Is Fasting Superior to Continuous Caloric Restriction for Weight Loss and Metabolic Outcomes in Obese Adults? A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. In- and Exclusion Criteria
2.3. Outcomes
2.4. Data Extraction and Statistical Analysis
2.5. Bias Assessment
3. Results
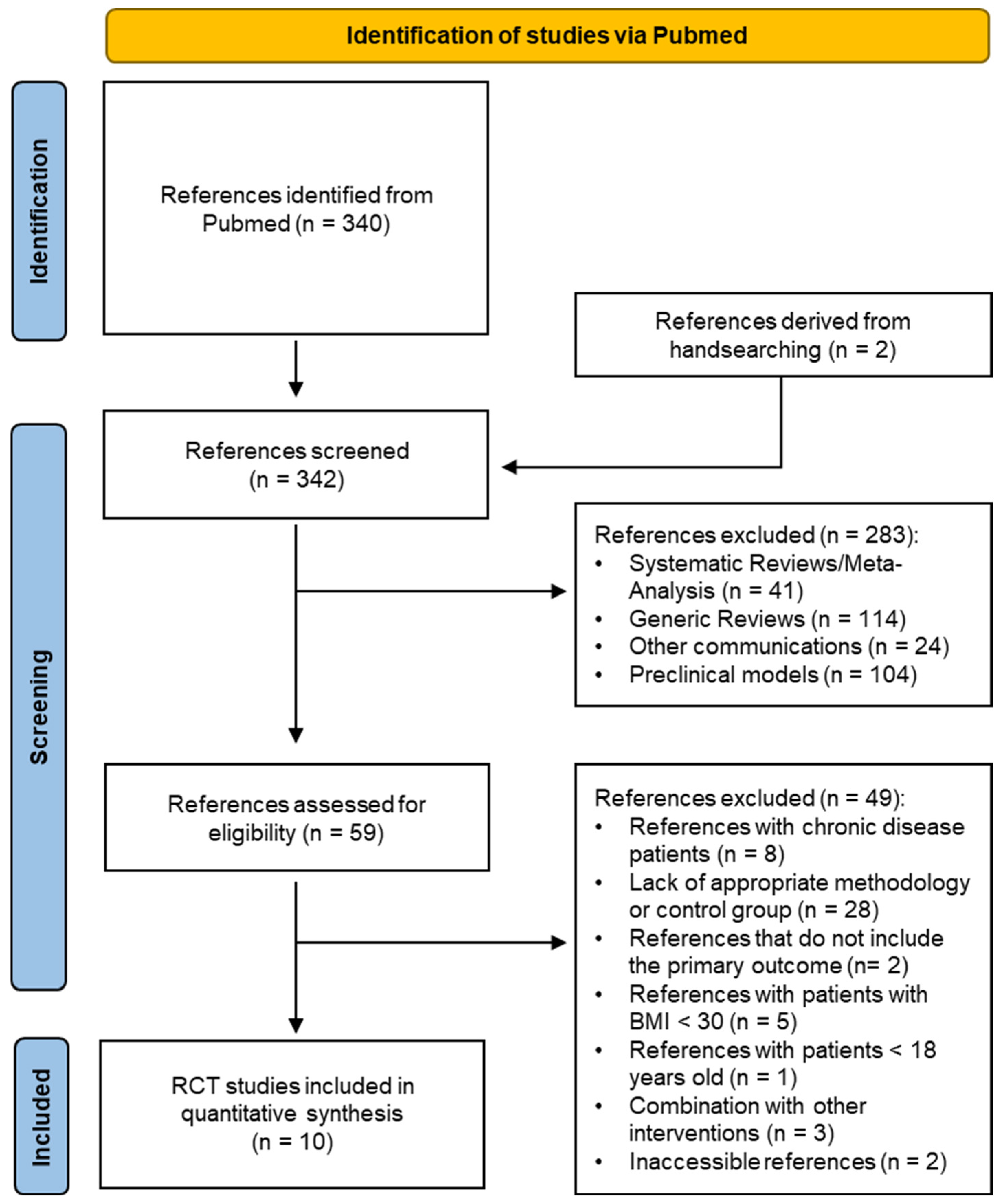
3.1. Study Selection and Characteristics
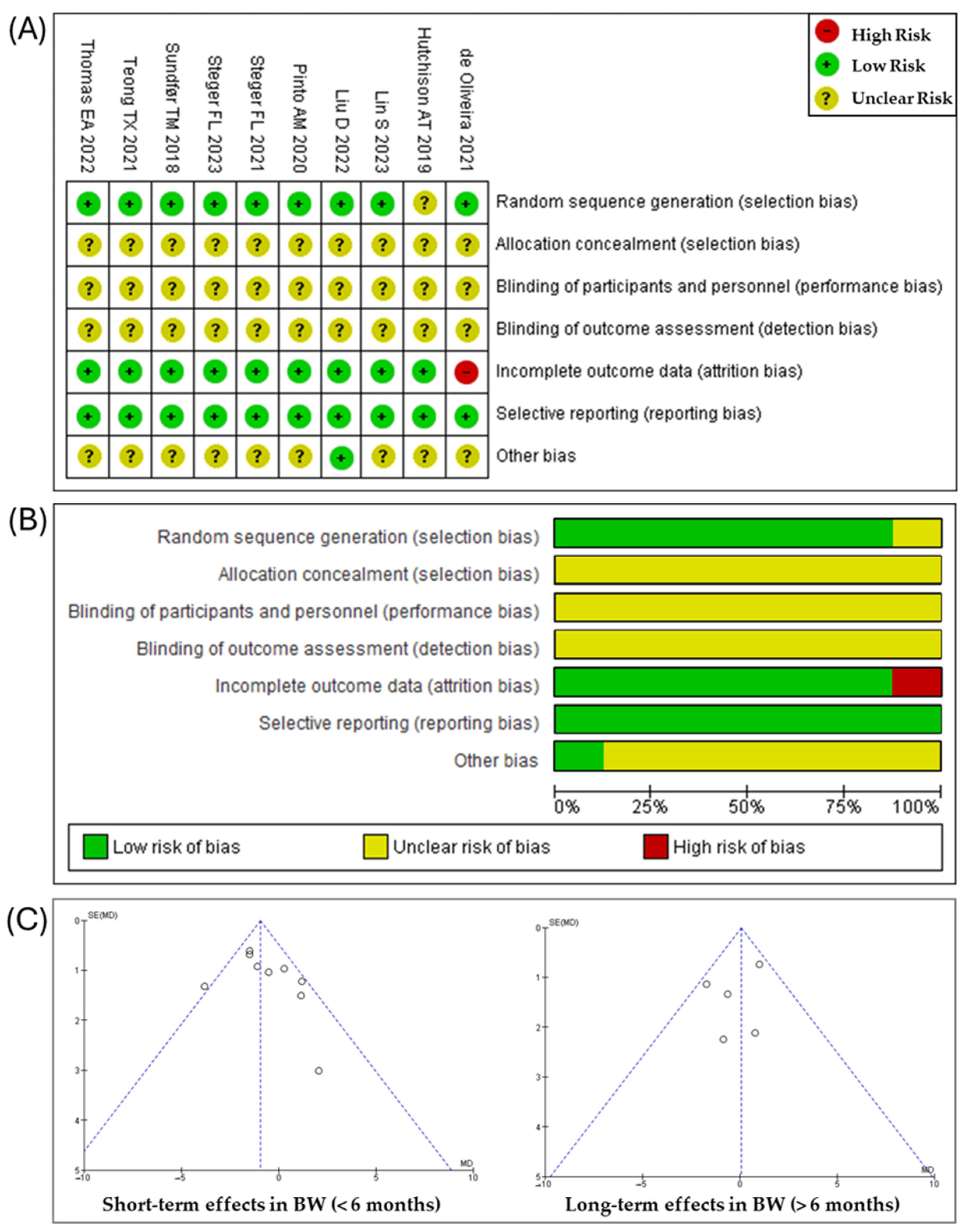
3.2. Risk of Bias
3.3. Effect of Fasting Strategies on Body Weight
3.4. Effect of Fasting Strategies on Body Composition, Waist and Hip Circunference
3.5. Effect of Fasting Strategies on Blood Pressure and Lipid Profiles
3.6. Effect of Fasting Strategies on Glucose Metabolism
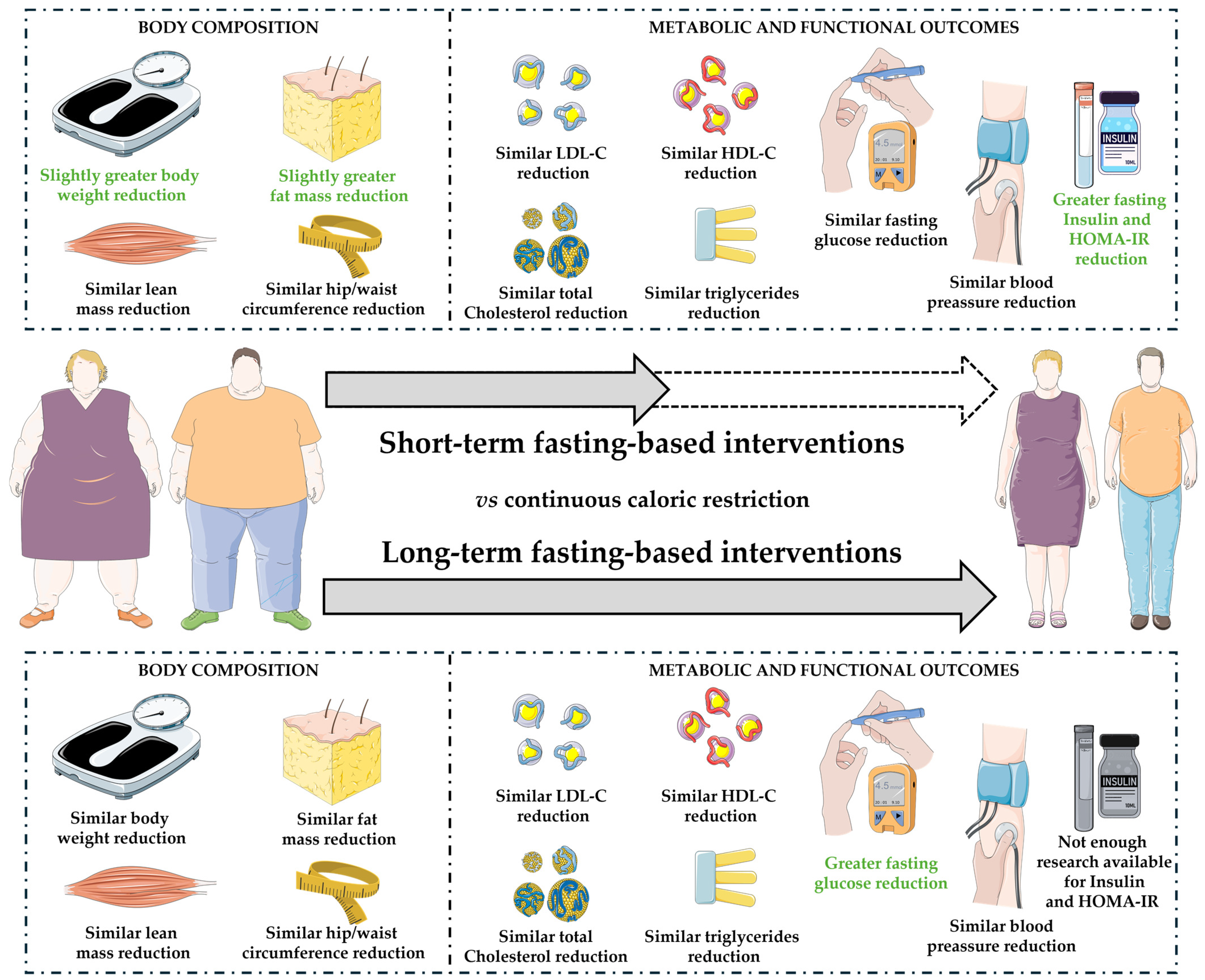
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 September 2024).
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Herz, D.; Karl, S.; Weiß, J.; Zimmermann, P.; Haupt, S.; Zimmer, R.T.; Schierbauer, J.; Wachsmuth, N.B.; Erlmann, M.P.; Niedrist, T.; et al. Effects of Different Types of Intermittent Fasting Interventions on Metabolic Health in Healthy Individuals (EDIF): A Randomised Trial with a Controlled-Run in Phase. Nutrients 2024, 16, 1114. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of Intermittent Fasting Diet on Glucose and Lipid Metabolism and Insulin Resistance in Patients with Impaired Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 6999907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, Q.; Hu, X.; Liu, M.; Zhang, A.-R. The Influence of Fasting and Caloric Restriction on Inflammation Levels in Humans. Medicine 2021, 100, e25509. [Google Scholar] [CrossRef]
- Petridou, A.; Rodopaios, N.E.; Mougios, V.; Koulouri, A.-A.; Vasara, E.; Papadopoulou, S.K.; Skepastianos, P.; Hassapidou, M.; Kafatos, A. Effects of Periodic Religious Fasting for Decades on Nutrient Intakes and the Blood Biochemical Profile. Nutrients 2021, 13, 3963. [Google Scholar] [CrossRef]
- Blumberg, J.; Hahn, S.L.; Bakke, J. Intermittent Fasting: Consider the Risks of Disordered Eating for Your Patient. Clin. Diabetes Endocrinol. 2023, 9, 4. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R. The Effect of Fasting on Human Metabolism and Psychological Health. Dis. Markers 2022, 2022, 5653739. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K.; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Soliman, G.A. Intermittent Fasting and Time-Restricted Eating Role in Dietary Interventions and Precision Nutrition. Front. Public Health 2022, 10, 1017254. [Google Scholar] [CrossRef]
- Shazman, S. Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach. Nutrients 2023, 15, 3926. [Google Scholar] [CrossRef]
- Duan, H.; Li, J.; Yu, L.; Fan, L. The Road Ahead of Dietary Restriction on Anti-Aging: Focusing on Personalized Nutrition. Crit. Rev. Food Sci. Nutr. 2024, 64, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Laferrère, B.; Panda, S. Calorie and Time Restriction in Weight Loss. N. Engl. J. Med. 2022, 386, 1572–1573. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Redman, L.M. Impact of Calorie Restriction on Energy Metabolism in Humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.A.M.; Most, M.; Howard, J.; Ravussin, E. Dietary Adherence to Long-Term Controlled Feeding in a Calorie-Restriction Study in Overweight Men and Women. Nutr. Clin. Pract. 2011, 26, 309–315. [Google Scholar] [CrossRef]
- Pannen, S.T.; Maldonado, S.G.; Nonnenmacher, T.; Sowah, S.A.; Gruner, L.F.; Watzinger, C.; Nischwitz, K.; Ulrich, C.M.; Kaaks, R.; Schübel, R.; et al. Adherence and Dietary Composition during Intermittent vs. Continuous Calorie Restriction: Follow-Up Data from a Randomized Controlled Trial in Adults with Overweight or Obesity. Nutrients 2021, 13, 1195. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) 7.7 Extracting Study Results and Converting to the Desired. In Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; Cochrane: London, UK, 2011; Available online: https://handbook-5-1.cochrane.org/chapter_7/7_7_extracting_study_results_and_converting_to_the_desired.htm (accessed on 23 August 2024).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- de Oliveira Maranhão Pureza, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; de Menezes Toledo Florêncio, T.M.; Bueno, N.B. Effects of Time-Restricted Feeding on Body Weight, Body Composition and Vital Signs in Low-Income Women with Obesity: A 12-Month Randomized Clinical Trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019, 27, 50–58. [Google Scholar] [CrossRef]
- Lin, S.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K.; Pavlou, V.; Mulas, A.; Chakos, K.; McStay, M.; Wu, J.; Tussing-Humphreys, L.; et al. Time-Restricted Eating Without Calorie Counting for Weight Loss in a Racially Diverse Population. Ann. Intern. Med. 2023, 176, 885–895. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent Energy Restriction Is Comparable to Continuous Energy Restriction for Cardiometabolic Health in Adults with Central Obesity: A Randomized Controlled Trial; the Met-IER Study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Steger, F.L.; Jamshed, H.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-J.; Peterson, C.M. Early Time-Restricted Eating Affects Weight, Metabolic Health, Mood, and Sleep in Adherent Completers: A Secondary Analysis. Obesity 2023, 31 (Suppl. 1), 96–107. [Google Scholar] [CrossRef]
- Steger, F.L.; Donnelly, J.E.; Hull, H.R.; Li, X.; Hu, J.; Sullivan, D.K. Intermittent and Continuous Energy Restriction Result in Similar Weight Loss, Weight Loss Maintenance, and Body Composition Changes in a 6 Month Randomized Pilot Study. Clin. Obes. 2021, 11, e12430. [Google Scholar] [CrossRef] [PubMed]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of Intermittent versus Continuous Energy Restriction on Weight Loss, Maintenance and Cardiometabolic Risk: A Randomized 1-Year Trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Teong, X.T.; Hutchison, A.T.; Liu, B.; Wittert, G.A.; Lange, K.; Banks, S.; Heilbronn, L.K. Eight Weeks of Intermittent Fasting versus Calorie Restriction Does Not Alter Eating Behaviors, Mood, Sleep Quality, Quality of Life and Cognitive Performance in Women with Overweight. Nutr. Res. 2021, 92, 32–39. [Google Scholar] [CrossRef]
- Thomas, E.A.; Zaman, A.; Sloggett, K.J.; Steinke, S.; Grau, L.; Catenacci, V.A.; Cornier, M.-A.; Rynders, C.A. Early Time-Restricted Eating Compared with Daily Caloric Restriction: A Randomized Trial in Adults with Obesity. Obesity 2022, 30, 1027–1038. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Wang, H.; Ma, Z.; Liu, D.; Guan, X.; Liu, Y.; Fu, Y.; Cui, M.; Dong, J. Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss? Nutrients 2022, 14, 1781. [Google Scholar] [CrossRef]
- Kushner, R.F. Weight Loss Strategies for Treatment of Obesity: Lifestyle Management and Pharmacotherapy. Prog. Cardiovasc. Dis. 2018, 61, 246–252. [Google Scholar] [CrossRef]
- Hall, K.D.; Kahan, S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med. Clin. N. Am. 2018, 102, 183–197. [Google Scholar] [CrossRef]
- Kheniser, K.; Saxon, D.R.; Kashyap, S.R. Long-Term Weight Loss Strategies for Obesity. J. Clin. Endocrinol. Metab. 2021, 106, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Salazar-Collier, C.L.; Pena, J.; Wilkinson, A.V.; Chavarria, E.A.; Reininger, B.M. Motivation for Weight Loss among Completers of a Free Community-Based Weight Loss Program in a US-Mexico Border Region: A Self-Determination Theory Perspective. Front. Public Health 2022, 10, 652271. [Google Scholar] [CrossRef] [PubMed]
- Elfhag, K.; Rössner, S. Who Succeeds in Maintaining Weight Loss? A Conceptual Review of Factors Associated with Weight Loss Maintenance and Weight Regain. Obes. Rev. 2005, 6, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Calugi, S.; Molinari, E.; Petroni, M.L.; Bondi, M.; Compare, A.; Marchesini, G.; QUOVADIS Study Group. Weight Loss Expectations in Obese Patients and Treatment Attrition: An Observational Multicenter Study. Obes. Res. 2005, 13, 1961–1969. [Google Scholar] [CrossRef]
- Davis, C.S.; Clarke, R.E.; Coulter, S.N.; Rounsefell, K.N.; Walker, R.E.; Rauch, C.E.; Huggins, C.E.; Ryan, L. Intermittent Energy Restriction and Weight Loss: A Systematic Review. Eur. J. Clin. Nutr. 2016, 70, 292–299. [Google Scholar] [CrossRef]
- Alhamdan, B.A.; Garcia-Alvarez, A.; Alzahrnai, A.H.; Karanxha, J.; Stretchberry, D.R.; Contrera, K.J.; Utria, A.F.; Cheskin, L.J. Alternate-Day versus Daily Energy Restriction Diets: Which Is More Effective for Weight Loss? A Systematic Review and Meta-Analysis. Obes. Sci. Pract. 2016, 2, 293–302. [Google Scholar] [CrossRef]
- Williamson, E.; Moore, D.R. A Muscle-Centric Perspective on Intermittent Fasting: A Suboptimal Dietary Strategy for Supporting Muscle Protein Remodeling and Muscle Mass? Front. Nutr. 2021, 8, 640621. [Google Scholar] [CrossRef] [PubMed]
- Kawaji, L.D.; Fontanilla, J.A. Accuracy of Waist Circumference Measurement Using the WHO versus NIH Protocol in Predicting Visceral Adiposity Using Bioelectrical Impedance Analysis among Overweight and Obese Adult Filipinos in a Tertiary Hospital. J. ASEAN Fed. Endocr. Soc. 2021, 36, 180–188. [Google Scholar] [CrossRef]
- Ross, R.; Berentzen, T.; Bradshaw, A.J.; Janssen, I.; Kahn, H.S.; Katzmarzyk, P.T.; Kuk, J.L.; Seidell, J.C.; Snijder, M.B.; Sørensen, T.I.A.; et al. Does the Relationship between Waist Circumference, Morbidity and Mortality Depend on Measurement Protocol for Waist Circumference? Obes. Rev. 2008, 9, 312–325. [Google Scholar] [CrossRef]
- Di Daniele, N.; Marrone, G.; Di Lauro, M.; Di Daniele, F.; Palazzetti, D.; Guerriero, C.; Noce, A. Effects of Caloric Restriction Diet on Arterial Hypertension and Endothelial Dysfunction. Nutrients 2021, 13, 274. [Google Scholar] [CrossRef]
- Caristia, S.; De Vito, M.; Sarro, A.; Leone, A.; Pecere, A.; Zibetti, A.; Filigheddu, N.; Zeppegno, P.; Prodam, F.; Faggiano, F.; et al. Is Caloric Restriction Associated with Better Healthy Aging Outcomes? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2290. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Parker, D.C.; Bhapkar, M.; Racette, S.B.; Martin, C.K.; Redman, L.M.; Das, S.K.; Connelly, M.A.; Pieper, C.F.; Orenduff, M.; et al. Calorie Restriction Improves Lipid-Related Emerging Cardiometabolic Risk Factors in Healthy Adults without Obesity: Distinct Influences of BMI and Sex from CALERIETM a Multicentre, Phase 2, Randomised Controlled Trial. eClinicalMedicine 2022, 43, 101261. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. Two Years of Calorie Restriction and Cardiometabolic Risk Factors. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mudgal, S.K.; Kalra, S.; Gaur, R.; Thakur, K.; Agarwal, R. Effect of Intermittent Fasting on Glycaemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. touchREV Endocrinol. 2023, 19, 25–32. [Google Scholar] [CrossRef]

| Sample (n; M/F) | Experimental Procedure | Population | |||||
|---|---|---|---|---|---|---|---|
| Studies | Int. | Control | Int. | Control | Duration | Basal Characteristics | Outcomes vs. Control |
| Lin S, 2023 [23] | 30 (5/25) | 30 (6/24) | TRE (8 h/16 h) + EW (12:00 p.m. to 08:00 p.m.) | 25% CCR | 24/48 weeks | Mid-aged (44 ± 12 years vs. 44 ± 9 years) obese (100 ± 17 kg bw vs. 102 ± 18 kg bw) patients | = BW, BLM, BFM, VFM, BP, WC, HDL, LDL, TC, TT, FG, HbA1c, HOMA-IR, and FI at 24/48 weeks |
| Steger FL, 2023 [26] | 15 (4/11) | 22 (6/15) | TRE (8 h/16 h) + EW (07:00 a.m. to 03:00 p.m.) + 25% CR | 25% CCR | 14 weeks | Mid-aged (46 ± 11 years vs. 42 ± 12 years) obese (111.1 ± 22.4 kg bw vs. 104.4 ± 21.7 kg bw) patients | ↓ BW, BFM, FG, and HOMA-IR = FFM, VFM, WC, BP, TC, FI, Hb1Ac, LDL, and HDL |
| Liu D, 2022 [24] | 69 (36/33) | 70 (35/35) | TRE (8 h/16 h) + EW (08:00 a.m. to 04:00 p.m.) + 25% CR | 25% CCR | 24/48 weeks | Adult (31.6 ± 9.3 years vs. 32.2 ± 8.8 years) obese (88.4 ± 10.2 kg bw vs. 87.9 ± 12.8 kg bw) patients | = BW, WC, BFM, BLM, VFM, BP, FG, HDL, LDL, TC, TT, and HOMA-IR at 24/48 weeks |
| Thomas EA, 2022 [30] | 41 (7/34) | 40 (5/35) | TRE (10 h/14 h) + 35% CR | 35% CCR | 12/39 weeks | Adult (38.3 ± 7.9 years vs. 37.8 ± 7.8 years) obese (96.1 ± 18.1 kg bw vs. 93.4 ± 18.4 kg bw) patients | = BW, BFM, BLM, TC, TT, LDL, HDL, and HbA1c at 12/39 weeks |
| de Oliveira Maranhão Pureza IR, 2021 [21] | 31 (0/31) | 27 (0/27) | TRE (12 h/12 h) + 25% CR | 25% CCR | 48 weeks | Adult (31.80 ± 6.96 years vs. 31.03 ± 7.16 years) obese (81.25 ± 13.51 kg bw vs. 80.25 ± 9.40 kg bw) patients | ↓ BFM at 48 weeks = BW, WC, and BP at 48 weeks |
| Steger FL, 2021 [27] | 18 (5/13) | 17 (3/14) | IF (3 non-CD of fasting (80% CR) + 4 days ad libitum) | 25–35% CCR | 12/24 weeks | Mid-aged (43.4 ± 11.8 years vs. 48.0 ± 10.0 years) obese (87.4 ± 11.5 kg bw vs. 91.0 ± 9.7 kg bw) patients | = BW, BFM, BLM, WC, HC, and BP at 12/24 weeks |
| Teong XT, 2021 [29] | 22 (0/22) | 24 (0/24) | IF (3 non-CD of fasting (70% CR) + 4 days of UE) | 30% CCR | 8 weeks | Mid-aged (50 ± 9 years vs. 50 ± 9 years) obese (89.2 ± 13.8 kg bw) patients | ↓ BW and BFM = WC and HC |
| Pinto AM, 2020 [25] | 21 (6/15) | 22 (6/16) | IF (2 CD of fasting (80% CR) + 4 days of UE) | 25% CCR | 4 weeks | Mid-aged (50 ± 12 years vs. 56 ± 8 years) obese (87.7 ± 11.5 kg bw vs. 89.2 ± 13.8 kg bw) patients | ↑ FG = BW, BFM, WC, FI, HOMA-IR, and HDL |
| Hutchison AT, 2019 [22] | 22 (0/22) | 24 (0/24) | IF (3 non-CD of fasting (70% CR) + 4 days of UE) | 30% CCR | 8 weeks | Mid-aged (49 ± 2 years vs. 51 ± 2 years) obese (89.4 ± 2.8 kg bw vs. 88.4 ± 2.8 kg bw) patients | ↓ BW, BFM, WC, TC, LDL, and TG = BLM, HC, HDL, FG, FI, HOMA-IR, and BP |
| Sundfør TM, 2018 [28] | 54 (28/26) | 58 (28/30) | IF (2 non-CD of fasting (75% CR) + 5 days ad libitum) | 25% CCR | 12/24/48 weeks | Mid-aged (49.9 ± 10.1 years vs. 47.5 ± 11.6 years) obese (108.6 ± 16.3 kg bw vs. 107.5 ± 16.1 kg bw) patients | = BW, WC, HC, BP, HDL, LDL, TT, FG, and HbA1c at 12/24/48 weeks |
| Outcome | Studies | Sample | Statistical Method | Overall Effect Size | p Value |
|---|---|---|---|---|---|
| Short-term effects | |||||
| Body lean mass (kg) | 6 | 364 | MD (IV, Fixed, 95% CI) | −0.29 [−0.70, 0.13] | 0.18 |
| Body fat mass (kg) | 8 | 453 | MD (IV, Fixed, 95% CI) | −1.08 [−1.63, −0.53] | 0.0001 |
| Waist circumference (cm) | 8 | 502 | MD (IV, Random, 95% CI) | 0.21 [−1.38, 1.80] | 0.80 |
| Hip circumference (cm) | 4 | 233 | MD (IV, Fixed, 95% CI) | −0.20 [−1.50, 1.10] | 0.76 |
| Long-term effects | |||||
| Body lean mass (kg) | 3 | 253 | MD (IV, Fixed, 95% CI) | 0.07 [−0.45, 0.58] | 0.80 |
| Body fat mass (kg) | 4 | 311 | MD (IV, Fixed, 95% CI) | −0.96 [−2.09, 0.17] | 0.10 |
| Waist circumference (cm) | 4 | 360 | MD (IV, Random, 95% CI) | −0.90 [−3.12, 1.32] | 0.42 |
| Outcome | Studies | Sample | Statistical Method | Overall Effect Size | p Value |
|---|---|---|---|---|---|
| Short-term effects | |||||
| Systolic blood pressure (mmHg) | 6 | 413 | MD (IV, Fixed, 95% CI) | −0.80 [−2.89, 1.29] | 0.45 |
| Diastolic blood pressure (mmHg) | 6 | 413 | MD (IV, Fixed, 95% CI) | −1.03 [−2.44, 0.38] | 0.15 |
| HDL-Cholesterol (mg/dL) | 5 | 378 | MD (IV, Fixed, 95% CI) | −0.51 [−1.90, 0.89] | 0.48 |
| LDL-Cholesterol (mg/dL) | 5 | 378 | MD (IV, Random, 95% CI) | −3.19 [−10.61, 4.22] | 0.40 |
| Total cholesterol (mg/dL) | 6 | 421 | MD (IV, Random, 95% CI) | −3.15 [−8.84, 2.54] | 0.28 |
| Triglycerides (mg/dL) | 5 | 378 | MD (IV, Fixed, 95% CI) | −6.51 [−16.30, 3.28] | 0.19 |
| Long-term effects | |||||
| Systolic blood pressure (mmHg) | 4 | 360 | MD (IV, Fixed, 95% CI) | 0.93 [−1.40, 3.27] | 0.43 |
| Diastolic blood pressure (mmHg) | 4 | 360 | MD (IV, Fixed, 95% CI) | −0.60 [−2.34, 1.13] | 0.49 |
| HDL-Cholesterol (mg/dL) | 3 | 302 | MD (IV, Fixed, 95% CI) | 1.09 [−1.06, 3.24] | 0.32 |
| LDL-Cholesterol (mg/dL) | 3 | 302 | MD (IV, Fixed, 95% CI) | −1.33 [−6.66, 4.00] | 0.62 |
| Total cholesterol (mg/dL) | 3 | 302 | MD (IV, Fixed, 95% CI) | −0.62 [−6.63, 5.39] | 0.84 |
| Triglycerides (mg/dL) | 3 | 302 | MD (IV, Fixed, 95% CI) | −5.00 [−17.82, 7.83] | 0.45 |
| Outcome | Studies | Sample | Statistical Method | Overall Effect Size | p Value |
|---|---|---|---|---|---|
| Short-term effects | |||||
| Fasting glucose (mg/dL) | 6 | 421 | MD (IV, Random, 95% CI) | −0.99 [−5.54, 3.56] | 0.67 |
| Fasting insulin (pmol/L) | 4 | 179 | MD (IV, Fixed, 95% CI) | −7.46 [−13.77, −1.15] | 0.02 |
| HbA1C (%) | 3 | 199 | MD (IV, Fixed, 95% CI) | −0.05 [−0.14, 0.05] | 0.36 |
| HOMA-IR | 5 | 209 | MD (IV, Fixed, 95% CI) | −0.14 [−0.27, −0.01] | 0.04 |
| Long-term effects | |||||
| Fasting glucose (mg/dL) | 3 | 311 | MD (IV, Fixed, 95% CI) | −3.59 [−3.92, −3.25] | 0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siles-Guerrero, V.; Romero-Márquez, J.M.; García-Pérez, R.N.; Novo-Rodríguez, C.; Guardia-Baena, J.M.; Hayón-Ponce, M.; Tenorio-Jiménez, C.; López-de-la-Torre-Casares, M.; Muñoz-Garach, A. Is Fasting Superior to Continuous Caloric Restriction for Weight Loss and Metabolic Outcomes in Obese Adults? A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2024, 16, 3533. https://doi.org/10.3390/nu16203533
Siles-Guerrero V, Romero-Márquez JM, García-Pérez RN, Novo-Rodríguez C, Guardia-Baena JM, Hayón-Ponce M, Tenorio-Jiménez C, López-de-la-Torre-Casares M, Muñoz-Garach A. Is Fasting Superior to Continuous Caloric Restriction for Weight Loss and Metabolic Outcomes in Obese Adults? A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients. 2024; 16(20):3533. https://doi.org/10.3390/nu16203533
Chicago/Turabian StyleSiles-Guerrero, Víctor, Jose M. Romero-Márquez, Rosa Natalia García-Pérez, Cristina Novo-Rodríguez, Juan Manuel Guardia-Baena, María Hayón-Ponce, Carmen Tenorio-Jiménez, Martín López-de-la-Torre-Casares, and Araceli Muñoz-Garach. 2024. "Is Fasting Superior to Continuous Caloric Restriction for Weight Loss and Metabolic Outcomes in Obese Adults? A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Nutrients 16, no. 20: 3533. https://doi.org/10.3390/nu16203533
APA StyleSiles-Guerrero, V., Romero-Márquez, J. M., García-Pérez, R. N., Novo-Rodríguez, C., Guardia-Baena, J. M., Hayón-Ponce, M., Tenorio-Jiménez, C., López-de-la-Torre-Casares, M., & Muñoz-Garach, A. (2024). Is Fasting Superior to Continuous Caloric Restriction for Weight Loss and Metabolic Outcomes in Obese Adults? A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients, 16(20), 3533. https://doi.org/10.3390/nu16203533










