Association Between Gut Microbiome Composition and Physical Characteristics in Patients with Severe Motor and Intellectual Disabilities: Perspectives from Microbial Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Survey Details and Fecal Sample Collection
2.3. BA Measurement
2.4. SCFA Measurement
2.5. DNA Extraction and 16S rRNA Gene Amplicon Sequencing
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Patients Overall and Each Group
3.2. Comparisons Between Groups
3.2.1. Nutrient Intake, Prescription Drugs, Blood Biochemical Tests, and Defecation Status
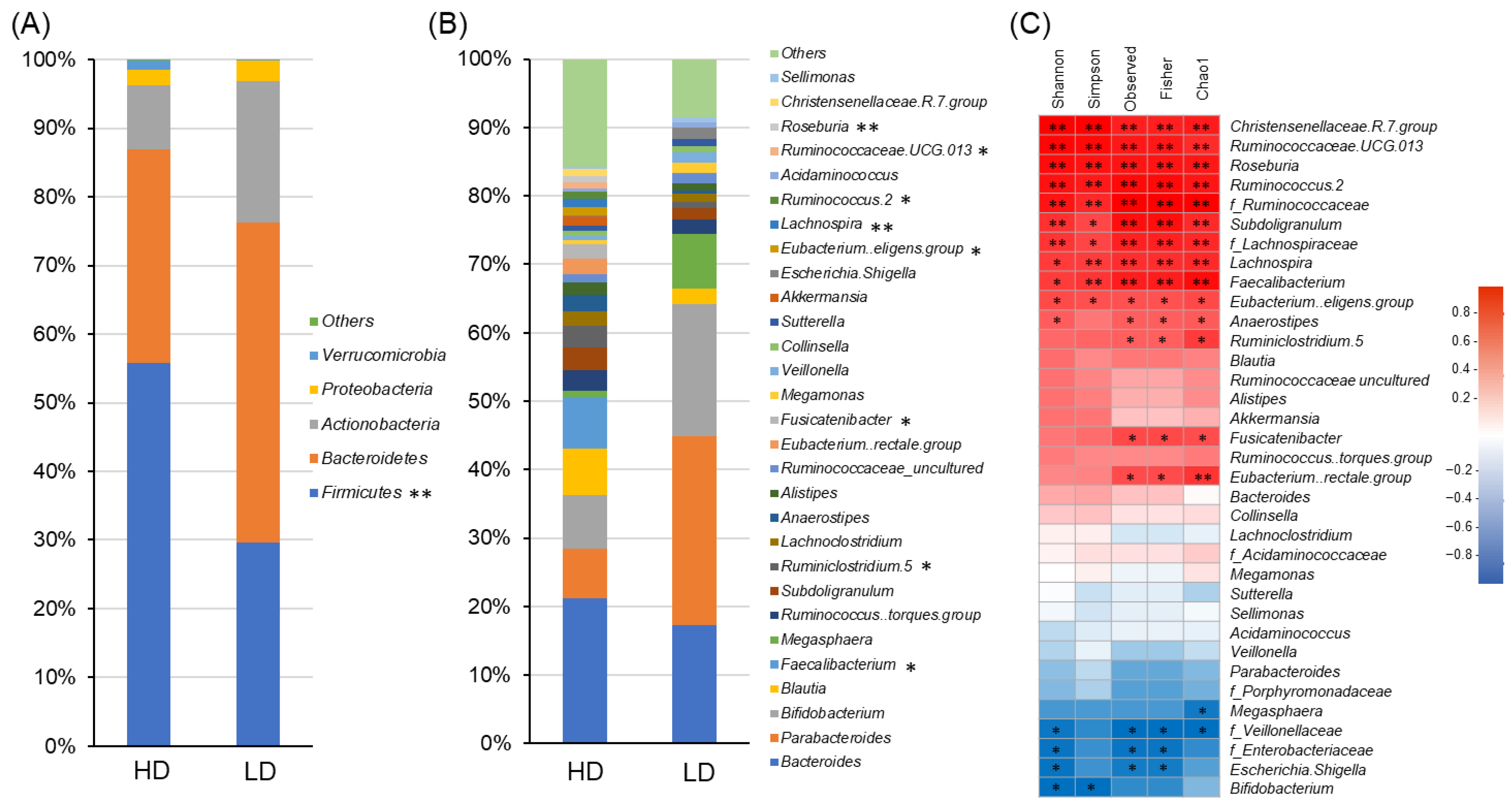
3.2.2. Gut Microbiome
3.2.3. Fecal Metabolites (BAs and SCFAs)
3.2.4. Covariates Adjustment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Timmeren, E.A.; van der Schans, C.P.; van der Putten, A.A.; Krijnen, W.P.; Steenbergen, H.A.; van Schrojenstein Lant-man-de Valk, H.M.; Waninge, A. Physical health issues in adults with severe or profound intellectual and motor disabilities: A systematic review of cross-sectional studies. J. Intellect. Disabil. Res. 2017, 61, 30–49. [Google Scholar] [CrossRef] [PubMed]
- van Timmeren, E.A.; van der Putten, A.A.; van Schrojenstein Lantman-de Valk, H.M.; van der Schans, C.P.; Waninge, A. Prevalence of reported physical health problems in people with severe or profound intellectual and motor disabilities: A cross-sectional study of medical records and care plans. J. Intellect. Disabil. Res. 2016, 60, 1109–1118. [Google Scholar] [CrossRef]
- van Timmeren, E.A.; Waninge, A.; van Schrojenstein Lantman-de, H.M.J.; van der Putten, A.A.J.; van der Schans, C.P. Patterns of multimorbidity in people with severe or profound intellectual and motor disabilities. Res. Dev. Disabil. 2017, 67, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Momma, E.; Koeda, M.; Tanabe, T.; Hoshikawa, Y.; Hoshino, S.; Kawami, N.; Kawagoe, T.; Tatsuguchi, A.; Kaise, M.; Iwakiri, K. Relationship between gastroesophageal reflux disease (GERD) and constipation: Laxative use is common in GERD patients. Esophagus 2021, 18, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Inoue, K.; Hosomi, K.; Park, J.; Yumioka, H.; Suka, T.; Kurohashi, Y.; Teramoto, K.; Syauki, A.Y.; Doi, M.; et al. Effects of malted rice amazake on constipation symptoms and gut microbiota in children and adults with severe motor and intellectual disabilities: A pilot study. Nutrients 2021, 13, 4466. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Shimizu, H.; Ohue-Kitano, R.; Kimura, I. Regulation of host energy metabolism by gut microbiota-derived short-chain fatty acids. Glycative Stress Res. 2019, 6, 181–191. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Inoue, R. Crucial role of microbiota in the pathogenesis of chronic constipation. J. Jpn. Soc. Gastroenterol. 2018, 115, 940–949. [Google Scholar] [CrossRef]
- Tanaka, M.; Sanefuji, M.; Morokuma, S.; Yoden, M.; Momoda, R.; Sonomoto, K.; Ogawa, M.; Kato, K.; Nakayama, J. The association between gut microbiota development and maturation of intestinal bile acid metabolism in the first 3 y of healthy Japanese infants. Gut Microbes 2020, 11, 205–216. [Google Scholar] [CrossRef]
- Kane, A.V.; Dinh, D.M.; Ward, H.D. Childhood malnutrition and the intestinal microbiome. Pediatr. Res. 2015, 77, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Stephens, D.A.; Moodie, E.E.M.; Prendergast, A.J.; Stoltzfus, R.J.; Humphrey, J.H.; Manges, A.R. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Akagawa, S.; Fujishiro, S.; Akagawa, Y.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Ohashi, A.; Hashiyada, M.; Akane, A.; et al. Dysbiosis of the gut microbiota in children with severe motor and intellectual disabilities receiving enteral nutrition: A pilot study. J. Parenter. Enter. Nutr. 2023, 47, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K. Basic issue on severe motor and intellectual disabilities. Nippon Koshu Eisei Zasshi 1971, 35, 648–655. [Google Scholar]
- McMillan, S.C.; Williams, F.A. Validity and reliability of the Constipation Assessment Scale. Cancer Nurs. 1989, 12, 183–188. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Kageyama, S.; Suka, T.; Kurohashi, Y.; Teramoto, K.; Ayabe, M.; Doi, M.; Syauki, A.Y.; Irie, Y. Changes in constipation symptoms associated with ingestion of malted-rice ‘amazake’ for 6 weeks in home-care patients with severe motor and intellectual disabilities. J. Child Heal. 2022, 81, 34–44. Available online: https://www.jschild.med-all.net/Contents/private/cx3child/2022/008101/007/0034-0044.pdf (accessed on 15 October 2024).
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Koo, S.H.; Deng, J.; Ang, D.S.W.; Heiang, J.C.; Lee, L.S.; Aazmi, S.; Mohamed, E.H.M.; Yang, H.; Yap, S.Y.; Teh, L.K.; et al. Effects of proton pump inhibitor on the human gut microbiome profile in multi-ethnic groups in Singapore. Singap. Med. J. 2019, 60, 512–521. [Google Scholar] [CrossRef]
- Morimoto, M.; Hamada, S.; Kitaoka, T.; Kyotani, S. Effects of prescription intervention by pharmacists on multidrug therapy in patients with severe motor and intellectual disabilities. J. Sev. Mot. Intellect. Disabil. 2018, 43, 457–464. [Google Scholar] [CrossRef]
- Takayama, N.; Suzuki, Y.; Morino, S. Guidance on Infection Control Measures in Wards for Patients with Severe Motor and Intellectual Disabilities; National Hospital Organization: Chiba, Japan, 2024; pp. 1–156. Available online: https://nho.hosp.go.jp/files/000213961.pdf (accessed on 15 October 2024).
- Akagawa, Y.; Kimata, T.; Akagawa, S.; Yamaguchi, T.; Kato, S.; Yamanouchi, S.; Hashiyada, M.; Akane, A.; Kino, M.; Tsuji, S.; et al. Impact of Long-Term Low Dose Antibiotic Prophylaxis on Gut Microbiota in Children. J. Urol. 2020, 204, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, H.; Kodama, H.; Yamasaki, M.; Murata, Y.; Fukuba, H.; Matsumoto, N.; Ichikawa, M.; Takemoto, M.; Ikeda, M.; Hirada, A.; et al. Fecal Microbiota and Fecal Characteristics of Patients with Severe Motor and Intellectual Disabilities Undergoing Long-Term Tube Feeding. J. Intest. Microbiol. 2013, 27, 1–6. [Google Scholar] [CrossRef]
- Kakiyama, G.; Huto, A.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Hofmann, A.F.; Pandak, W.M.; Bajaj, J. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: Validation by GC-MS and LC-MS. J. Lipid Res. 2014, 55, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Sheweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Hosomi, K.; Murakami, H.; Natsume-Kitatani, Y.; Tanisawa, K.; Hirata, S.; Suzuki, H.; Nagatake, T.; Nishino, T.; Mizuguchi, K.; Miyachi, M.; et al. Method for preparing DNA from feces in guanidine thiocyanate solution affects 16S rRNA-based profiling of human microbiota diversity. Sci. Rep. 2017, 7, 4339. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Mohsen, A.; Park, J.; Chen, Y.A.; Kawashima, H.; Mizuguchi, K. Impact of quality trimming on the efficiency of reads joining and diversity analysis of Illumina paired-end reads in the context of QIIME1 and QIIME2 microbiome analysis frameworks. BMC Bioinform. 2019, 20, 581. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Blake, M.R.; Raker, J.M.; Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, T.J. Rarefaction and extrapolation of species richness using an area-based Fisher’s logseries. Ecol. Evol. 2017, 7, 10066–10078. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, R.; Masuoka, H.; Suda, W. Advances in gut microbiome analysis. Mod. Media 2020, 66, 133–138. Available online: https://www.eiken.co.jp/uploads/modern_media/literature/P1-6.pdf (accessed on 15 October 2024).
- Million, M.; Diallo, A.; Raoult, D. Gut microbiota and malnutrition. Microb. Pathog. 2017, 106, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wijeyesekera, A.; Wagner, J.; Goffau, M.D.; Thurston, S.; Sabino, A.R.; Zaher, S.; White, D.; Ridout, J.; Peters, M.J.; Ramnarayan, P.; et al. Multi-compartment profiling of bacterial and host metabolites identifies intestinal dysbiosis and its functional consequences in the critically ill child. Crit. Care Med. 2019, 47, E727–E734. [Google Scholar] [CrossRef]
- Sakata, T.; Ichikawa, H. Physiological Actions of Short-Chain Fatty Acids. J. Jpn. Oil Chem. Soc. 1997, 46, 1205–1212. [Google Scholar] [CrossRef]
- Yoshii, K.; Hosomi, K.; Kunisawa, J. Microbial Metabolites for the Control of Host Immunity in the Gut. J. Intest. Microbiol. 2022, 36, 1–11. Available online: https://www.jstage.jst.go.jp/article/jim/36/1/36_1/_pdf (accessed on 15 October 2024).
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Pascal, V.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Medema, M.H.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut.microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Vital, M.; Rud, T.; Rath, S.; Pieper, D.H.; Schlüter, D. Diversity of Bacteria Exhibiting Bile Acid-inducible 7α-dehydroxylation Genes in the Human Gut. Comput. Struct. Biotechnol. J. 2019, 17, 1016–1019. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Cross-talk between host and intestinal bacteria via bile acids—Bile acids are the key to understanding the relationship between host and gut bacteria. Kagakutoseibutsu 2022, 60, 79–88. [Google Scholar] [CrossRef]
- Kuno, T.; Hirayama-Kurogi, M.; Ito, S.; Ohtsuki, S. Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci. Rep. 2018, 8, 1253. [Google Scholar] [CrossRef]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut microbiota and diarrhea: An updated review. Front. Cell. Infect. Microbiol. 2021, 15, 625210. [Google Scholar] [CrossRef]
- Ren, J.; Ren, Y.; Mu, Y.; Zhang, L.; Chen, B.; Li, S.; Fang, Q.; Zhang, Z.; Zhang, K.; Li, S.; et al. Microbial imbalance in Chinese children with diarrhea or constipation. Sci. Rep. 2024, 14, 13516. [Google Scholar] [CrossRef]
- Yang, D.; Lyu, C.; He, K.; Pang, K.; Guo, Z.; Wu, D. Bile acid diarrhea: From molecular mechanisms to clinical diagnosis and treatment in the era of precision medicine. Int. J. Mol. Sci. 2024, 25, 1544. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Endo, Y.; Kanazawa, M. Progress in the treatment of chronic constipation. Topics: VII. How to treat chronic constipation with intestinal secretagogues or inhibitor of ileal bile acid transporter. J. Jpn. Soc. Int. Med. 2019, 108, 46–54. [Google Scholar] [CrossRef]
- Watabe, J. Carbohydrate fermentation in the colon. J. Intest. Microbiol. 2005, 19, 169–177. Available online: https://www.jstage.jst.go.jp/article/jim/19/3/19_3_169/_pdf (accessed on 15 October 2024).
- Matute, A.; Sshurink, S.; Krijnen, S.; Florijn, A.; Rozenberg-Arska, M.; Hoepelman, I. Double-Blind, Placebo-Controlled Study Comparing the Effect of Azithromycin with Clarithromycin on Oropharyngeal and Bowel Microflora in Volunteers. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 427–431. [Google Scholar] [CrossRef]
- Mitsuoka, T. Prebiotics and Intestinal Flora. Biosci. Microflora 2002, 1621, 1–10. [Google Scholar] [CrossRef]
- Matsumotom, K. Effectsof Transgalactosylated Oligosaccharides Mixture (N-GOS) on Human Intestinal Microflora. J. Intest. Microbiol. 2004, 18, 25–35. [Google Scholar] [CrossRef]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Endtz, H.P.; Cravioto, A.; Ali, S.I.; Nakaya, T.; et al. Gut microbiota of healthy and malnourished children in Bangladesh. Front. Microbiol. 2011, 2, 228. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, D. Understanding the development and function of the gut microbiota in health and inflammation. Frontline Gastroenterol. 2022, 13, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Paudel, D.; Nair, D.V.T.; Joseph, G.; Castro, R.; Tiwari, A.K.; Singh, V. Gastrointestinal microbiota-directed nutritional and therapeutic interventions for inflammatory bowel disease: Opportunities and challenges. Gastroenterol. Rep. 2024, 12, goae033. [Google Scholar] [CrossRef]
- Lubin, J.B.; Green, J.; Maddux, S.; Denu, L.; Duranova, T.; Lanza, M.; Wynosky-Dolfi, M.; Flores, J.N.; Grimes, L.P.; Brodsky, I.E.; et al. Arresting microbiome development limits immune system maturation and resistance to infection in mice. Cell Host Microbe. 2023, 31, 554–570.e7. [Google Scholar] [CrossRef]
- Ghoshal, N.; Niyo, Y.; Trenkle, A. Growth hormone concentrations in plasma of healthy pigs and pigs with atrophic rhinitis. Am. J. Vet. Res. 1991, 52, 1684–1687. Available online: https://avmajournals.avma.org/downloadpdf/view/journals/ajvr/52/10/ajvr.1991.52.10.1684.pdf (accessed on 15 October 2024). [CrossRef]

| 1 Sex | Age (Year) | 2 Ht (m) | 2 Wt (kg) | 3 H/A (%) | 3 W/H (%) | 4 Motor Function | 5 Intake Method | Meal Details | Weaning Experience | Diagnosis | 6 Group | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 6 | 1.03 | 16.2 | 91.5 | 101.3 | Gait disturbance | Gastro (Oral) | Paste diet | No | Cerebral palsy, Autism spectrum | HD |
| 2 | M | 6 | 1.15 | 17.5 | 101.8 | 85.4 | Bedridden | Oral (Gasto) | Mashed diet | Yes | Cerebral palsy, Intractable epilepsy | HD |
| 3 | M | 7 | 1.21 | 20.0 | 101.7 | 87.0 | Bedridden | Gastro | Enteral formula | No | Sequelae of hypoxic encephalopathy, Epilepsy | LD |
| 4 | M | 9 | 1.20 | 28.3 | 92.3 | 125.8 | Gait disturbance | Oral | Mashed diet | Yes | Fukuyama congenital muscular dystrophy | HD |
| 5 | M | 11 | 1.12 | 24.0 | 78.9 | 126.3 | Bedridden | Gastro | Enteral formula | Yes | Cerebral palsy, Microcephaly, Epilepsy | LD |
| 6 | M | 12 | 1.41 | 33.8 | 94.6 | 98.0 | Gait disturbance | Oral | Mashed diet | Yes | Symptomatic epilepsy | HD |
| 7 | F | 12 | 1.28 | 22.2 | 85.3 | 85.4 | Bedridden | Gastro | Enteral formula | No | Cerebral atrophy, Infantile spasms | LD |
| 8 | F | 13 | 1.48 | 32.0 | 96.1 | 80.0 | Sit with support | Gastro | Enteral formula and paste diet | Yes | Epilepsy sequelae | HD |
| 9 | M | 13 | 1.38 | 35.1 | 87.9 | 106.4 | Gait disturbance | Oral | Regular diet | Yes | Cerebral palsy, Periventricular Leukomalacia | HD |
| 10 | F | 14 | 1.18 | 21.1 | 75.6 | 95.9 | Bedridden | Gastro | Enteral formula | Yes | Sequelae of acute encephalopathy | LD |
| 11 | F | 14 | 1.35 | 19.9 | 86.5 | 66.3 | Bedridden | Gastro | Enteral formula | Yes | Dentato-ruburo- pallido-luysian atrophy, Epilepsy | LD |
| 12 | F | 14 | 1.46 | 50.3 | 93.3 | 132.4 | Gait disturbance | Oral | Regular diet | Yes | Cerebral palsy, Extremely low birth weight | HD |
| 13 | M | 15 | 1.42 | 22.5 | 84.8 | 63.4 | Bedridden | Gastro | Enteral formula | No | Sequelae of hypoxic encephalopathy | LD |
| 14 | F | 17 | 1.45 | 36.0 | 92.1 | 97.3 | Gait disturbance | Oral | Regular diet | Yes | Cerebral palsy, Epilepsy, Extremely low birth weight | HD |
| Item | HD Group | LD Group | p-Value | |
|---|---|---|---|---|
| Number (men) | 8 (5) | 6 (3) | a 1.000 | |
| Age (year) | 12.5 (6, 17) | 13.0 (7, 15) | b 0.573 | |
| Height (m) | 1.40 (1.03, 1.48) | 1.25 (1.12, 1.42) | b 0.414 | |
| Weight (kg) | 32.9 (16.2, 50.3) | 21.7 (19.9, 24.0) | b 0.142 | |
| Height for age (H/A) (%) | 92.8 (87.9, 101.8) | 85.1 (75.6, 101.7) | b 0.029 * | |
| Weight for height (W/H) (%) | 99.6 (80.0, 132.4) | 86.2 (63.4, 126.3) | b 0.181 | |
| Motor function (n) | Bedridden | 1 | 6 | a 0.005 ** |
| Sit with support or gait disturbance | 7 | 0 | ||
| Experience of weaning food (n) | Yes | 8 | 3 | a 0.055 † |
| No | 0 | 3 | ||
| Main nutritional intake method (n) | Oral | 6 | 0 | a 0.0097 ** |
| Gastronomy | 2 | 6 | ||
| Types of meals (n) | Meals with or without enteral formula (regular, mashed, paste) | 8 | 0 | a < 0.001 ** |
| Enteral formula only | 0 | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kageyama, S.; Inoue, R.; Hosomi, K.; Park, J.; Yumioka, H.; Doi, M.; Miyake, M.; Nagashio, Y.; Shibuya, Y.; Oka, N.; et al. Association Between Gut Microbiome Composition and Physical Characteristics in Patients with Severe Motor and Intellectual Disabilities: Perspectives from Microbial Diversity. Nutrients 2024, 16, 3546. https://doi.org/10.3390/nu16203546
Kageyama S, Inoue R, Hosomi K, Park J, Yumioka H, Doi M, Miyake M, Nagashio Y, Shibuya Y, Oka N, et al. Association Between Gut Microbiome Composition and Physical Characteristics in Patients with Severe Motor and Intellectual Disabilities: Perspectives from Microbial Diversity. Nutrients. 2024; 16(20):3546. https://doi.org/10.3390/nu16203546
Chicago/Turabian StyleKageyama, Suzumi, Rikako Inoue, Koji Hosomi, Jonguk Park, Hitomi Yumioka, Miki Doi, Miyuu Miyake, Yuka Nagashio, Yoshiko Shibuya, Nobue Oka, and et al. 2024. "Association Between Gut Microbiome Composition and Physical Characteristics in Patients with Severe Motor and Intellectual Disabilities: Perspectives from Microbial Diversity" Nutrients 16, no. 20: 3546. https://doi.org/10.3390/nu16203546
APA StyleKageyama, S., Inoue, R., Hosomi, K., Park, J., Yumioka, H., Doi, M., Miyake, M., Nagashio, Y., Shibuya, Y., Oka, N., Akazawa, H., Kanzaki, S., Mizuguchi, K., Kunisawa, J., & Irie, Y. (2024). Association Between Gut Microbiome Composition and Physical Characteristics in Patients with Severe Motor and Intellectual Disabilities: Perspectives from Microbial Diversity. Nutrients, 16(20), 3546. https://doi.org/10.3390/nu16203546







