] [CrossRef]
- Ghulmiyyah, L.; Sibai, B. Maternal Mortality From Preeclampsia/Eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar] [CrossRef]
- Rengarajan, A.; Mauro, A.K.; Boeldt, D.S. Maternal disease and gasotransmitters. Nitric Oxide 2020, 96, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Ahmed, A. Dysregulation of hydrogen sulfide producing enzyme cysta-thionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013, 127, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Roles of Hydrogen Sulfide in Hypertension Development and Its Complications: What, So What, Now What. Hypertension 2023, 80, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Fauste, E.; Rodrigo, S.; Aguirre, R.; Donis, C.; Rodríguez, L.; Álvarez-Millán, J.J.; Panadero, M.I.; Otero, P.; Bocos, C. Maternal Fructose Intake Increases Liver H2S Synthesis but Exarcebates its Fructose-Induced Decrease in Female Progeny. Mol. Nutr. Food Res. 2020, 64, e2000628. [Google Scholar] [CrossRef] [PubMed]
- Roglans, N.; Fauste, E.; Bentanachs, R.; Velázquez, A.M.; Pérez-Armas, M.; Donis, C.; Laguna, J.C. Bempedoic Acid Restores Liver H2S Pro-duction in a Female Sprague-Dawley Rat Dietary Model of Non-Alcoholic Fatty Liver. Int. J. Mol. Sci. 2022, 24, 473. [Google Scholar] [CrossRef]
- Rodríguez, L.; Panadero, M.I.; Roglans, N.; Otero, P.; Alvarez-Millán, J.J.; Laguna, J.C.; Bocos, C. Fructose during preg-nancy affects maternal and fetal leptin signaling. J. Nutr. Biochem. 2013, 24, 1709–1716. [Google Scholar] [CrossRef]
- Fauste, E.; Panadero, M.I.; Donis, C.; Otero, P.; Bocos, C. Pregnancy Is Enough to Provoke Deleterious Effects in Descendants of Fructose-Fed Mothers and Their Fetuses. Nutrients 2021, 13, 3667. [Google Scholar] [CrossRef]
- Hine, C.; Mitchell, J.R. Endpoint or Kinetic Measurement of Hydrogen Sulfide Production Capacity in Tissue Extracts. Bio-protocol 2017, 7, e2382. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing Acts: Molecular Control of Mammalian Iron Metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Nakagawa, T.; Andres-Hernando, A.; Kosugi, T.; Sanchez-Lozada, L.G.; Stenvinkel, P.; Kublickiene, K.; Karumanchi, S.A.; Kang, D.-H.; Kojima, H.; Rodriguez-Iturbe, B.; et al. Fructose might be a clue to the origin of preeclampsia insights from nature and evolution. Hypertens. Res. 2023, 46, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, S.; Rodríguez, L.; Otero, P.; Panadero, M.I.; García, A.; Barbas, C.; Roglans, N.; Ramos, S.; Goya, L.; Laguna, J.C.; et al. Fructose during pregnancy provokes fetal oxidative stress: The key role of the placental heme oxygenase-1. Mol. Nutr. Food Res. 2016, 60, 2700–2711. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Clayton, Z.E.; Yap, C.; Sloboda, D.M. Maternal fructose intake during pregnancy and lactation alters placental growth and leads to sex-specific changes in fetal and neonatal endocrine function. Endocrinology. 2011, 152, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Altıntas, A.; Yavuz, F.; Copur, S.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Johnson, R.J. Responses to Hypoxia: How Fructose Me-tabolism and Hypoxia-Inducible Factor-1a Pathways Converge in Health and Disease. Curr. Nutr. Rep. 2023, 12, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, T.; Schönenberger, M.J.; Walter, K.M.; Charles, K.N.; Faust, P.L.; Kovacs, W.J. Peroxisome-Deficiency and HIF-2α Signaling Are Negative Regulators of Ketohexokinase Expression. Front. Cell Dev. Biol. 2020, 8, 566. [Google Scholar] [CrossRef]
- Perez-Pinera, P.; Berenson, J.R.; Deuel, T.F. Pleiotrophin, a multifunctional angiogenic factor: Mechanisms and pathways in normal and pathological angiogenesis. Curr. Opin. Hematol. 2008, 15, 210–214. [Google Scholar] [CrossRef]
- He, Y.; Smith, S.K.; Day, K.A.; Clark, D.E.; Licence, D.R.; Charnock-Jones, D.S. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol. Endocrinol. 1999, 13, 537–545. [Google Scholar] [CrossRef]
- Patel, P.; Vatish, M.; Heptinstall, J.; Wang, R.; Carson, R.J. The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod. Biol. Endocrinol. 2009, 7, 10. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Herrera, E.A.; Niu, Y.; Kingdom, J.; Giussani, D.A.; Burton, G.J. Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am. J. Pathol. 2013, 182, 1448–1458. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Jing, M.-R.; Cai, C.-B.; Zhu, S.-G.; Zhang, C.-J.; Wang, Q.-M.; Zhai, Y.-K.; Ji, X.-Y.; Wu, D.-D. Role of hydrogen sulphide in physiological and pathological angiogenesis. Cell Prolif. 2023, 56, e13374. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Moore, P.K. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol. Sci. 2008, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]

| CC | FC | FF | |
|---|---|---|---|
| Fructose Metabolism | |||
| KHK | 0.88 ± 0.10 | 1.76 ± 0.56 | 1.40 ± 0.207 |
| Aldo B | 1.86 ± 0.89 | 3.49 ± 2.35 | 0.88 ± 0.38 |
| TKFC | 1.00 ± 0.16 | 0.85 ± 0.19 | 0.78 ± 0.21 |
| SDH | 1.04 ± 0.17 | 1.10 ± 0.41 | 0.70 ± 0.04 |
| HIF1α Target genes | |||
| HIF1α | 1.00 ± 0.05 | 1.42 ± 0.16 | 1.20 ± 0.12 |
| GLUT1 | 1.16 ± 0.10 | 0.91 ± 0.02 | 0.92 ± 0.18 |
| PDK1 | 0.92 ± 0.06 | 1.11 ± 0.07 | 1.20 ± 0.12 |
| LDHA | 0.99 ± 0.03 | 1.00 ± 0.10 | 0.87 ± 0.13 |
| MCT1 | 1.02 ± 0.12 | 1.06 ± 0.19 | 0.81 ± 0.02 |
| PFKB3 | 1.03 ± 0.15 | 1.04 ± 0.25 | 0.59 ± 0.07 |
| BNIP3 | 0.99 ± 0.05 | 1.14 ± 0.11 | 1.07 ± 0.13 |
| ADORA2B | 1.02 ± 0.11 | 0.72 ± 0.11 | 0.91± 0.12 |
| IL1β | 0.82 ± 0.06 | 1.71 ± 0.44 | 1.61 ± 0.34 |
| VEGF | 0.90 ± 0.02 | 1.31 ± 0.02 | 1.25 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Armas, M.; Fauste, E.; Donis, C.; Rodrigo, S.; Rodríguez, L.; Álvarez-Millán, J.J.; Panadero, M.I.; Otero, P.; Bocos, C. Fructose Consumption Affects Placental Production of H2S: Impact on Preeclampsia-Related Parameters. Nutrients 2024, 16, 309. https://doi.org/10.3390/nu16020309
Pérez-Armas M, Fauste E, Donis C, Rodrigo S, Rodríguez L, Álvarez-Millán JJ, Panadero MI, Otero P, Bocos C. Fructose Consumption Affects Placental Production of H2S: Impact on Preeclampsia-Related Parameters. Nutrients. 2024; 16(2):309. https://doi.org/10.3390/nu16020309
Chicago/Turabian StylePérez-Armas, Madelín, Elena Fauste, Cristina Donis, Silvia Rodrigo, Lourdes Rodríguez, Juan J. Álvarez-Millán, María I. Panadero, Paola Otero, and Carlos Bocos. 2024. "Fructose Consumption Affects Placental Production of H2S: Impact on Preeclampsia-Related Parameters" Nutrients 16, no. 2: 309. https://doi.org/10.3390/nu16020309
APA StylePérez-Armas, M., Fauste, E., Donis, C., Rodrigo, S., Rodríguez, L., Álvarez-Millán, J. J., Panadero, M. I., Otero, P., & Bocos, C. (2024). Fructose Consumption Affects Placental Production of H2S: Impact on Preeclampsia-Related Parameters. Nutrients, 16(2), 309. https://doi.org/10.3390/nu16020309








