Effect of Oral Administration of Collagen Peptide OG-5 on Advanced Atherosclerosis Development in ApoE−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Animal Model and Environmental Conditions
2.3. Treatment of ApoE−/− Mice with OG-5
2.4. ELISA Analysis
2.5. Dissection and Examination of Aorta for Atherosclerotic Lesions
2.6. VSMC Culture and Phenotype Switch
2.7. Western Blot
2.8. Peptide Transport Experiment
2.9. Peptide Stability in Plasma
2.10. In Vivo Absorption Experiment on Rats
2.11. Statistical Analysis
3. Results
3.1. Effect of OG-5 on Body Weight, Organ Index, and Serum Lipid Levels in ApoE−/− Mice
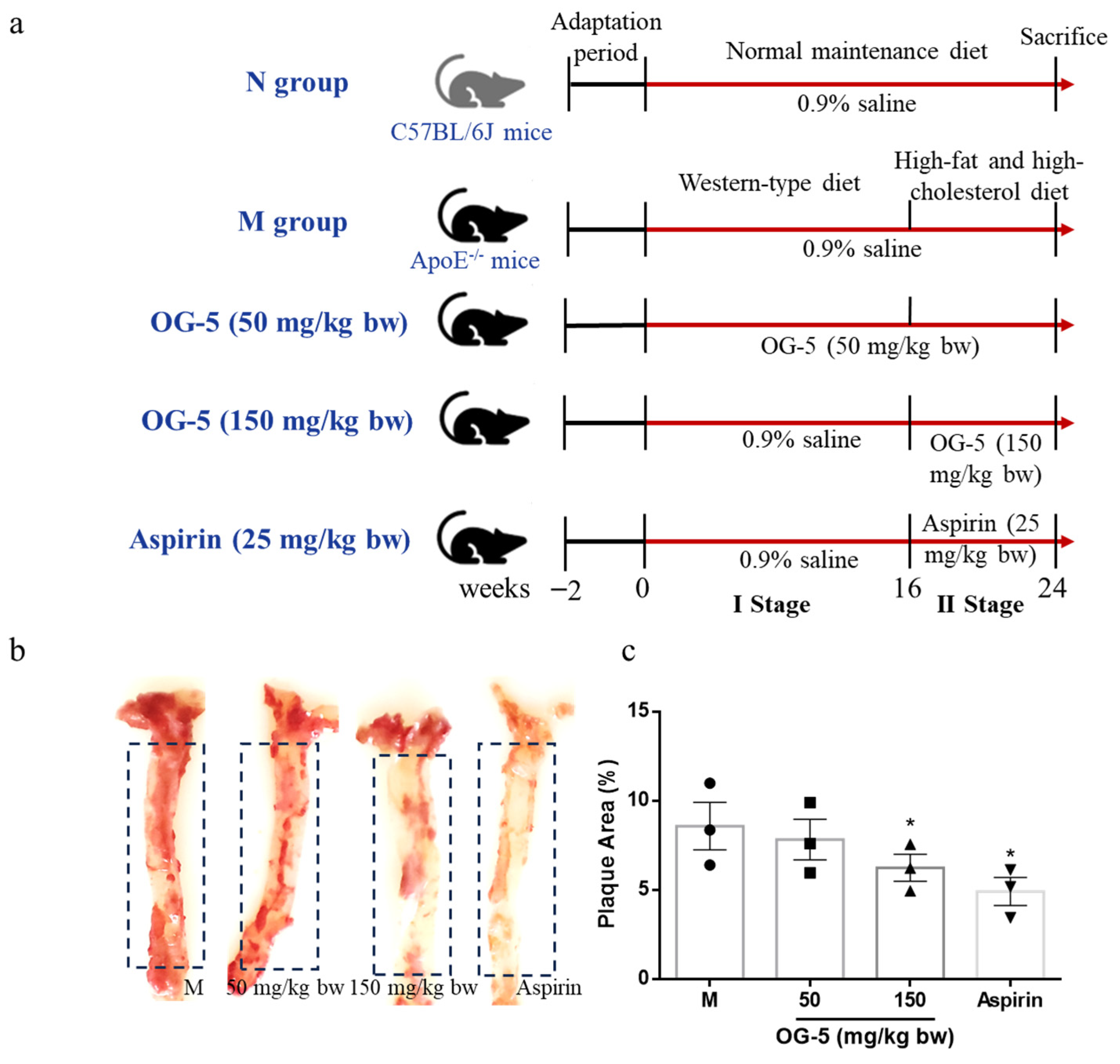
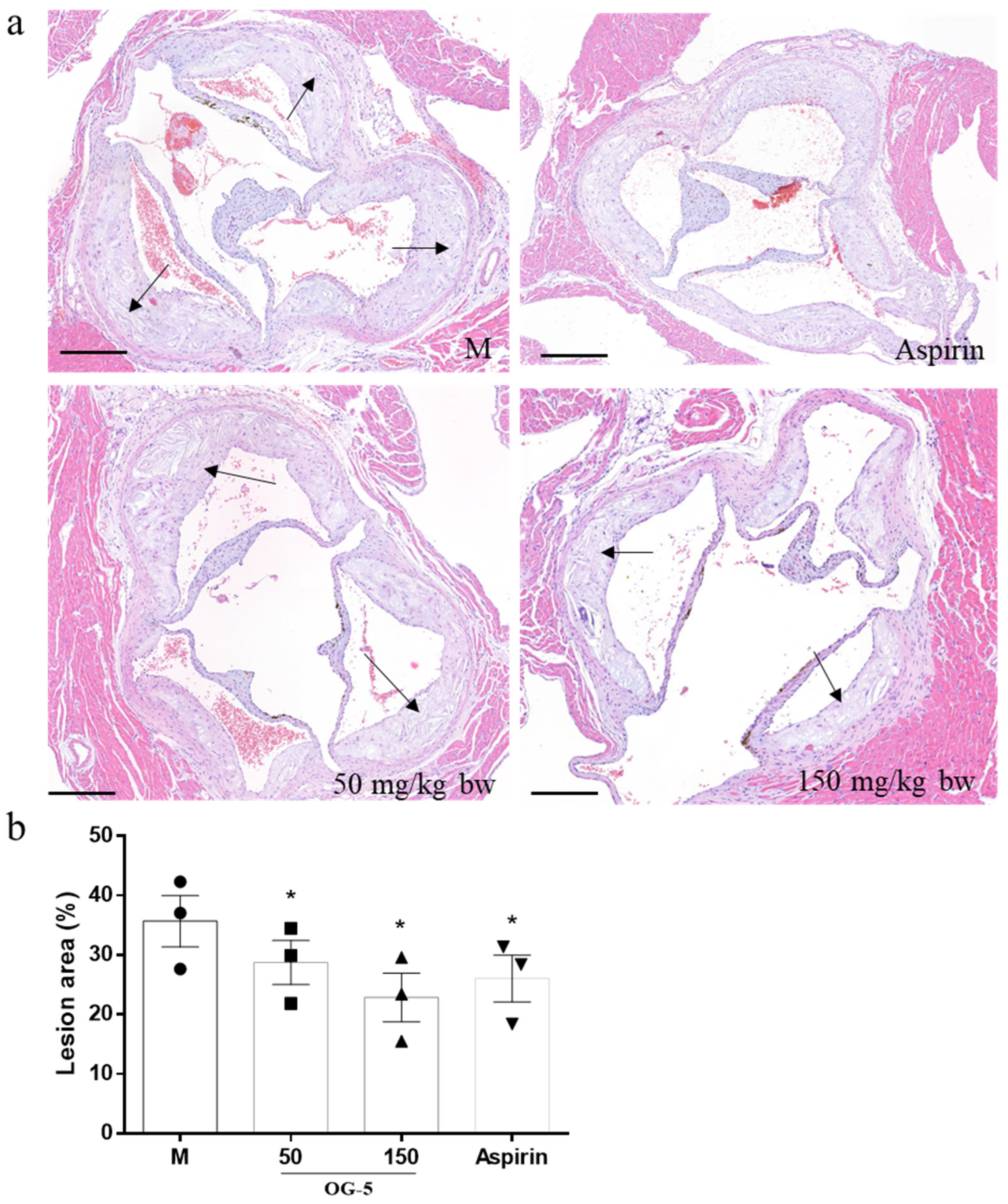
3.2. Peptide OG-5 Attenuated Atherogenesis in ApoE−/− Mice
3.3. Peptide OG-5 Inhibited Inflammatory Cytokines and Platelet Activation in Plasma
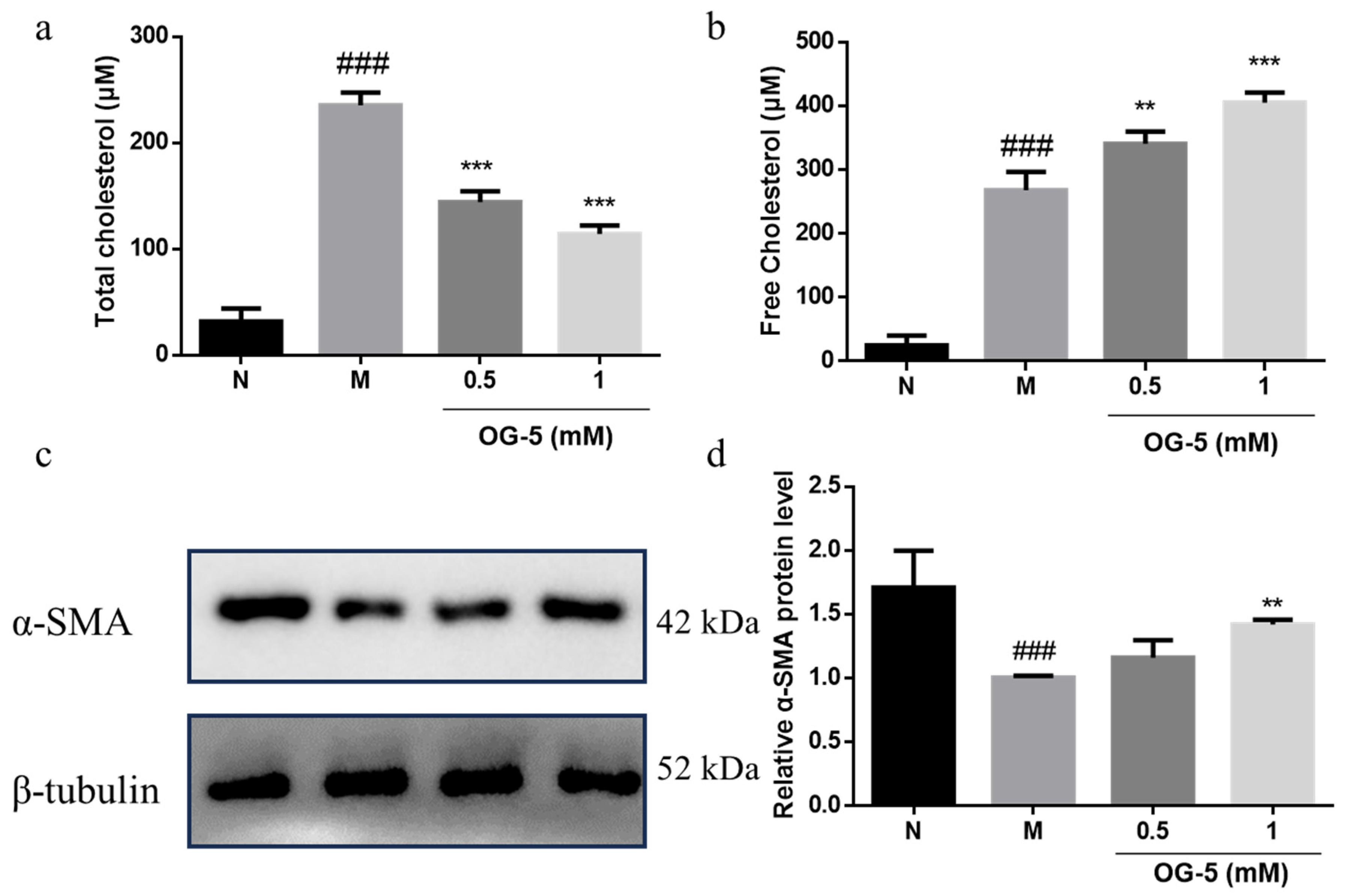
3.4. Peptide OG-5 Inhibited VSMC Phenotype Switch Induced by Ox-LDL
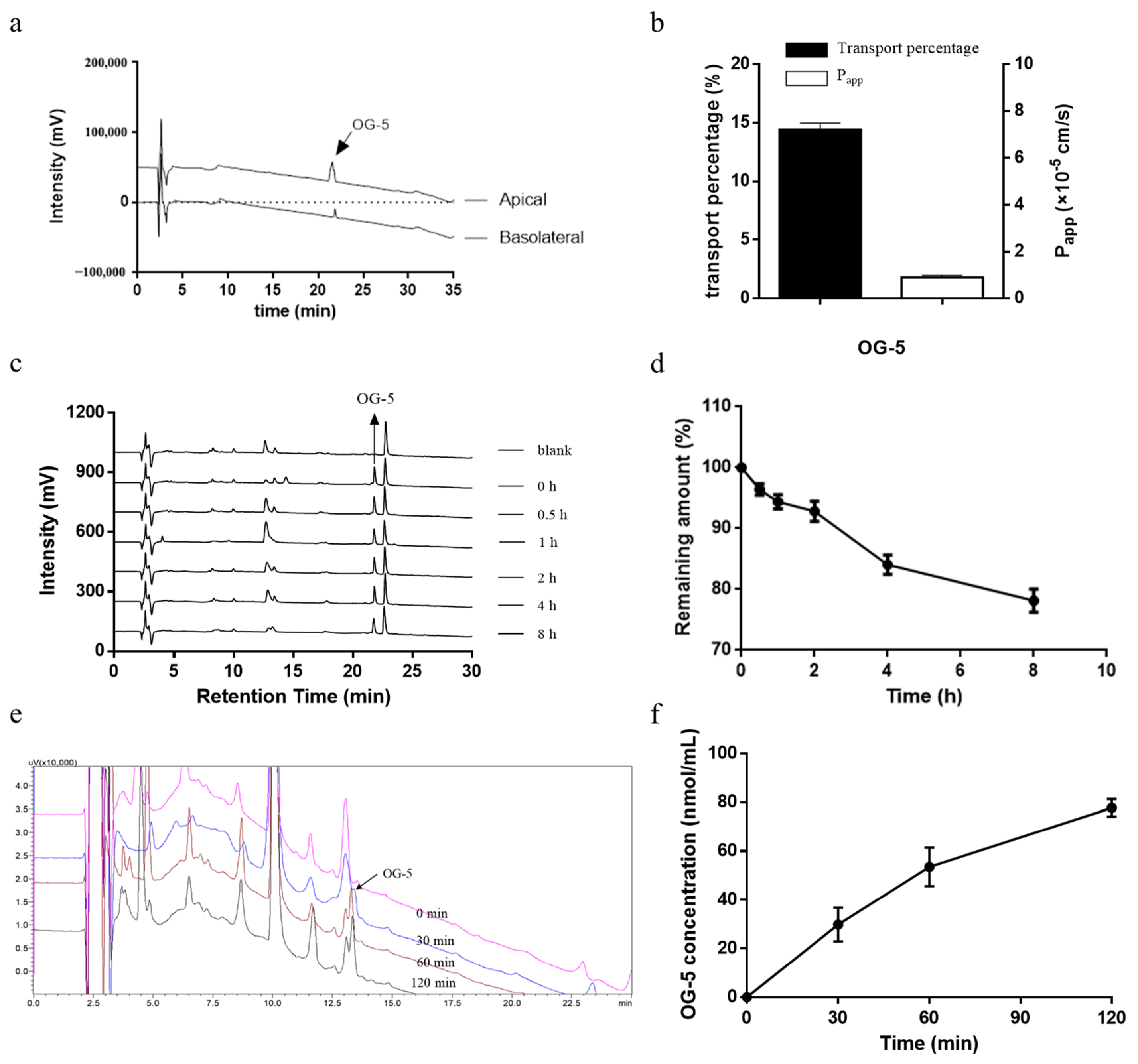
3.5. Transport of OG-5 Across Caco-2 Cell Monolayers
3.6. Stability of Peptide In Vitro Plasma
3.7. In Vivo Absorption of Peptide OG-5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; McVey, D.G.; Shen, D.; Huang, X.; Ye, S. Phenotypic Switching of Vascular Smooth Muscle Cells in Atherosclerosis. J. Am. Heart Assoc. 2023, 12, e031121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis. Cell. Mol. Life Sci. 2021, 79, 6. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2021, 45, 100694. [Google Scholar] [CrossRef]
- Lindemann, S.; Krmer, B.; Seizer, P.; Gawaz, M. Platelets, inflammation and atherosclerosis. J. Thromb. Haemost. 2007, 5 (Suppl. S1), 203–211. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Eckert, T.; Jährling-Butkus, M.; Louton, H.; Burg-Roderfeld, M.; Zhang, R.; Zhang, N.; Hesse, K.; Petridis, A.K.; Kožár, T.; Steinmeyer, J.; et al. Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms. Mar. Drugs 2021, 19, 542. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobenecker, B.; Böswald, L.F.; Reese, S.; Steigmeier-Raith, S.; Trillig, L.; Oesser, S.; Schunck, M.; Meyer-Lindenberg, A.; Hugenberg, J. The oral intake of specific Bioactive Collagen Peptides (BCP) improves gait and quality of life in canine osteoarthritis patients—A translational large animal model for a nutritional therapy option. PLoS ONE 2024, 19, e0308378. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Tian, Q.; Li, B. Purification and Characterization of Novel Collagen Peptides against Platelet Aggregation and Thrombosis from Salmo salar. ACS Omega 2020, 5, 19995–20003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Cui, L.; Liu, Y.; Fu, L.; Li, B. A Collagen-Derived Oligopeptide from Salmo salar Collagen Hydrolysates Restrains Atherogenesis in ApoE(-/-) Mice via Targeting P2Y12 Receptor. Mol. Nutr. Food Res. 2022, 66, e2200166. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Pi, S.-l.; Baral, S.; Xia, Y.-p.; He, Q.-w.; Li, Y.-n.; Jin, H.-j.; Li, M.; Wang, M.-d.; Mao, L.; et al. P2Y12 Promotes Migration of Vascular Smooth Muscle Cells Through Cofilin Dephosphorylation During Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.H.; Filep, J.G. Purinergic receptors and atherosclerosis: Emerging role for vessel wall P2Y12. Cardiovasc. Res. 2014, 102, 339–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, L.; Zhao, F.; Dai, M.; Li, H.; Chen, C.; Nie, J.; Wang, P.; Wang, D.W. P2y12 Receptor Promotes Pressure Overload-Induced Cardiac Remodeling via Platelet-Driven Inflammation in Mice. Hypertension 2017, 70, 759–769. [Google Scholar] [CrossRef]
- Pi, S.; Mao, L.; Chen, J.; Shi, H.; Liu, Y.; Guo, X.; Li, Y.; Zhou, L.; He, H.; Yu, C.; et al. The P2RY12 receptor promotes VSMC-derived foam cell formation by inhibiting autophagy in advanced atherosclerosis. Autophagy 2021, 17, 980–1000. [Google Scholar] [CrossRef]
- Cao, H.; Jia, Q.; Shen, D.; Yan, L.; Chen, C.; Xing, S. Quercetin has a protective effect on atherosclerosis via enhancement of autophagy in ApoE(-/-) mice. Exp. Ther. Med. 2019, 18, 2451–2458. [Google Scholar] [CrossRef]
- Ma, S.; Motevalli, S.M.; Chen, J.; Xu, M.Q.; Wang, Y.; Feng, J.; Qiu, Y.; Han, D.; Fan, M.; Ding, M.; et al. Precise theranostic nanomedicines for inhibiting vulnerable atherosclerotic plaque progression through regulation of vascular smooth muscle cell phenotype switching. Theranostics 2018, 8, 3693–3706. [Google Scholar] [CrossRef]
- Yang, Y.; Li, B. Novel Peptide Motifs Containing Asp-Glu-Gly Target P2Y12 and Thromboxane A2 Receptors to Inhibit Platelet Aggregation and Thrombus Formation. J. Agric. Food Chem. 2022, 70, 785–793. [Google Scholar] [CrossRef]
- Yang, Y.-J.; He, H.-Y.; Wang, F.-Z.; Ju, X.-R.; Yuan, J.; Wang, L.-F.; Aluko, R.E.; He, R. Transport of angiotensin converting enzyme and renin dual inhibitory peptides LY, RALP and TF across Caco-2 cell monolayers. J. Funct. Foods 2017, 35, 303–314. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Li, B. Structural Requirement of Casein Peptides for Transcytosis through the Caco-2 Cell Monolayer: Hydrophobicity and Charge Property Affect the Transport Pathway and Efficiency. J. Agric. Food Chem. 2019, 67, 11778–11787. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tian, Q.; Li, B. Novel Hyp-Gly-containing antiplatelet peptides from collagen hydrolysate after simulated gastrointestinal digestion and intestinal absorption. Food Funct. 2020, 11, 5553–5564. [Google Scholar] [CrossRef] [PubMed]
- Pryma, C.S.; Ortega, C.; Dubland, J.A.; Francis, G.A. Pathways of smooth muscle foam cell formation in atherosclerosis. Curr. Opin. Lipidol. 2019, 30, 117–124. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Satake, M.; Enjoh, M.; Nakamura, Y.; Takano, T.; Kawamura, Y.; Arai, S.; Shimizu, M. Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2002, 66, 378–384. [Google Scholar] [CrossRef]
- Bejjani, S.; Wu, J. Transport of IRW, an Ovotransferrin-Derived Antihypertensive Peptide, in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2013, 61, 1487–1492. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Zhang, Y.; Liu, J. Transport of Antihypertensive Peptide RVPSL, Ovotransferrin 328–332, in Human Intestinal Caco-2 Cell Monolayers. J. Agric. Food Chem. 2015, 63, 8143–8150. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Yu, Z.; Zhang, T.; Liu, J. Digestion and absorption of an egg white ACE-inhibitory peptide in human intestinal Caco-2 cell monolayers. Int. J. Food Sci. Nutr. 2016, 67, 111–116. [Google Scholar] [CrossRef]
- Yee, S.Y. In Vitro Permeability Across Caco-2 Cells (Colonic) Can Predict In Vivo (Small Intestinal) Absorption in Man—Fact or Myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- Kontermann, R.E. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011, 22, 868–876. [Google Scholar] [CrossRef]
- Werle, M.; Bernkop-Schnurch, A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids 2006, 30, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Liu, Y.; Cui, L.; Fu, L.; Li, B. Various bioactive peptides in collagen hydrolysate from salmo salar skin and the combined inhibitory effects on atherosclerosis in vitro and in vivo. Food Res. Int. 2022, 157, 111281. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth Muscle Cells Contribute the Majority of Foam Cells in ApoE (Apolipoprotein E)-Deficient Mouse Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef]
- Korybalska, K.; Rutkowski, R.; Luczak, J.; Czepulis, N.; Karpinski, K.; Witowski, J. The role of purinergic P2Y12 receptor blockers on the angiogenic properties of endothelial cells: An in vitro study. J. Physiol. Pharmacol. 2018, 69, 4. [Google Scholar] [CrossRef]
- Satonaka, H.; Nagata, D.; Takahashi, M.; Kiyosue, A.; Myojo, M.; Fujita, D.; Ishimitsu, T.; Nagano, T.; Nagai, R.; Hirata, Y. Involvement of P2Y12 receptor in vascular smooth muscle inflammatory changes via MCP-1 upregulation and monocyte adhesion. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H853–H861. [Google Scholar] [CrossRef]
- Mansour, A.; Bachelot-Loza, C.; Nesseler, N.; Gaussem, P.; Gouin-Thibault, I. P2Y12 Inhibition beyond Thrombosis: Effects on Inflammation. Int. J. Mol. Sci. 2020, 21, 1391. [Google Scholar] [CrossRef]
- Rauch, B.H.; Rosenkranz, A.C.; Ermler, S.; Bohm, A.; Driessen, J.; Fischer, J.W.; Sugidachi, A.; Jakubowski, J.A.; Schror, K. Regulation of functionally active P2Y12 ADP receptors by thrombin in human smooth muscle cells and the presence of P2Y12 in carotid artery lesions. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2434–2442. [Google Scholar] [CrossRef]


| Parameters | N | M-I-Stage | M-II-Stage | OG-5 (50 mg/kg bw) | OG-5 (150 mg/kg bw) | Aspirin |
|---|---|---|---|---|---|---|
| Initial Body weight (g) | 24.0 ± 1.3 | 24.9 ± 0.9 | 27.4 ± 2.5 | 24.5 ± 1.0 | 27.8 ± 2.5 | 27.7 ± 2.8 |
| Final Body weight (g) | 36.6 ± 3.3 | 27.4 ± 3.0 # | 28.2 ± 1.4 | 27.7 ± 2.2 | 28.9 ± 2.0 | 28.8 ± 1.7 |
| Thymus index (mg/g bw) | 0.78 ± 0.09 | 0.81 ± 0.24 | 0.83 ± 0.24 | 0.69 ± 0.15 | 0.79 ± 0.13 | 0.83 ± 0.12 |
| Spleen index (mg/g bw) | 3.05 ± 0.52 | 6.60 ± 2.91 # | 5.01 ± 1.98 | 4.16 ± 0.99 | 4.80 ± 2.13 | 4.99 ± 2.64 |
| TC (mM) | 5.21 ± 0.88 | 35.16 ± 3.74 # | 45.52 ± 3.79 † | 36.48 ± 4.91 ** | 40.99 ± 5.78 | 41.09 ± 6.05 |
| TG (mM) | 0.44 ± 0.09 | 1.56 ± 0.02 # | 3.29 ± 0.42 † | 3.04 ± 0.31 | 3.25 ± 0.78 | 3.43 ± 0.96 |
| HDL-C (mM) | 2.10 ± 0.52 | 0.73 ± 0.12 # | 0.56 ± 0.10 | 0.66 ± 0.10 | 0.61 ± 0.27 | 0.64 ± 0.37 |
| LDL-C (mM) | 0.31 ± 0.12 | 6.81 ± 0.98 # | 10.28 ± 0.79 † | 8.31 ± 0.71 * | 9.39 ± 2.03 | 9.55 ± 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, B. Effect of Oral Administration of Collagen Peptide OG-5 on Advanced Atherosclerosis Development in ApoE−/− Mice. Nutrients 2024, 16, 3752. https://doi.org/10.3390/nu16213752
Yang Y, Li B. Effect of Oral Administration of Collagen Peptide OG-5 on Advanced Atherosclerosis Development in ApoE−/− Mice. Nutrients. 2024; 16(21):3752. https://doi.org/10.3390/nu16213752
Chicago/Turabian StyleYang, Yijie, and Bo Li. 2024. "Effect of Oral Administration of Collagen Peptide OG-5 on Advanced Atherosclerosis Development in ApoE−/− Mice" Nutrients 16, no. 21: 3752. https://doi.org/10.3390/nu16213752
APA StyleYang, Y., & Li, B. (2024). Effect of Oral Administration of Collagen Peptide OG-5 on Advanced Atherosclerosis Development in ApoE−/− Mice. Nutrients, 16(21), 3752. https://doi.org/10.3390/nu16213752








