Iron Deficiency: Global Trends and Projections from 1990 to 2050
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources, Definitions
2.2. Data Analysis
3. Results
3.1. Sensitivity Analysis, Modeling Fitting, and Validation
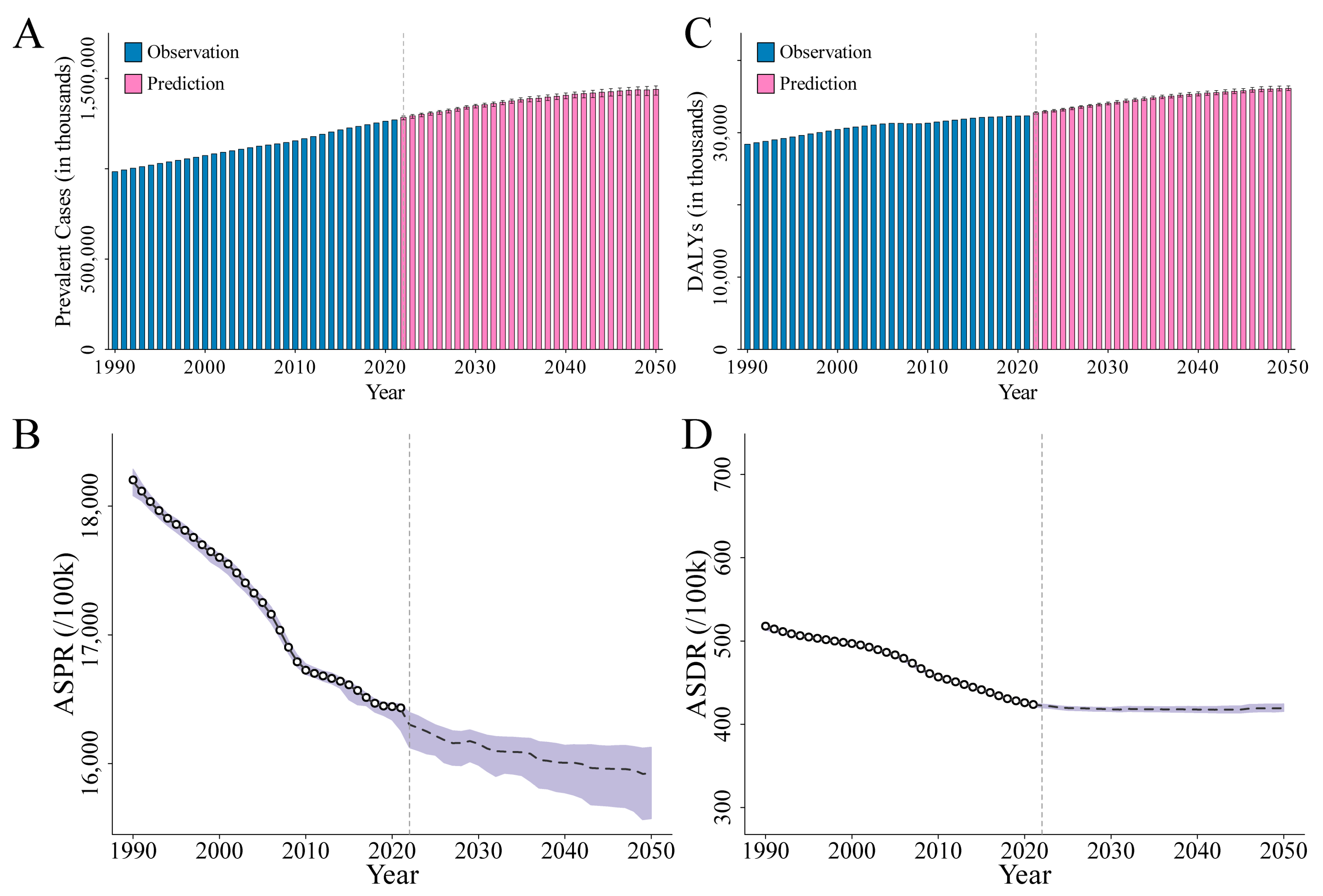
3.2. Overall Burden of ID
3.2.1. Prevalence of ID
3.2.2. DALYs of ID
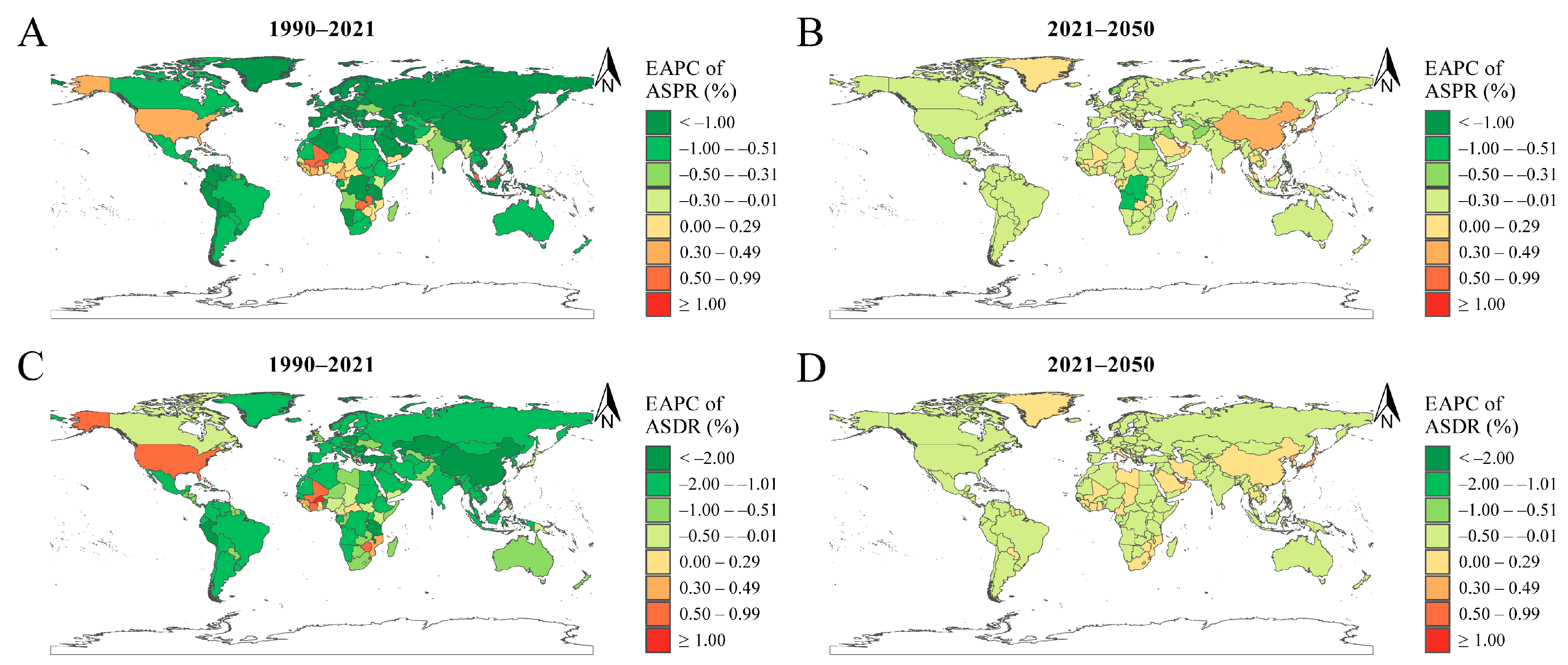
3.3. APC Analysis of ID Prevalence and DALYs
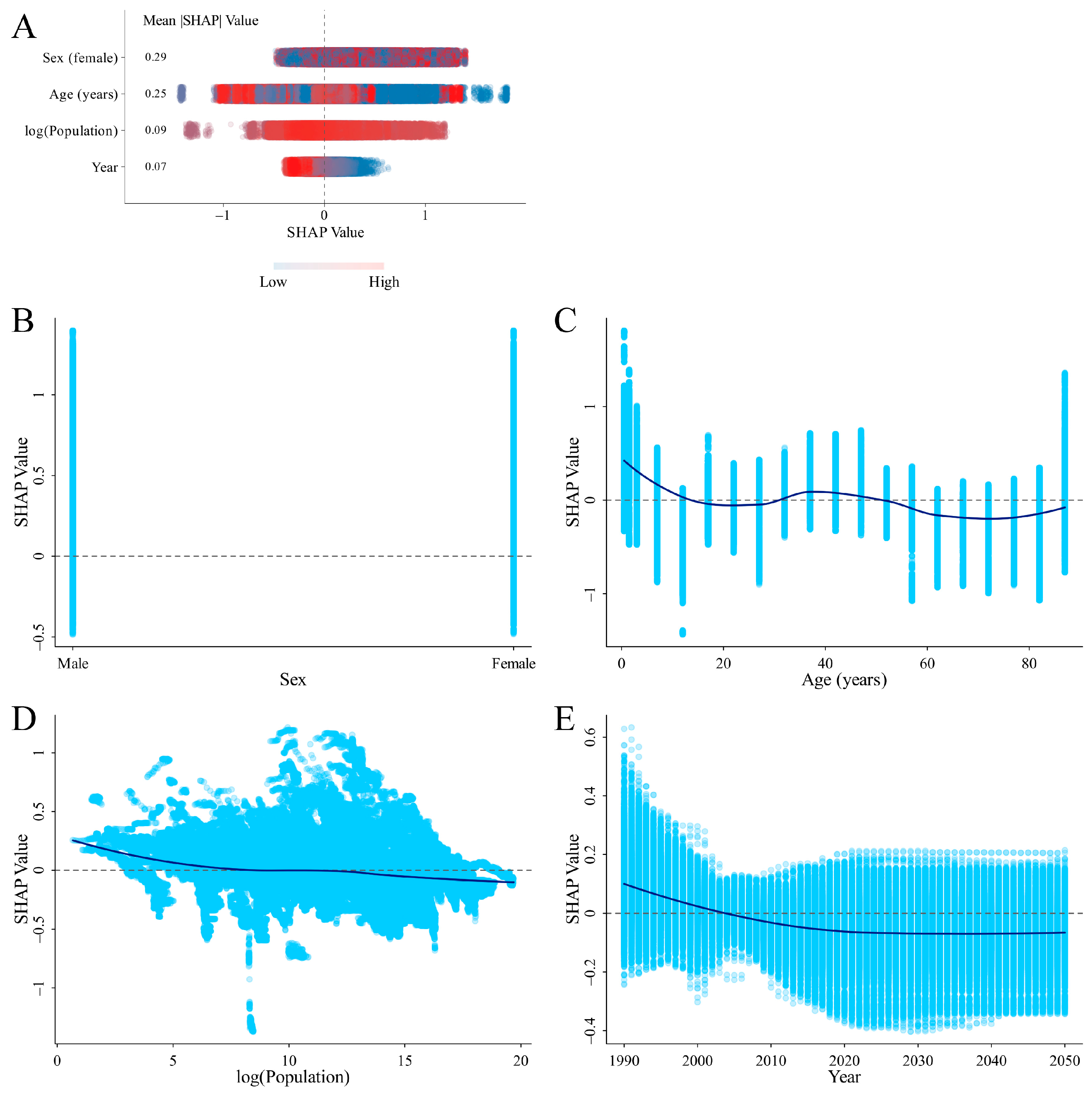
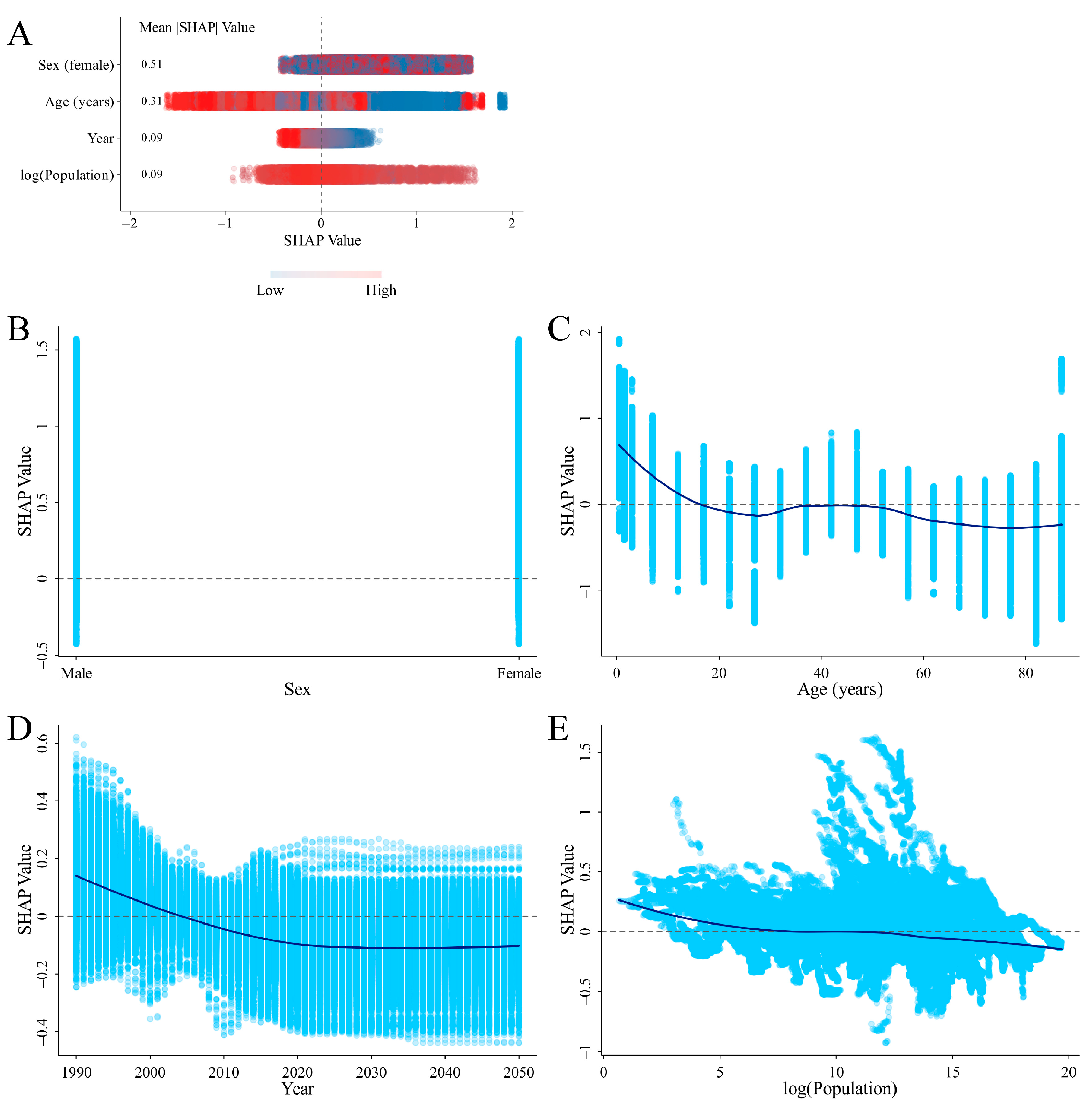
3.4. SHAP Analysis on the Variables Influencing ID Prevalence and DALYs Rates
3.5. Correlation between SDI and ASPR or ASDR of ID
3.6. Correlation between the ASPR of ID and Anemia
4. Discussion
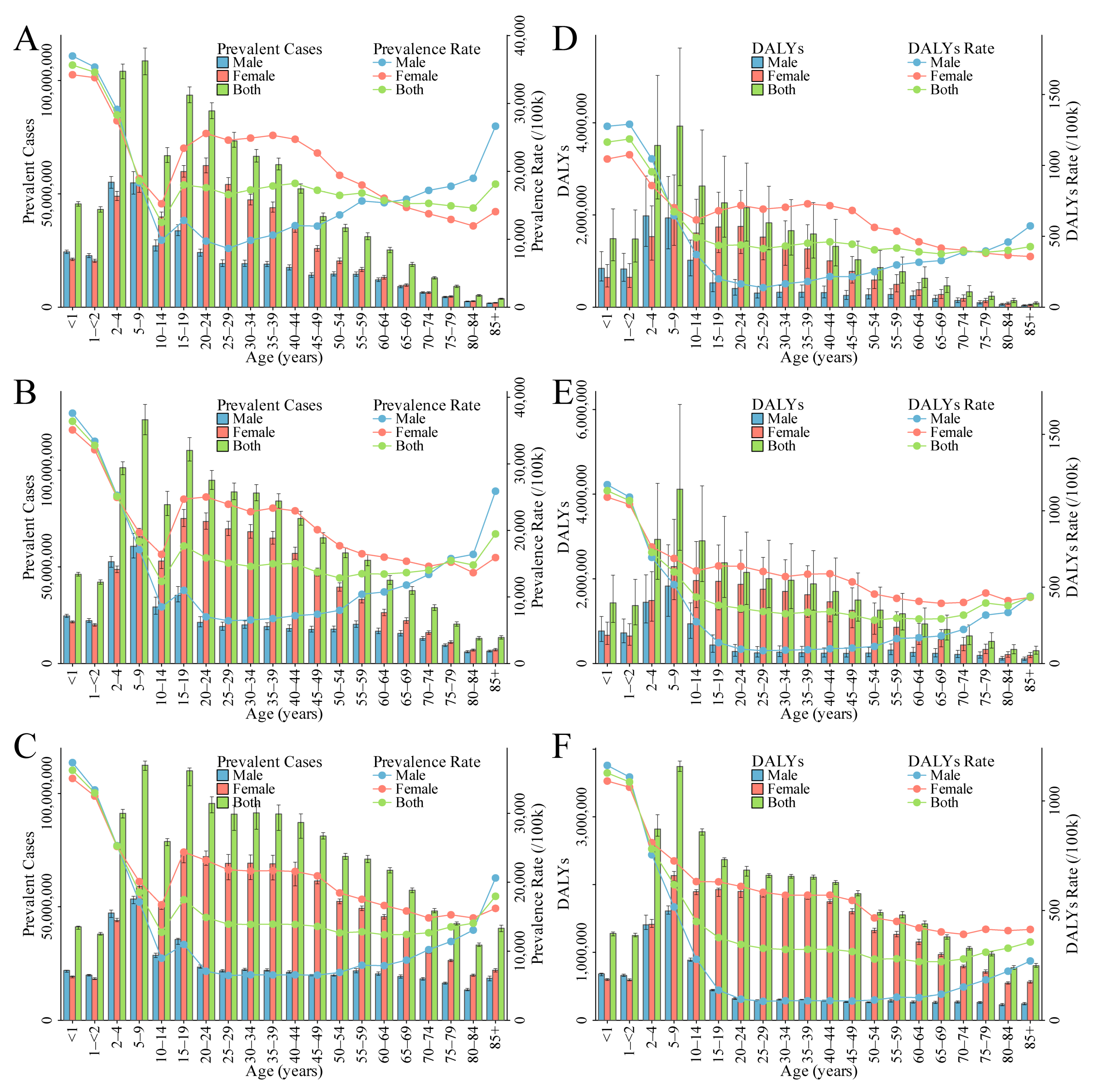
4.1. The Burden of ID: Groups with Higher Burden among Children, Women, and Elderly Men
4.2. The Impact of Social and Economic Development on ID Burden: ASPR and ASDR Trends across SDI Level
4.3. SHAP Analysis for Identifying Critical Factors Influencing ID
4.4. Implications of the Correlation between ID and Anemia
4.5. Challenges in Modeling ID with XGBoost and SHAP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A. Infants: Weaning. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 418–423. [Google Scholar]
- World Health Organization. New Thresholds for the Use of Ferritin Concentrations to Access Iron Status in Individuals and Populations. Available online: https://www.who.int/docs/default-source/micronutrients/ferritin-guideline/ferritin-guidelines-brochure.pdf?sfvrsn=76a71b5a_4 (accessed on 1 September 2024).
- World Health Organization. Anaemia in Children <5 Years. 2017. Available online: http://apps.who.int/gho/data/view.main.ANEMIACHILDRENREGv?lang=en (accessed on 1 September 2024).
- World Health Organization. Prevalence of Anaemia in Women. 2017. Available online: http://apps.who.int/gho/data/node.main.ANAEMIAWOMEN?lang=en (accessed on 1 September 2024).
- Di Renzo, G.C.; Spano, F.; Giardina, I.; Brillo, E.; Clerici, G.; Roura, L.C. Iron Deficiency Anemia in Pregnancy. Women’s Health 2015, 11, 891–900. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidance Helps Detect Iron Deficiency and Protect Brain Development. 2020. Available online: https://www.who.int/news/item/20-04-2020-who-guidance-helps-detect-iron-deficiency-and-protect-brain-development#:~:text=Iron%20deficiency%20occurs%20mainly%20when%20the%20requirements%20of,anaemia%2C%20reduced%20birth%20weight%20and%20reduced%20gestation%20periods (accessed on 1 September 2024).
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Long, D.; Mao, C.; Liu, Y.; Zhou, T.; Xu, Y.; Zhu, Y. Evolving trends and burden of iron deficiency among children, 1990–2019: A systematic analysis for the global burden of disease study 2019. Front. Nutr. 2023, 10, 1275291. [Google Scholar] [CrossRef]
- Chernyavskiy, P.; Little, M.P.; Rosenberg, P.S. Correlated Poisson models for age-period-cohort analysis. Stat. Med. 2018, 37, 405–424. [Google Scholar] [CrossRef]
- Trächsel, B.; Rousson, V.; Bulliard, J.L.; Locatelli, I. Comparison of statistical models to predict age-standardized cancer incidence in Switzerland. Biom. J. 2023, 65, 2200046. [Google Scholar] [CrossRef]
- Alim, M.; Ye, G.-H.; Guan, P.; Huang, D.-S.; Zhou, B.-S.; Wu, W. Comparison of ARIMA model and XGBoost model for prediction of human brucellosis in mainland China: A time-series study. BMJ Open 2020, 10, e039676. [Google Scholar] [CrossRef]
- Fang, Z.-G.; Yang, S.-Q.; Lv, C.-X.; An, S.-Y.; Wu, W. Application of a data-driven XGBoost model for the prediction of COVID-19 in the USA: A time-series study. BMJ Open 2022, 12, e056685. [Google Scholar] [CrossRef]
- Aas, K.; Jullum, M.; Løland, A. Explaining individual predictions when features are dependent: More accurate approximations to Shapley values. Artif. Intell. 2021, 298, 103502. [Google Scholar] [CrossRef]
- Wang, K.; Tian, J.; Zheng, C.; Yang, H.; Ren, J.; Liu, Y.; Han, Q.; Zhang, Y. Interpretable prediction of 3-year all-cause mortality in patients with heart failure caused by coronary heart disease based on machine learning and SHAP. Comput. Biol. Med. 2021, 137, 104813. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Hu, Y.; Zhu, L.; Mao, Y.; Liu, J. Predicting mortality in intensive care unit patients with heart failure using an interpretable machine learning model: Retrospective cohort study. J. Med. Internet Res. 2022, 24, e38082. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd ElHafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Chase, R.C.; Li, T.; Ramai, D.; Li, S.; Huang, X.; Antwi, S.O.; Keaveny, A.P.; Pang, M. Global burden of digestive diseases: A systematic analysis of the global burden of diseases study, 1990 to 2019. Gastroenterology 2023, 165, 773–783.e715. [Google Scholar] [CrossRef]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar]
- Brauer, M.; Roth, G.A.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME). Global Fertility, Mortality, Migration, and Population Forecasts 2017–2100. Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2020; Available online: https://ghdx.healthdata.org/record/ihme-data/global-population-forecasts-2017-2100 (accessed on 16 August 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Socio-Demographic Index (SDI) 1950–2021. Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2024; Available online: https://ghdx.healthdata.org/record/global-burden-disease-study-2021-gbd-2021-socio-demographic-index-sdi-1950%E2%80%932021 (accessed on 16 August 2024).
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Lundberg, S. A unified approach to interpreting model predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar]
- Rosenberg, P.S.; Check, D.P.; Anderson, W.F. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2296–2302. [Google Scholar] [CrossRef]
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. _xgboost: Extreme Gradient Boosting_. R Package Version 1.7.7.1. 2024. Available online: https://CRAN.R-project.org/package=xgboost (accessed on 1 September 2024).
- Liu, Y.; Just, A. _SHAPforxgboost: SHAP Plots for ‘XGBoost’_. R Package Version 0.1.3. 2023. Available online: https://cran.r-project.org/web/packages/SHAPforxgboost/index.html (accessed on 1 September 2024).
- Lozoff, B.; Georgieff, M.K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 2006, 13, 158–165. [Google Scholar] [CrossRef]
- Ziegler, E.E. Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr. Rev. 2011, 69, S37–S42. [Google Scholar] [CrossRef]
- Rao, R.; Georgieff, M.K. Iron in fetal and neonatal nutrition. Semin. Fetal Neonatal Med. 2007, 12, 54–63. [Google Scholar] [CrossRef]
- Krebs, N.F.; Hambidge, K.M. Complementary feeding: Clinically relevant factors affecting timing and composition23. Am. J. Clin. Nutr. 2007, 85, 639S–645S. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef]
- Shill, K.B.; Karmakar, P.; Kibria, M.G.; Das, A.; Rahman, M.A.; Hossain, M.S.; Sattar, M.M. Prevalence of iron-deficiency anaemia among university students in Noakhali region, Bangladesh. J. Health Popul. Nutr. 2014, 32, 103–110. [Google Scholar]
- Milman, N. Postpartum anemia I: Definition, prevalence, causes, and consequences. Ann. Hematol. 2011, 90, 1247–1253. [Google Scholar] [CrossRef]
- Romano, A.D.; Paglia, A.; Bellanti, F.; Villani, R.; Sangineto, M.; Vendemiale, G.; Serviddio, G. Molecular Aspects and Treatment of Iron Deficiency in the Elderly. Int. J. Mol. Sci. 2020, 21, 3821. [Google Scholar] [CrossRef]
- Yavuz, B.B.; Cankurtaran, M.; Haznedaroğlu, İ.C.; Halil, M.G.; Ulger, Z.; Altun, B.; Arıoğul, S. Iron deficiency can cause cognitive impairment in geriatric patients. J. Nutr. Health Aging 2012, 16, 220–224. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Wawer, A.A.; Gillings, R.; Jennings, A.; Myint, P.K. Iron status in the elderly. Mech. Ageing Dev. 2014, 136–137, 22–28. [Google Scholar] [CrossRef]
- Domellöf, M.; Braegger, C.P.; Campoy, C.; Colomb, V.; Decsi, T.s.; Fewtrell, M.S.; Hojsak, I.; Mihatsch, W.; Mølgaard, C.; Shamir, R.; et al. Iron Requirements of Infants and Toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Berglund, S.K.; Westrup, B.; Domellöf, M. Iron Supplements Reduce the Risk of Iron Deficiency Anemia in Marginally Low Birth Weight Infants. Pediatrics 2010, 126, e874–e883. [Google Scholar] [CrossRef]
- Thankachan, P.; Walczyk, T.; Muthayya, S.; Kurpad, A.V.; Hurrell, R.F. Iron absorption in young Indian women: The interaction of iron status with the influence of tea and ascorbic acid. Am. J. Clin. Nutr. 2008, 87, 881–886. [Google Scholar] [CrossRef]
- Wawer, A.A.; Jennings, A.; Fairweather-Tait, S.J. Iron status in the elderly: A review of recent evidence. Mech. Ageing Dev. 2018, 175, 55–73. [Google Scholar] [CrossRef]
- Detzel, P.; Wieser, S. Food Fortification for Addressing Iron Deficiency in Filipino Children: Benefits and Cost-Effectiveness. Ann. Nutr. Metab. 2015, 66, 35–42. [Google Scholar] [CrossRef]
- Larsen, B.; Baller, E.B.; Boucher, A.A.; Calkins, M.E.; Laney, N.; Moore, T.M.; Roalf, D.R.; Ruparel, K.; Gur, R.C.; Gur, R.E.; et al. Development of Iron Status Measures during Youth: Associations with Sex, Neighborhood Socioeconomic Status, Cognitive Performance, and Brain Structure. Am. J. Clin. Nutr. 2023, 118, 121–131. [Google Scholar] [CrossRef]
- Levi, M.; Rosselli, M.; Simonetti, M.; Brignoli, O.; Cancian, M.; Masotti, A.; Pegoraro, V.; Cataldo, N.; Heiman, F.; Chelo, M.; et al. Epidemiology of iron deficiency anaemia in four European countries: A population-based study in primary care. Eur. J. Haematol. 2016, 97, 583–593. [Google Scholar] [CrossRef]
- Malhotra, U.; Roy, M.; Sontakke, M.; Choudhary, P. A recent paradigm on iron absorption, prevalence, and emerging dietary approaches to eradicate iron deficiency. Food Bioeng. 2023, 2, 53–63. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Chouraqui, J.-P. Dietary Approaches to Iron Deficiency Prevention in Childhood—A Critical Public Health Issue. Nutrients 2022, 14, 1604. [Google Scholar] [CrossRef]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.; Moses, J.A.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Stoltzfus, R.J. Iron Deficiency: Global Prevalence and Consequences. Food Nutr. Bull. 2003, 24, S103–S199. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- PJ, H. Neglected tropical diseases in sub-saharan Africa: Review of their prevalence, distribution, and disease burden. PLoS Negl. Trop Dis. 2009, 3, e412. [Google Scholar]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P. A systematic analysis of global anemia burden from 1990 to 2010. Blood J. Am. Soc. Hematol. 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef]
- Balarajan, Y.; Ramakrishnan, U.; Özaltin, E.; Shankar, A.H.; Subramanian, S. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- Habib, A.; Kureishy, S.; Soofi, S.; Hussain, I.; Rizvi, A.; Ahmed, I.; Ahmed, K.M.; Achakzai, A.B.K.; Bhutta, Z.A. Prevalence and Risk Factors for Iron Deficiency Anemia among Children under Five and Women of Reproductive Age in Pakistan: Findings from the National Nutrition Survey 2018. Nutrients 2023, 15, 3361. [Google Scholar] [CrossRef]
- Estecha Querol, S.; Iqbal, R.; Kudrna, L.; Al-Khudairy, L.; Gill, P. The double burden of malnutrition and associated factors among south Asian adolescents: Findings from the global school-based student health survey. Nutrients 2021, 13, 2867. [Google Scholar] [CrossRef]
- Chhabra, S.; Kashyap, A.; Bhagat, M.; Mahajan, R.; Sethi, S. Anemia and nutritional status in tuberculosis patients. Int. J. Appl. Basic Med. Res. 2021, 11, 226–230. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low-and middle-income countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef]
- Sunuwar, D.R.; Singh, D.R.; Pradhan, P.M.S.; Shrestha, V.; Rai, P.; Shah, S.K.; Adhikari, B. Factors associated with anemia among children in South and Southeast Asia: A multilevel analysis. BMC Public Health 2023, 23, 343. [Google Scholar] [CrossRef]
- Tesema, G.A.; Worku, M.G.; Tessema, Z.T.; Teshale, A.B.; Alem, A.Z.; Yeshaw, Y.; Alamneh, T.S.; Liyew, A.M. Prevalence and determinants of severity levels of anemia among children aged 6–59 months in sub-Saharan Africa: A multilevel ordinal logistic regression analysis. PLoS ONE 2021, 16, e0249978. [Google Scholar] [CrossRef]
- Stoltzfus, R.J.; Chwaya, H.M.; Tielsch, J.M.; Schulze, K.J.; Albonico, M.; Savioli, L. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: The importance of hookworms. Am. J. Clin. Nutr. 1997, 65, 153–159. [Google Scholar] [CrossRef]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Iron fortification: Its efficacy and safety in relation to infections. Food Nutr. Bull. 2007, 28, S585–S594. [Google Scholar] [CrossRef]
- Cairncross, S.; Hunt, C.; Boisson, S.; Bostoen, K.; Curtis, V.; Fung, I.C.; Schmidt, W.-P. Water, sanitation and hygiene for the prevention of diarrhoea. Int. J. Epidemiol. 2010, 39, i193–i205. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Schneider, A.; Mertes, C.; Tatem, A.J.; Tan, B.; Sulla-Menashe, D.; Graves, S.J.; Patel, N.N.; Horton, J.A.; Gaughan, A.E.; Rollo, J.T.; et al. A new urban landscape in East–Southeast Asia, 2000–2010. Environ. Res. Lett. 2015, 10, 034002. [Google Scholar] [CrossRef]
- Wieringa, F.T.; Dijkhuizen, M.A.; Berger, J. Micronutrient deficiencies and their public health implications for South-East Asia. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 479–482. [Google Scholar] [CrossRef]
- Al Hasan, S.M.; Saulam, J.; Mikami, F.; Kanda, K.; Ngatu, N.R.; Yokoi, H.; Hirao, T. Trends in per Capita Food and Protein Availability at the National Level of the Southeast Asian Countries: An Analysis of the FAO’s Food Balance Sheet Data from 1961 to 2018. Nutrients 2022, 14, 603. [Google Scholar] [CrossRef]



| Location | Number in Thousands (ASR per 100,000) | EAPC (95% CI) (%) | |||
|---|---|---|---|---|---|
| 1990 | 2021 | 2050 | 1990–2021 | 2021–2050 | |
| Prevalence | |||||
| Overall | 984,607.52 (18,204) | 1,270,637.34 (16,433) | 1,439,994.05 (15,922) | −0.36 (−0.38 to −0.34) | −0.09 (−0.10 to −0.08) |
| Sex | |||||
| Male | 396,759.00 (14,700) | 444,691.17 (11,675) | 484,424.28 (11,042) | −0.78 (−0.82 to −0.75) | −0.18 (−0.19 to −0.16) |
| Female | 587,848.51 (21,892) | 825,946.17 (21,336) | 955,569.76 (21,000) | −0.10 (−0.13 to −0.08) | −0.02 (−0.03 to −0.02) |
| Region | |||||
| Andean Latin America | 7082.95 (17,489) | 6821.55 (10,382) | 8028.49 (9728) | −1.81 (−1.97 to −1.64) | −0.22 (−0.23 to −0.22) |
| Australasia | 943.61 (4832) | 1110.59 (3620) | 1268.77 (3285) | −0.91 (−1.04 to −0.78) | −0.24 (−0.28 to −0.20) |
| Caribbean | 7053.05 (19,457) | 8961.35 (19,491) | 8457.69 (19,163) | 0.03 (−0.07 to 0.14) | −0.04 (−0.05 to −0.03) |
| Central Asia | 17,249.54 (23,839) | 19,799.63 (20,386) | 23,470.23 (20,014) | −0.64 (−0.70 to −0.58) | −0.06 (−0.06 to −0.06) |
| Central Europe | 18,944.21 (15,569) | 11,687.33 (10,623) | 8902.88 (10,318) | −1.28 (−1.35 to −1.21) | −0.10 (−0.11 to −0.09) |
| Central Latin America | 16,343.81 (9372) | 17,116.68 (7011) | 20,406.94 (6691) | −0.87 (−0.94 to −0.81) | −0.14 (−0.16 to −0.12) |
| Central Sub-Saharan Africa | 14,108.07 (25,700) | 25,333.57 (18,599) | 39,115.37 (16,030) | −1.05 (−1.11 to −1.00) | −0.49 (−0.51 to −0.46) |
| East Asia | 139,772.15 (11,751) | 83,926.60 (5418) | 84,822.11 (5883) | −2.71 (−2.80 to −2.62) | 0.35 (0.32 to 0.38) |
| Eastern Europe | 28,550.56 (12,586) | 20,900.01 (9869) | 17,403.56 (9623) | −0.96 (−1.10 to −0.83) | −0.07 (−0.08 to −0.06) |
| Eastern Sub-Saharan Africa | 48,124.79 (23,921) | 89,598.69 (20,374) | 158,241.73 (19,802) | −0.57 (−0.61 to −0.53) | −0.08 (−0.10 to −0.06) |
| High-income Asia Pacific | 11,366.25 (6551) | 9661.41 (4459) | 8807.25 (4607) | −1.09 (−1.33 to −0.85) | 0.16 (0.12 to 0.20) |
| High-income North America | 10,707.62 (3676) | 13,933.83 (3581) | 14,606.84 (3420) | 0.25 (0.04 to 0.46) | −0.13 (−0.16 to −0.10) |
| North Africa and Middle East | 67,719.38 (19,409) | 88,916.15 (14,363) | 114,782.16 (13,217) | −0.91 (−0.94 to −0.88) | −0.24 (−0.26 to −0.21) |
| Oceania | 1544.13 (23,765) | 3038.61 (21,731) | 4750.17 (21,120) | −0.23 (−0.31 to −0.15) | −0.11 (−0.11 to −0.10) |
| South Asia | 393,349.18 (36,430) | 569,464.64 (31,696) | 615,459.06 (30,074) | −0.48 (−0.50 to −0.46) | −0.18 (−0.20 to −0.17) |
| Southeast Asia | 88,446.72 (19,578) | 99,767.83 (14,574) | 111,458.90 (13,929) | −1.05 (−1.13 to −0.98) | −0.16 (−0.17 to −0.15) |
| Southern Latin America | 4380.46 (8888) | 4061.43 (6454) | 4061.79 (5972) | −0.94 (−1.10 to −0.77) | −0.26 (−0.28 to −0.23) |
| Southern Sub-Saharan Africa | 11,097.39 (20,322) | 14,257.31 (17,563) | 18,328.74 (17,133) | −0.50 (−0.52 to −0.49) | −0.06 (−0.07 to −0.05) |
| Tropical Latin America | 29,083.48 (18,890) | 31,992.38 (14,253) | 31,131.27 (14,046) | −0.88 (−0.93 to −0.83) | −0.04 (−0.05 to −0.03) |
| Western Europe | 17,794.45 (4857) | 14,795.69 (3418) | 14,135.56 (3340) | −1.05 (−1.21 to −0.89) | −0.08 (−0.10 to −0.05) |
| Western Sub-Saharan Africa | 50,945.72 (24,938) | 135,492.08 (26,159) | 259,429.69 (26,045) | 0.19 (0.15 to 0.23) | −0.01 (−0.01 to −0.01) |
| High SDI | 53,685.35 (6152) | 51,611.55 (4579) | 59,591.16 (4510) | −0.78 (−0.94 to −0.61) | 0.03 (−0.01 to 0.06) |
| High–middle SDI | 128,441.90 (12,197) | 102,978.16 (8005) | 117,788.73 (7779) | −1.50 (−1.56 to −1.44) | −0.10 (−0.11 to −0.09) |
| Middle SDI | 295,665.49 (17,387) | 324,486.32 (13,564) | 414,938.03 (12,840) | −0.83 (−0.85 to −0.81) | −0.19 (−0.20 to −0.17) |
| Low–middle SDI | 349,563.99 (30,212) | 485,130.96 (25,686) | 716,530.29 (24,659) | −0.54 (−0.56 to −0.52) | −0.14 (−0.15 to −0.13) |
| Low SDI | 156,394.36 (30,844) | 305,505.41 (27,288) | 617,457.33 (26,374) | −0.44 (−0.47 to −0.41) | −0.11 (−0.11 to −0.10) |
| DALYs | |||||
| Overall | 28,406.13 (518) | 32,315.75 (424) | 36,131.94 (419) | −0.68 (−0.72 to −0.64) | −0.02 (−0.04 to −0.01) |
| Sex | |||||
| Male | 10,389.00 (372) | 9382.60 (253) | 9515.37 (242) | −1.32 (−1.40 to −1.24) | −0.15 (−0.17 to −0.12) |
| Female | 18,017.13 (668) | 22,933.15 (598) | 26,616.56 (603) | −0.37 (−0.39 to −0.34) | 0.05 (0.04 to 0.06) |
| Region | |||||
| Andean Latin America | 167.41 (394) | 135.59 (209) | 160.23 (199) | −2.18 (−2.23 to −2.13) | −0.14 (−0.16 to −0.12) |
| Australasia | 11.93 (59) | 14.56 (46) | 16.48 (43) | −0.78 (−0.91 to −0.66) | −0.22 (−0.25 to −0.19) |
| Caribbean | 193.90 (527) | 209.49 (465) | 192.52 (469) | −0.44 (−0.54 to −0.34) | 0.04 (0.03 to 0.05) |
| Central Asia | 529.88 (716) | 538.54 (551) | 626.23 (544) | −1.07 (−1.18 to −0.96) | −0.05 (−0.05 to −0.05) |
| Central Europe | 396.51 (329) | 203.38 (184) | 154.75 (177) | −1.98 (−2.07 to −1.90) | −0.17 (−0.19 to −0.14) |
| Central Latin America | 369.79 (205) | 352.33 (145) | 403.49 (138) | −0.99 (−1.06 to −0.92) | −0.19 (−0.21 to −0.17) |
| Central Sub-Saharan Africa | 378.66 (633) | 571.64 (398) | 948.47 (386) | −1.45 (−1.56 to −1.33) | −0.11 (−0.12 to −0.10) |
| East Asia | 3300.13 (285) | 1772.46 (117) | 1650.65 (123) | −3.12 (−3.23 to −3.01) | 0.21 (0.19 to 0.23) |
| Eastern Europe | 666.54 (299) | 431.67 (212) | 346.42 (205) | −1.42 (−1.60 to −1.23) | −0.14 (−0.15 to −0.13) |
| Eastern Sub-Saharan Africa | 1523.52 (700) | 2288.52 (496) | 3827.83 (479) | −1.22 (−1.30 to −1.14) | −0.11 (−0.12 to −0.10) |
| High-income Asia Pacific | 111.77 (66) | 110.03 (47) | 102.00 (50) | −0.87 (−1.07 to −0.66) | 0.16 (0.12 to 0.21) |
| High-income North America | 137.08 (47) | 200.97 (53) | 214.45 (51) | 0.70 (0.55 to 0.85) | −0.10 (−0.11 to −0.09) |
| North Africa and Middle East | 1841.63 (499) | 2241.35 (360) | 2933.00 (351) | −1.03 (−1.05 to −1.01) | −0.07 (−0.08 to −0.06) |
| Oceania | 31.50 (465) | 60.72 (419) | 92.57 (407) | −0.08 (−0.18 to 0.02) | −0.11 (−0.11 to −0.10) |
| South Asia | 13,548.86 (1235) | 15,671.28 (885) | 16,495.17 (837) | −1.09 (−1.13 to −1.04) | −0.20 (−0.22 to −0.18) |
| Southeast Asia | 2072.55 (440) | 2096.97 (310) | 2300.82 (309) | −1.16 (−1.20 to −1.13) | −0.06 (−0.08 to −0.05) |
| Southern Latin America | 51.84 (106) | 43.95 (69) | 42.74 (62) | −1.21 (−1.41 to −1.00) | −0.32 (−0.34 to −0.29) |
| Southern Sub-Saharan Africa | 362.16 (629) | 445.09 (546) | 549.17 (532) | −0.45 (−0.55 to −0.35) | −0.06 (−0.08 to −0.05) |
| Tropical Latin America | 841.06 (520) | 783.99 (363) | 718.40 (359) | −1.14 (−1.19 to −1.08) | −0.05 (−0.05 to −0.05) |
| Western Europe | 266.41 (79) | 210.48 (53) | 205.60 (53) | −1.27 (−1.42 to −1.12) | −0.02 (−0.03 to −0.01) |
| Western Sub-Saharan Africa | 1602.99 (733) | 3932.72 (708) | 7060.90 (696) | −0.08 (−0.17 to 0.01) | −0.06 (−0.06 to −0.06) |
| High SDI | 821.79 (98) | 768.62 (71) | 911.54 (71) | −0.92 (−1.04 to −0.79) | −0.03 (−0.04 to −0.02) |
| High–middle SDI | 2925.49 (281) | 1994.56 (158) | 2335.54 (157) | −2.09 (−2.18 to −1.99) | −0.05 (−0.05 to −0.04) |
| Middle SDI | 7977.35 (461) | 7621.49 (326) | 9855.23 (311) | −1.14 (−1.18 to −1.10) | −0.17 (−0.17 to −0.17) |
| Low–middle SDI | 11,517.45 (975) | 13,195.16 (702) | 19,556.11 (675) | −1.06 (−1.11 to −1.00) | −0.14 (−0.14 to −0.13) |
| Low SDI | 5143.75 (969) | 8715.89 (757) | 17,419.40 (723) | −0.81 (−0.84 to −0.78) | −0.13 (−0.14 to −0.12) |
| Year | ASPR (per 100,000) | ASDR (per 100,000) |
|---|---|---|
| 1990 | ||
| India (36,853) | Yemen (1440) | |
| Nepal (36,048) | Pakistan (1313) | |
| Pakistan (35,972) | Senegal (1269) | |
| 2021 | ||
| Senegal (34,421) | Yemen (1405) | |
| Mali (34,233) | Mozambique (1149) | |
| Pakistan (33,942) | Mali (1093) | |
| 2050 | ||
| Mali (35,070) | Yemen (1388) | |
| Senegal (34,132) | Mali (1181) | |
| Zambia (33,149) | Mozambique (1177) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liang, D.; Huangfu, H.; Shi, X.; Liu, S.; Zhong, P.; Luo, Z.; Ke, C.; Lai, Y. Iron Deficiency: Global Trends and Projections from 1990 to 2050. Nutrients 2024, 16, 3434. https://doi.org/10.3390/nu16203434
Wang L, Liang D, Huangfu H, Shi X, Liu S, Zhong P, Luo Z, Ke C, Lai Y. Iron Deficiency: Global Trends and Projections from 1990 to 2050. Nutrients. 2024; 16(20):3434. https://doi.org/10.3390/nu16203434
Chicago/Turabian StyleWang, Li, Dan Liang, Hengqian Huangfu, Xinfu Shi, Shuang Liu, Panpan Zhong, Zhen Luo, Changwen Ke, and Yingsi Lai. 2024. "Iron Deficiency: Global Trends and Projections from 1990 to 2050" Nutrients 16, no. 20: 3434. https://doi.org/10.3390/nu16203434






