The Effect of the Oral Contraceptive Pill on Acute Glycaemic Response to an Oral Glucose Bolus in Healthy Young Women: A Randomised Crossover Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Experimental Design and Study Visits
2.3. Plasma Analyses
2.4. Data Analyses
3. Results
3.1. Participant Characteristics
3.2. Fasting (Baseline) Levels of Hormones and Biomarkers of Metabolism
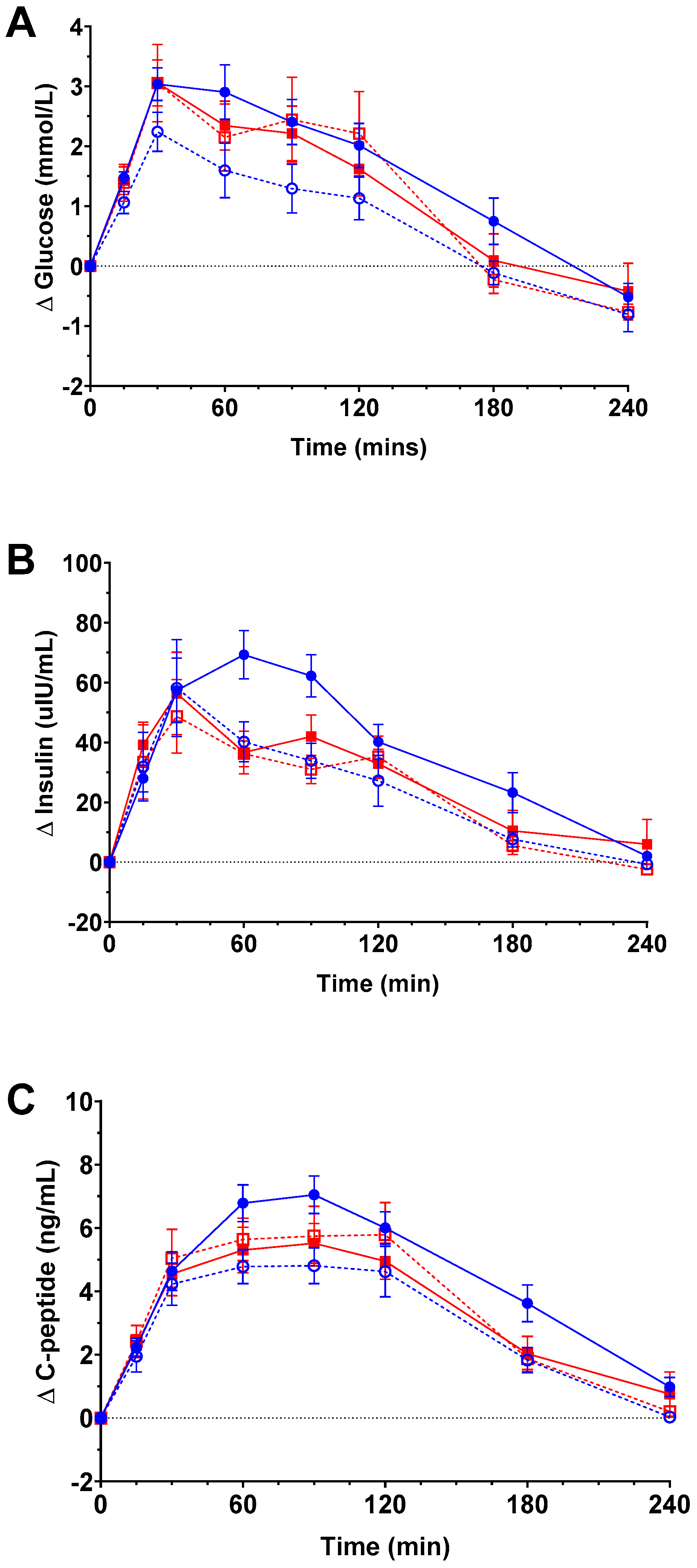
3.3. Postprandial Glycaemic Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, E.S.; Giles, W.H.; Mokdad, A.H. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care 2004, 27, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Majmundar, M.K.; Becker, T. (Eds.) High and Rising Mortality Rates among Working-Age Adults; National Academies Press: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Moore, J.X.; Chaudhary, N.; Akinyemiju, T. Metabolic syndrome prevalence by race/ethnicity and sex in the united states, national health and nutrition examination survey, 1988–2012. Prev. Chronic Dis. 2017, 14, E24. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.G.; Olden, K. The prevalence of metabolic syndrome among US women of childbearing age. Am. J. Public Health 2008, 98, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P. The key role of epigenetics in human disease prevention and mitigation. N. Engl. J. Med. 2018, 378, 1323–1334. [Google Scholar] [CrossRef]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef]
- United Nations. Contraceptive Use by Method 2019: Data Booklet [Internet]. 2019. Available online: https://www.un.org/development/desa/pd/content/contraceptive-use-method-2019 (accessed on 25 November 2020).
- Perseghin, G.; Scifo, P.; Pagliato, E.; Battezzati, A.; Benedini, S.; Soldini, L.; Testolin, G.; Del Maschio, A.; Luzi, L. Gender factors affect fatty acids-induced insulin resistance in nonobese humans: Effects of oral steroidal contraception. J. Clin. Endocrinol. Metab. 2001, 86, 3188–3196. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grimes, D.A.; Schulz, K.F. Steroidal contraceptives: Effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst. Rev. 2019, 2019, CD006133. [Google Scholar] [CrossRef]
- Schoenfield, D.A. Statistical Considerations for Clinical Trials and Scientific Experiments. 2015. Available online: https://hedwig.mgh.harvard.edu/sample_size/size.html#ssize (accessed on 26 March 2018).
- Mooy, J.M.; Grootenhuis, P.A.; De Vries, H.; Kostense, P.J.; Popp-Snijders, C.; Bouter, L.M.; Heine, R.J. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: The Hoorn Study. Diabetologia 1996, 39, 298–305. [Google Scholar] [CrossRef]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8, 3–63. [Google Scholar] [CrossRef]
- Ministry of Health. Food and Nutrition Guidelines for Healthy Adults: A Background Paper; Ministry of Health: Wellington, New Zealand, 2003. [Google Scholar]
- Schutz, Y.; Dulloo, A. Resting Metabolic Rate, Thermic Effect of Food, and Obesity. In Handbook of Obesity: Epidemiology, Etiology and Pathophysiology, 4th ed.; Bray, G.A., Bouchard, C., Eds.; CRC Press: Boca Raton, FL, USA, 2024; Volume 1, pp. 286–295. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef] [PubMed]
- Jandrain, B.J.; Humblet, D.M.P.; Jaminet, C.B.; Scheen, A.J.; Gaspard, U.J.; Lefebvre, P.J. Effects of ethinyl estradiol combined with desogestrel and cyproterone acetate on glucose tolerance and insulin response to an oral glucose load: A one-year randomized, prospective, comparative trial. Am. J. Obstet. Gynecol. 1990, 163, 378–381. [Google Scholar] [CrossRef]
- Ågren, U.M.; Anttila, M.; Mäenpää-Liukko, K.; Rantala, M.L.; Rautiainen, H.; Sommer, W.F.; Mommers, E. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol compared with one containing levonorgestrel and ethinylestradiol on haemostasis, lipids and carbohydrate metabolism. Eur. J. Contracept. Reprod. Health Care 2011, 16, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.A.; Casey, E.; Crossley, E.; Williams, N.; Dhaher, Y.Y. The hormonal profile in women using combined monophasic oral contraceptive pills varies across the pill cycle: A temporal analysis of serum endogenous and exogenous hormones using liquid chromatography with tandem mass spectroscopy. Am. J. Physiology. Endocrinol. Metab. 2024, 327, E121–E133. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Blaak, E. Sex differences in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 500–504. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Tatulashvili, S.; Gusto, G.; Cosson, E.; Balkau, B.; Gourdy, P.; Bonnet, F.; Bihan, H.; Fagherazzi, G. Gonadal hormonal factors before menopause and incident type 2 diabetes in women: A 22-year follow-up of 83 799 women from the E3N cohort study. J. Diabetes 2021, 13, 330–338. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, R.; Jeon, E.; Lee, J.H.; Shon, H.S. Use of Oral Contraceptives at Child-Bearing Age Are Associated with the Prevalence of Diabetes in Postmenopausal Women. Diabetes 2018, 67, 177-OR. [Google Scholar] [CrossRef]
- Mosorin, M.E.; Haverinen, A.; Ollila, M.M.; Nordström, T.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Puukka, K.; Ruokonen, A.; Auvinen, J.; Piltonen, T.; et al. Current use of combined hormonal contraception is associated with glucose metabolism disorders in perimenopausal women. Eur. J. Endocrinol. 2020, 183, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Mosorin, M.E.; Ollila, M.M.; Nordström, T.; Jokelainen, J.; Piltonen, T.; Auvinen, J.; Morin-Papunen, L.; Tapanainen, J. Former long-term use of combined hormonal contraception and glucose metabolism disorders in perimenopausal women: A prospective, population-based cohort study. Acta Obstet. Gynecol. Scand. 2023, 102, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Teal, S.; Edelman, A. Contraception Selection, Effectiveness, and Adverse Effects: A Review. JAMA 2021, 326, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Doggen, C.J.M.; Smith, N.L.; Lemaitre, R.N.; Heckbert, S.R.; Rosendaal, F.R.; Psaty, B.M. Serum lipid levels and the risk of venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1970–1975. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Ellison, J.; Tait, R.C.; Walker, I.D.; Packard, C.J.; Greer, I.A.; McColl, M.D. Lipoprotein (a), cholesterol and triglycerides in women with venous thromboembolism. Blood Coagul. Fibrinolysis 2000, 11, 225–229. [Google Scholar]
- Klipping, C.; Duijkers, I.; Mawet, M.; Maillard, C.; Bastidas, A.; Jost, M.; Foidart, J.M. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception 2021, 103, 213–221. [Google Scholar] [CrossRef]
- Graham, I.; Cooney, M.T.; Bradley, D.; Dudina, A.; Reiner, Z. Dyslipidemias in the prevention of cardiovascular disease: Risks and causality. Curr. Cardiol. Rep. 2012, 14, 709–720. [Google Scholar] [CrossRef]
- Coussa, A.; Hasan, H.A.; Barber, T.M. Impact of contraception and IVF hormones on metabolic, endocrine, and inflammatory status. J. Assist. Reprod. Genet. 2020, 37, 1267–1272. [Google Scholar] [CrossRef]
- Cagnacci, A.; Paoletti, A.M.; Renzi, A.; Orrù, M.; Pilloni, M.; Melis, G.B.; Volpe, A. Glucose metabolism and insulin resistance in women with polycystic ovary syndrome during therapy with oral contraceptives containing cyproterone acetate or desogestrel. J. Clin. Endocrinol. Metab. 2003, 88, 3621–3625. [Google Scholar] [CrossRef][Green Version]
- Cagnacci, A.; Ferrari, S.; Tirelli, A.; Zanin, R.; Volpe, A. Insulin sensitivity and lipid metabolism with oral contraceptives containing chlormadinone acetate or desogestrel: A randomized trial. Contraception 2009, 79, 111–116. [Google Scholar] [CrossRef]
- Scheen, A.J.; Jandrain, B.J.; Humblet, D.M.P.; Jaminet, C.B.; Gaspard, U.J.; Lefebvre, P.J. Effects of a 1-year treatment with a low-dose combined oral contraceptive containing ethinyl estradiol and cyproterone acetate on glucose and insulin metabolism. Fertil. Steril. 1993, 59, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Godsland, I.F.; Walton, C.; Felton, C.; Proudler, A.; Patel, A.; Wynn, V. Insulin resistance, secretion, and metabolism in users of oral contraceptives. J. Clin. Endocrinol. Metab. 1992, 74, 64–70. [Google Scholar] [PubMed]
- Godsland, I.F.; Crook, D.; Simpson, R.; Proudler, T.; Felton, C.; Lees, B.; Anyaoku, V.; Devenport, M.; Wynn, V. The Effects of Different Formulations of Oral Contraceptive Agents on Lipid and Carbohydrate Metabolism. N. Engl. J. Med. 1990, 323, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R.; Nath, A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Silva-Bermudez, L.S.; Toloza, F.J.K.; Perez-Matos, M.C.; de Souza, R.J.; Banfield, L.; Vargas-Villanueva, A.; Mendivil, C.O. Effects of oral contraceptives on metabolic parameters in adult premenopausal women: A meta-analysis. Endocr. Connect. 2020, 9, 978–998. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, G.; Lundberg, P.A.; Lapidus, L.; Lundgren, H.; Bengtsson, C.; Björntorp, P. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM: 12-yr follow-up of population study of women in Gothenburq, Sweden. Diabetes 1991, 40, 123–128. [Google Scholar] [CrossRef]
- Zimmerman, Y.; Eijkemans, M.J.C.; Coelingh Bennink, H.J.T.; Blankenstein, M.A.; Fauser, B.C.J.M. The effect of combined oral contraception on testosterone levels in healthy women: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 76–105. [Google Scholar] [CrossRef]
- Panzer, C.; Wise, S.; Fantini, G.; Kang, D.; Munarriz, R.; Guay, A.; Goldstein, I. Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: A retrospective study in women with sexual dysfunction. J. Sex. Med. 2006, 3, 104–113. [Google Scholar] [CrossRef]
- Tappy, L. Basics in clinical nutrition: Carbohydrate metabolism. e-SPEN 2008, 3, e192–e195. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; Beserra, J.B.; de Oiveira, A.R.S.; Cruz, K.J.C.; de Sousa Melo, S.R.; do Nascimento, G.V.R.; de Macedo, G.F.S.; do Nascimento Marreiro, D. Association Between Cortisol, Insulin Resistance and Zinc in Obesity: A Mini-Review. Biol. Trace Elem. Res. 2019, 191, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Khani, S.; Tayek, J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001, 101, 739–747. [Google Scholar] [CrossRef] [PubMed]

| OCP Brand Name | No. of Participants | Estrogen (Dose µg) | Progestogen (Dose µg) | Progestin Class | Progestin Generation |
|---|---|---|---|---|---|
| Androgenic | |||||
| Ava 20 | 2 | EE (20) | Levonorgestrel (100) | 19-nortestosterone | 2 |
| Brevinor | 1 | EE (35) | Levonorgestrel (150) | 19-nortestosterone | 2 |
| Levlen | 7 | EE (30) | Levonorgestrel (150) | 19-nortestosterone | 2 |
| Marvellon | 1 | EE (30) | Desogestrel (150) | 19-nortestosterone | 3 |
| Microgynon | 1 | EE (30) | Levonorgestrel (150) | 19-nortestosterone | 2 |
| Norimin | 1 | EE (35) | Norethisterone (500) | 19-nortestosterone | 1 |
| Anti-androgenic | |||||
| Ginet | 7 | EE (35) | Cyproterone Acetate (2000) | 17α-hydroxyprogesterone | 3 |
| Jeanine | 1 | EE (30) | Dienogest (2000) | 19-nortestosterone | 4 |
| Total (n) | 21 |
| Characteristic | All | Androgenic OCP Users | Anti-Androgenic OCP Users | p-Value |
|---|---|---|---|---|
| n | 21 | 13 | 8 | |
| Age (y) | 24.4 ± 6.5 | 23.1 ± 6.0 | 26.6 ± 7.1 | 0.23 |
| Weight (kg) | 61.4 ± 5.0 | 60.6 ± 4.0 | 62.5 ± 6.5 | 0.41 |
| Height (cm) | 166 ± 4 | 166 ± 5 | 166 ± 4 | 1.00 |
| BMI (kg/m2) | 22.4 ±1.8 | 22.1 ± 1.7 | 22.8 ± 2.1 | 0.43 |
| FFM (kg) | 42.2 ±3.9 | 41.6 ± 3.8 | 43.3 ± 4.2 | 0.33 |
| % Body fat | 31.4 ± 5.2 | 31.2 ± 5.6 | 31.7 ± 4.9 | 0.84 |
| Measure | Androgenic OCP Users | Anti-Androgenic OCP Users | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Inactive | Active | Inactive | Active | OCP Phase | OCP Type | Inter-Action | |
| n | n = 13 | n = 13 | n = 8 | n = 8 | |||
| Glucose (mmol/L) | 4.91 ± 0.12 | 4.84 ± 0.10 | 4.76 ± 0.09 | 4.83 ± 0.07 | 0.979 | 0.573 | 0.416 |
| Insulin (µIU/mL) | 6.77 ± 1.27 | 6.99 ± 1.00 | 7.79 ± 1.63 | 7.73 ± 1.98 | 0.893 | 0.655 | 0.810 |
| C-peptide (ng/mL) | 1.88 ± 0.17 | 1.74 ± 0.11 | 1.79 ± 0.19 | 1.93 ± 0.21 | 0.542 | 0.981 | 0.021 |
| HOMA1-IR | 1.51 ± 0.30 | 1.53 ± 0.24 | 1.69 ± 0.38 | 1.66 ± 0.43 | 0.978 | 0.720 | 0.850 |
| HOMA2-IR | 1.36 ± 0.12 | 1.26 ± 0.09 | 1.29 ± 0.14 | 1.39 ± 0.15 | 0.531 | 0.999 | 0.019 |
| Estradiol (pg/mL) | 24.5 ± 4.6 | 11.0 ± 2.0 | 51.9 ± 13.7 | 14.3 ± 3.0 | <0.001 | 0.020 | 0.081 |
| Progesterone (ng/mL) | 0.26 ± 0.04 | 0.26 ± 0.04 | 0.27 ± 0.04 | 0.31 ± 0.08 | 0.540 | 0.636 | 0.572 |
| Testosterone (ng/mL) | 0.25 ± 0.03 | 0.26 ± 0.04 | 0.38 ± 0.06 | 0.24 ± 0.05 | 0.021 | 0.351 | 0.008 |
| SHBG (nmol/L) | 117 ± 15 | 133 ± 20 | 266 ± 11 | 335 ± 25 | <0.001 | <0.001 | 0.003 |
| Cortisol (nmol/L) | 854 ± 63 | 985 ± 79 | 869 ± 122 | 1086 ± 119 | 0.006 | 0.630 | 0.456 |
| HDL-C (mmol/L) | 1.49 ± 0.08 | 1.34 ± 0.05 | 1.84 ± 0.15 | 1.77 ± 0.18 | 0.011 | 0.015 | 0.396 |
| LDL-C (mmol/L) | 3.30 ± 0.31 | 2.86 ± 0.30 | 2.51 ± 0.20 | 2.15 ± 0.20 | <0.001 | 0.081 | 0.621 |
| Triglycerides (mmol/L) | 1.08 ± 0.07 | 1.21 ± 0.09 | 1.00 ± 0.10 | 1.17 ± 0.10 | 0.012 | 0.640 | 0.734 |
| Total Cholesterol (mmol/L) | 5.06 ± 0.29 | 4.61 ± 0.28 | 4.52 ± 0.21 | 4.20 ± 0.31 | 0.002 | 0.262 | 0.542 |
| Total Cholesterol/HDL-C ratio | 3.48 ± 0.25 | 3.50 ± 0.26 | 2.52 ± 0.15 | 2.44 ± 0.15 | 0.590 | 0.009 | 0.410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cree, J.M.E.; Brennan, N.M.; Poppitt, S.D.; Miles-Chan, J.L. The Effect of the Oral Contraceptive Pill on Acute Glycaemic Response to an Oral Glucose Bolus in Healthy Young Women: A Randomised Crossover Study. Nutrients 2024, 16, 3490. https://doi.org/10.3390/nu16203490
Cree JME, Brennan NM, Poppitt SD, Miles-Chan JL. The Effect of the Oral Contraceptive Pill on Acute Glycaemic Response to an Oral Glucose Bolus in Healthy Young Women: A Randomised Crossover Study. Nutrients. 2024; 16(20):3490. https://doi.org/10.3390/nu16203490
Chicago/Turabian StyleCree, Julia M. E., Niamh M. Brennan, Sally D. Poppitt, and Jennifer L. Miles-Chan. 2024. "The Effect of the Oral Contraceptive Pill on Acute Glycaemic Response to an Oral Glucose Bolus in Healthy Young Women: A Randomised Crossover Study" Nutrients 16, no. 20: 3490. https://doi.org/10.3390/nu16203490
APA StyleCree, J. M. E., Brennan, N. M., Poppitt, S. D., & Miles-Chan, J. L. (2024). The Effect of the Oral Contraceptive Pill on Acute Glycaemic Response to an Oral Glucose Bolus in Healthy Young Women: A Randomised Crossover Study. Nutrients, 16(20), 3490. https://doi.org/10.3390/nu16203490






