Nutritional Profile and Chlorophyll Intake of Collard Green as a Convenience Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Freeze-Dried CGLs
2.3. Determination of Water Activity
2.4. Proximate Composition of CGLs
2.5. Amino Acids Profile Determination
2.6. Fatty Acids Profile Determination
2.7. Laboratory Sous Vide Cooking of CGLs
2.8. Colorimetric Properties of CGLs
2.9. Determination of Chlorophyll
2.10. Data Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Soluble Sugars
3.3. Mineral Composition
3.4. Amino Acid Profile
3.5. Fatty Acid Composition and Content
3.6. Chlorophyll Content in CGL Convenience Food
3.7. Impact of Sous Vide-Cooking Processing on Color and Chlorophyll Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francisco, M.; Tortosa, M.; Martínez-Ballesta, M.d.C.; Velasco, P.; García-Viguera, C.; Moreno, D.A. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 273–285. [Google Scholar] [CrossRef]
- Raza, A.; Hafeez, M.B.; Zahra, N.; Shaukat, K.; Umbreen, S.; Tabassum, J.; Charagh, S.; Khan, R.S.A. The Plant Family Brassicaceae: Introduction, Biology, And Importance. In The Plant Family Brassicaceae: Introduction, Biology, And Importance; Springer: Singapore, 2020; pp. 1–43. [Google Scholar]
- Li, Y.Z.; Yang, Z.Y.; Gong, T.T.; Liu, Y.S.; Liu, F.H.; Wen, Z.Y.; Li, X.Y.; Gao, C.; Luan, M.; Zhao, Y.H.; et al. Cruciferous vegetable consumption and multiple health outcomes: An umbrella review of 41 systematic reviews and meta-analyses of 303 observational studies. Food Funct. 2022, 13, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef] [PubMed]
- Aǧagündüz, D.; Şahin, T.Ö.; Yilmaz, B.; Ekenci, K.D.; Duyar Özer, Ş.; Capasso, R. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid.-Based Complement. Altern. Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Picchi, V.; Tava, A.; Doria, F.; Walley, P.G.; Dever, L.; di Bella, M.C.; Arena, D.; Ammar, H.B.; Scalzo, R.L.; et al. Insights into the phytochemical composition of selected genotypes of organic kale (Brassica oleracea L. var. acephala). J. Food Compos. Anal. 2024, 125, 105721. [Google Scholar] [CrossRef]
- Giorgetti, L.; Giorgi, G.; Cherubini, E.; Gervasi, P.G.; Della Croce, C.M.; Longo, V.; Longo, V.; Bellani, L. Screening identification of major phytochemical compounds in seeds sprouts leaves of Tuscan black kale Brassica oleracea (L.) ssp. acephala (DC) var. sabellica, L. Nat. Prod. Res. 2018, 32, 1617–1626. [Google Scholar] [CrossRef]
- Ljubej, V.; Radojčić Redovniković, I.; Salopek-Sondi, B.; Smolko, A.; Roje, S.; Šamec, D. Chilling and freezing temperature stress differently influence glucosinolates content in Brassica oleracea var. acephala. Plants 2021, 10, 1305. [Google Scholar] [CrossRef]
- Hai, H.D. The Worldwide Vegetables. Brassica oleracea var. acephala Collards or Collard Greens. 2015. Available online: https://theworldwidevegetables.weebly.com/collard-green.html (accessed on 11 October 2024).
- Appiah, J.; Knust, O. Utilization of Agricultural Food Waste Products for Bioethanol Generation, Kiambu county, Kenya. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2021. [Google Scholar]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Pérez-gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Roca, M.; Pérez-Gálvez, A. Application of EFSA EU menu database and R computing language to calculate the green chlorophyll intake in the European population. Food Chem. 2024, 461, 140912. [Google Scholar] [CrossRef]
- Roca, M.; Chen, K.; Pérez-Gálvez, A. Chlorophylls. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 193–226. [Google Scholar]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green natural colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food additives. Off. J. Eur. Union 2008, 354, 16–33. [Google Scholar]
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological applications of natural colorants in food systems: A review. Foods 2021, 10, 634. [Google Scholar] [CrossRef]
- Caponio, F.; Piga, A.; Poiana, M. Valorization of Food Processing By-Products. Foods 2022, 11, 3246. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Cui, Z.W.; Xu, S.Y.; Sun, D.W. Effect of microwave-vacuum drying on the carotenoids retention of carrot slices and chlorophyll retention of Chinese chive leaves. Dry. Technol. 2004, 22, 563–575. [Google Scholar] [CrossRef]
- Korus, A. Effect of preliminary and technological treatments on the content of chlorophylls and carotenoids in kale (Brassica oleracea L. var. acephala). J. Food Process Preserv. 2013, 37, 335–344. [Google Scholar] [CrossRef]
- Saidi, I.A.; Azara, R.; Efendi, N.; Kartika, S.D. Physicochemical characteristics of homemade ice cream with mustard green (Brassica juncea) powder or puree. Afr. J. Food Agric. Nutr. Dev. 2023, 23, 24680–24695. [Google Scholar] [CrossRef]
- Waseem, M.; Akhtar, S.; Mehmood, T.; Qamar, M.; Saeed, W.; Younis, M.; Perveen, S.; Ismail, T.; Esatbeyoglu, T. Nutritional, safety and sensory quality evaluation of unleavened flatbread supplemented with thermal and non-thermal processed spinach powder. J. Agric. Food Res. 2024, 16, 101114. [Google Scholar] [CrossRef]
- Fanesi, B.; Ismaiel, L.; Nartea, A.; Orhotohwo, O.L.; Kuhalskaya, A.; Pacetti, D.; Lucci, P.; Falcone, P.M. Bioactives and Technological Quality of Functional Biscuits Containing Flour and Liquid Extracts from Broccoli By-Products. Antioxidants 2023, 12, 2115. [Google Scholar] [CrossRef]
- WHO. WHO European Childhood Obesity Surveillance Initiative (COSI); WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Istituto Superiore di Sanità EE per la SP. OKkio Alla Salute. 2024. Available online: https://www.epicentro.iss.it/okkioallasalute/ (accessed on 31 August 2024).
- WHO Healthy Diet. 2019. Available online: https://applications.emro.who.int/docs/EMROPUB_2019_en_23536.pdf (accessed on 13 October 2024).
- CREA Linee Guida per Una Sana Alimentazione. 2018. Available online: https://www.crea.gov.it/documents/59764/0/Dossier+Scientifico+Linee+Guida+2018+%281%29.pdf (accessed on 13 October 2024).
- Gruppo Prodotti a Base Vegetale-Unione Italiana Food. Solo il 7% Degli Italiani Consuma 5 Porzioni di Frutta e Verdura al Giorno. 2024. Available online: https://agricolae.eu/unione-italiana-food-solo-il-7-degli-italiani-consuma-5-porzioni-di-frutta-e-verdura-ogni-giorno/?print=print (accessed on 1 September 2024).
- Landry, M.J.; Burgermaster, M.; van den Berg, A.E.; Asigbee, F.M.; Vandyousefi, S.; Ghaddar, R.; Jeans, M.R.; Yau, A.; Davis, J.N. Barriers to preparing and cooking vegetables are associated with decreased home availability of vegetables in low-income households. Nutrients 2020, 12, 1823. [Google Scholar] [CrossRef]
- Contini, C.; Boncinelli, F.; Marone, E.; Scozzafava, G.; Casini, L. Drivers of plant-based convenience foods consumption: Results of a multicomponent extension of the theory of planned behaviour. Food Qual. Prefer. 2020, 84, 103931. [Google Scholar] [CrossRef]
- Drewnowski, A.; Monsivais, P. Taste, cost, convenience, and food choices. In Present Knowledge in Nutrition: Clinical and Applied Topics in Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–200. [Google Scholar]
- Peltner, J.; Thiele, S. Convenience-based food purchase patterns: Identification and associations with dietary quality, sociodemographic factors and attitudes. Public. Health Nutr. 2018, 21, 558–570. [Google Scholar] [CrossRef]
- Brunner, T.A.; van der Horst, K.; Siegrist, M. Convenience food products. Driv. Consum. Appet. 2010, 55, 498–506. [Google Scholar]
- Raj, S.; Suvadarshini, A.; Mishra, B.B. Role, Relevance and Significance of Convenience Food—A Literature Review Approach. Glob. Media J. 2021, 19, 1–6. [Google Scholar]
- Dos Reis, L.C.R.; De Oliveira, V.R.; Hagen, M.E.K.; Jablonski, A.; Flôres, S.H.; De Oliveira Rios, A. Effect of cooking on the concentration of bioactive compounds in broccoli (Brassica oleracea var. Avenger) and cauliflower (Brassica oleracea var. alphina F1) grown in an organic system. Food Chem. 2015, 172, 770–777. [Google Scholar] [CrossRef]
- Guillén, S.; Mir-Bel, J.; Oria, R.; Salvador, M.L. Influence of cooking conditions on organoleptic and health-related properties of artichokes, green beans, broccoli and carrots. Food Chem. 2017, 217, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pero, M.; Askari, G.; Skåra, T.; Skipnes, D.; Kiani, H. Change in the color of heat-treated, vacuum-packed broccoli stems and florets during storage: Effects of process conditions and modeling by an artificial neural network. J. Sci. Food Agric. 2018, 98, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Zavadlav, S.; Blažić, M.; de Velde FVan Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a technique for preparing healthy and high-quality vegetable and seafood products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- Misu, G.A.; Canja, C.M.; Lupu, M.; Matei, F. Advances and Drawbacks of Sous-Vide Technique—A Critical Review. Foods 2024, 13, 2217. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Schifani, G.; Migliore, G. Understanding consumers’ convenience orientation. An exploratory study of fresh-cut fruit in Italy. Sustainability 2021, 13, 1027. [Google Scholar] [CrossRef]
- Koli, R.; Gyana Prasuna, R.; Watturakar, R. Consumer behavior for ready-to-eat salad food with special reference to working people. J. Postharvest Technol. 2024, 12, 68–81. [Google Scholar]
- Avató, J.L.; Mannheim, V. Life Cycle Assessment Model of a Catering Product: Comparing Environmental Impacts for Different End-of-Life Scenarios. Energies 2022, 15, 5423. [Google Scholar] [CrossRef]
- FAO. Chapter 2: Methods of Food Analysis; FAO: Rome, Italy, 2002. [Google Scholar]
- Agarwal, A.; Raj, N.; Chaturvedi, N.; Scholar, R.; Professor, A. A Comparative Study on Proximate and Antioxidant Activity of Brassica oleracea (Kale) and Spinacea oleracea (Spinach) Leaves. Int. J. Adv. Res. Biol. Sci. 2017, 4, 22–29. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Mihaylova, D.; Marangon, C.M.; Grigoletto, L.; Lante, A. Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves. Molecules 2022, 27, 8110. [Google Scholar] [CrossRef]
- Bosch, L.; Alegría, A.; Farré, R. Application of the 6-aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) reagent to the RP–HPLC determination of amino acids in infant foods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 831, 176–183. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Bayram, I.; Lante, A.; Decker, E.A. Acid-hydrolyzed phenolic extract of parsley (Petroselinum crispum L.) leaves inhibits lipid oxidation in soybean oil-in-water emulsions. Food Res. Int. 2024, 187, 114452. [Google Scholar] [CrossRef] [PubMed]
- Hartmut, K.; Lichtenthaler, C.B. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- CREA. Tabelle di Composizione degli Alimenti; CREA: New Delhi, India, 2019. [Google Scholar]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Hayırlıoglu-Ayaz, S. Nutrient contents of kale (Brassica oleraceae L. var. acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Hahn, C.; Ruiz-Matute, A.I.; Behrends, B.; Albach, D.C.; Kuhnert, N. Changes in low molecular weight carbohydrates in kale during development and acclimation to cold temperatures determined by chromatographic techniques coupled to mass spectrometry. Food Res. Int. 2020, 127, 108727. [Google Scholar] [CrossRef]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive compounds in brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules 2018, 23, 15. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EU) No 1169/2011 of the European Parliament and of the Council. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 136, 1–40. [Google Scholar]
- Ayaz, A.; Zaman, W.; Radák, Z.; Gu, Y. Green strength: The role of micronutrients in plant-based diets for athletic performance enhancement. Heliyon 2024, 10, e32803. [Google Scholar] [CrossRef]
- Baroni, L.; Pelosi, E.; Giampieri, F.; Battino, M. The VegPlate for Sports: A Plant-Based Food Guide for Athletes. Nutrients 2023, 15, 1746. [Google Scholar] [CrossRef]
- Liu, D.; Tian, Y.; Wang, R.; Zhang, T.; Shen, S.; Zeng, P.; Zou, T. Sodium, potassium intake, and all-cause mortality: Confusion and new findings. BMC Public. Health 2024, 24, 180. [Google Scholar]
- Papadakis, I.E.; Antonopoulou, C.; Sotiropoulos, T.; Chatzissavvidis, C.; Therios, I. Effect of Magnesium on Mineral Nutrition, Chlorophyll, Proline and Carbohydrate Concentrations of Sweet Orange (Citrus sinensis cv. Newhall) Plants. Appl. Sci. 2023, 13, 7995. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and the Hallmarks of Aging. Nutrients 2024, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls; 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557845/ (accessed on 2 September 2024).
- Xiao, F.; Guo, F. Impacts of essential amino acids on energy balance. Mol. Metab. 2022, 57, 101393. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO/UNU. Dietary Protein Quality Evaluation in Human Nutrition Report of an FAO Expert Consultation; FAO Food and Nutrition Paper; FAO: Rome, Italy, 2007. [Google Scholar]
- He, Y.; Qin, H.; Wen, J.; Wang, L.; Cao, W.; Fan, S.; Lu, W.; Li, J.; Li, C. Characterization of Amino Acid Composition, Nutritional Value, and Taste of Fruits from Different Actinidia arguta Resources. J. Food Qual. 2024, 2024, 1005194. [Google Scholar] [CrossRef]
- Thalacker-Mercer, A.E.; Gheller, M.E. Benefits and adverse effects of histidine supplementation. J. Nutr. 2020, 150, 2588S–2592S. [Google Scholar] [CrossRef]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive evaluation on chemical components of leaves, stalks and stems of sweet potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Lisiewska, Z.; Kmiecik, W.; Korus, A. The amino acid composition of kale (Brassica oleracea L. var. acephala), fresh and after culinary and technological processing. Food Chem. 2008, 108, 642–648. [Google Scholar] [CrossRef]
- Korus, A. Amino acid retention and protein quality in dried kale (Brassica oleracea L. var. acephala). J. Food Process Preserv. 2014, 38, 676–683. [Google Scholar] [CrossRef]
- Eppendorfer, W.H.; Bille, S.W. Free and total amino acid composition of edible parts of beans, kale, spinach, cauliflower and potatoes as influenced by nitrogen fertilisation and phosphorus and potassium deficiency. J. Sci. Food Agric. 1996, 71, 449–458. [Google Scholar] [CrossRef]
- Pitura, K.; Jarosz, Z. Skład chemiczny i wartość biologiczna jarmużu średniowysokiego w zależności od zróżnicowanego nawożenia mineralnego. Agron. Sci. 2020, 75, 97–107. [Google Scholar] [CrossRef]
- Tessari, P.; Lante, A.; Mosca, G. Essential amino acids: Master regulators of nutrition and environmental footprint? Sci. Rep. 2016, 6, 26074. [Google Scholar] [CrossRef] [PubMed]
- Lisiewska, Z.; Kmiecik, W.; Gbczyński, P.; Sobczyńska, L. Amino acid profile of raw and as-eaten products of spinach (Spinacia oleracea L.). Food Chem. 2011, 126, 460–465. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of soybean products in terms of essential amino acids composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Giulia Romualdi. AgroNotizie. Couve Manteiga, Dal Brasile Alla Romagna. 2020. Available online: https://plantgest.imagelinenetwork.com/it/news/2020/02/13/couve-manteiga-dal-brasile-alla-romagna/65778 (accessed on 30 August 2024).
- Czarnowska-Kujawska, M.; Draszanowska, A.; Chróst, M.; Starowicz, M. The Effect of Sous-Vide Processing Time on Chemical and Sensory Properties of Broccoli, Green Beans and Beetroots. Appl. Sci. 2023, 13, 4086. [Google Scholar] [CrossRef]
- Sun, X.; Yon, D.K.; Nguyen, T.T.; Tanisawa, K.; Son, K.; Zhang, L.; Shu, J.; Peng, W.; Yang, Y.; Branca, F.; et al. Dietary and other lifestyle factors and their influence on non-communicable diseases in the Western Pacific region. Lancet Reg. Health-West. Pac. 2023, 43, 100842. [Google Scholar] [CrossRef]
- Gherasim, A.; Arhire, L.I.; Nițǎ, O.; Popa, A.D.; Graur, M.; Mihalache, L. The relationship between lifestyle components and dietary patterns. Proc. Nutr. Soc. 2020, 79, 311–323. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids. 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Znyk, M.; Raciborski, F.; Kaleta, D. Dietary Behavior and Determinants of Diet Quality among Primary Health Care Patients in Poland. Nutrients 2024, 16, 925. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Carbone, S.; Lavie, C.J. Dietary fats and chronic noncommunicable diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Heseker, H.; Kiesswetter, E.; Koletzko, B. Dietary fat and fatty foods in the prevention of non-communicable diseases: A review of the evidence. Trends Food Sci. Technol. 2022, 128, 173–184. [Google Scholar] [CrossRef]
- Steele, E.M.; Batis, C.; Cediel, G.; da Costa Louzada, M.L.; Khandpur, N.; Machado, P.; Moubarac, J.C.; Rauber, F.; Jedlicki, M.R.; Levy, R.B.; et al. The burden of excessive saturated fatty acid intake attributed to ultra-processed food consumption: A study conducted with nationally representative cross-sectional studies from eight countries. J. Nutr. Sci. 2021, 10, e43. [Google Scholar] [CrossRef] [PubMed]
- Eichelmann, F.; Prada, M.; Sellem, L.; Jackson, K.G.; Salas Salvadó, J.; Razquin Burillo, C.; Estruch, R.; Friedén, M.; Rosqvist, F.; Risérus, U.; et al. Lipidome changes due to improved dietary fat quality inform cardiometabolic risk reduction and precision nutrition. Nat. Med. 2024, 30, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Iafelice, G.; Caboni, M.F.; Cubadda, R.; di Criscio, T.; Trivisonno, M.C.; Marconi, E. Development of functional spaghetti enriched with long chain omega-3 fatty acids. Cereal Chem. 2008, 85, 146–151. [Google Scholar] [CrossRef]
- Wongprawmas, R.; Mora, C.; Pellegrini, N.; Guiné, R.P.F.; Carini, E.; Sogari, G.; Vittadini, E. Food choice determinants and perceptions of a healthy diet among Italian consumers. Foods 2021, 10, 318. [Google Scholar] [CrossRef]
- Decreto del Presidente della Repubblica. Legge n.77 del 13 Maggio 2011, Disposizioni Concernenti la Preparazione, il Confezionamento e la Distribuzione dei Prodotti Ortofrutticoli di Quarta Gamma, art.2.Legge n.77 del 13 Maggio 2011, Disposizioni Concernenti la Preparazione, il Confezionamento e la Distribuzione dei Prodotti Ortofrutticoli di Quarta Gamma, art.2. 2011. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:2011;77 (accessed on 3 September 2024).
- Stringer, M.; Dennis, C. Chilled foods: A Comprehensive Guide; CRC Press: Boca Raton, FL, USA, 2000; 486p. [Google Scholar]
- Onyeaka, H.; Nwabor, O.; Jang, S.; Obileke, K.C.; Hart, A.; Anumudu, C.; Miri, T. Sous vide processing: A viable approach for the assurance of microbial food safety. J. Sci. Food Agric. 2022, 102, 3503–3512. [Google Scholar] [CrossRef]
- Forsido, S.F.; Welelaw, E.; Belachew, T.; Hensel, O. Effects of storage temperature and packaging material on physico-chemical, microbial and sensory properties and shelf life of extruded composite baby food flour. Heliyon 2021, 7, e06821. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (aw) on Microbial Stability: As a Hurdle in Food Preservation. In Water Activity in Foods: Fundamentals and Applications; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 239–271. [Google Scholar]
- Dilrukshi, S.H.B.; Senarath, H.P.S. Development and Quality Evaluation of Freeze-Dried Instant Green Smoothie Powder. Int. J. Food Sci. 2021, 2021, 6634764. [Google Scholar] [CrossRef] [PubMed]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. Freeze-dried snacks obtained from frozen vegetable by-products and apple pomace—Selected properties, energy consumption and carbon footprint. Innov. Food Sci. Emerg. Technol. 2022, 77, 102949. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, L.; Martin, P.; Shiwaku, I.; Nogueira, G.; Aguayo, E. Exploration of By-Products with Bioactive Compounds by Freeze Drying. 2016. Available online: https://www.researchgate.net/publication/350845327 (accessed on 9 September 2024).
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of Vegetable Fresh-Processing Residues as Functional Powdered Ingredients. A Review on the Potential Impact of Pretreatments and Drying Methods on Bioactive Compounds and Their Bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- Tan, C.H.; Hii, C.L.; Borompichaichartkul, C.; Phumsombat, P.; Kong, I.; Pui, L.P. Valorization of fruits, vegetables, and their by-products: Drying and bio-drying. Dry. Technol. 2022, 40, 1514–1538. [Google Scholar] [CrossRef]
- Paciulli, M.; Palermo, M.; Chiavaro, E.; Pellegrini, N. Chlorophylls and colour changes in cooked vegetables. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley Blackwell: New York, NY, USA, 2017; pp. 703–719. [Google Scholar]
- Armesto, J.; Gómez–Limia, L.; Carballo, J.; Martínez, S. Effects of different cooking methods on some chemical and sensory properties of Galega kale. Int. J. Food Sci. Technol. 2016, 51, 2071–2080. [Google Scholar] [CrossRef]
- WHO Ageing and Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=In%202020%2C%20the%20number%20of,from%2012%25%20to%2022%25 (accessed on 5 September 2024).
- Nicklett, E.J.; Kadell, A.R. Fruit and vegetable intake among older adults: A scoping review. Maturitas 2013, 75, 305–312. [Google Scholar] [CrossRef]
- Whitelock, E.; Ensaff, H. On your own: Older adults’ food choice and dietary habits. Nutrients 2018, 10, 413. [Google Scholar] [CrossRef]
- Kouiti, M.; Ortega-Rico, C.; Arrebola, J.P.; Gracia-Arnaiz, M.; Larrea-Killinger, C. Demographic and Socioeconomic Factors Associated to Fruits and Vegetables Consumption in Elderly Europeans: A Systematic Review. Int. J. Environ. Res. Public. Health 2023, 20, 3442. [Google Scholar] [CrossRef]
- Delerue Matos, A.; Barbosa, F.; Cunha, C.; Voss, G.; Correia, F. Social isolation, physical inactivity and inadequate diet among European middle-aged and older adults. BMC Public. Health 2021, 21, 924. [Google Scholar] [CrossRef]
- Tansey, F.; Gormley, R.; Butler, F. The effect of freezing compared with chilling on selected physico-chemical and sensory properties of sous vide cooked carrots. Innov. Food Sci. Emerg. Technol. 2010, 11, 137–145. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Sous-vide cooking as a systematic approach for quality maintenance and shelf-life extension of crab lump meat. LWT 2021, 142, 111004. [Google Scholar] [CrossRef]
- Hyytia-Tree, E.; Skytta, E.; Mokkila, M.; Kinnunen, A.; Lindstro, M.; La, L.; Ahvenainen, R.; Korkeala, H. Safety Evaluation of Sous Vide-Processed Products with Respect to Nonproteolytic Clostridium botulinum by Use of Challenge Studies and Predictive Microbiological Models. Appl. Environ. Microbiol. 2000, 66, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Sedat Velioglu, Y. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Technol. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- Armesto, J.; Gómez-Limia, L.; Carballo, J.; Martínez, S. Impact of vacuum cooking and boiling and refrigerated storage on the quality of galega kale (Brassica oleracea var. acephala cv. Galega). LWT 2017, 79, 267–277. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Pareek, S.; Sagar, N.A.; Sharma, S.; Agarwal, T. Fruit and Vegetable Phytochemicals: Chemistry and Human Health. 2017. Available online: https://www.researchgate.net/publication/319703059 (accessed on 5 September 2024).
- Wang, X.; Chen, G.; Du, S.; Wu, H.; Fu, R.; Yu, X. Light Intensity Influence on Growth and Photosynthetic Characteristics of Horsfieldia hainanensis. Front. Ecol. Evol. 2021, 9, 636804. [Google Scholar] [CrossRef]
- Danowska-Oziewicz, M.; Narwojsz, A.; Draszanowska, A.; Marat, N. The effects of cooking method on selected quality traits of broccoli and green asparagus. Int. J. Food Sci. Technol. 2020, 55, 127–135. [Google Scholar] [CrossRef]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of chlorophyll degradation and color loss in heated broccoli juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Shivhare, U.S.; Sarkar, B.C.; Ahmed, J. Thermal chlorophyll degradation kinetics of mint leaves puree. Int. J. Food Prop. 2007, 10, 853–865. [Google Scholar] [CrossRef]
- Gibson, M. Food Science and the Culinary Arts; Elsevier: Amsterdam, The Netherlands, 2018; 292p. [Google Scholar]
- Tijskens, L.M.M.; Schijvens, E.P.H.M.; Biekman, E.S.A. Modelling the change in colour of broccoli and green beans during blanching. Innov. Food Sci. Emerg. Technol. 2001, 2, 303–313. [Google Scholar] [CrossRef]
- Lau, M.H.; Tang, J.; Swanson, B.G. Kinetics of Textural and Color Changes in Green Asparagus During Thermal Treatments. J. Food Eng. 2000, 45, 231–236. [Google Scholar] [CrossRef]
- Severini, C.; Giuliani, R.; De Filippis, A.; Derossi, A.; De Pilli, T. Influence of different blanching methods on colour, ascorbic acid and phenolics content of broccoli. J. Food Sci. Technol. 2016, 53, 501–510. [Google Scholar] [CrossRef]
- Canet, W.; Alvarez, M.D.; Luna, P.; Fernández, C.; Tortosa, M.E. Blanching effects on chemistry, quality and structure of green beans (cv. Moncayo). Eur. Food Res. Technol. 2005, 220, 421–430. [Google Scholar] [CrossRef]
- Diamante, M.S.; Vanz Borges, C.; Minatel, I.O.; Jacomino, A.P.; Basílio, L.S.P.; Monteiro, G.C.; Corrêa, C.R.; de Oliveira, R.A.; Lima, G.P.P. Domestic cooking practices influence the carotenoid and tocopherol content in colored cauliflower. Food Chem. 2021, 340, 127901. [Google Scholar] [CrossRef]
- Hwang, E.S. Influence of Cooking Methods on Bioactive Compound Content and Antioxidant Activity of Brussels Sprouts. Prev. Nutr. Food Sci. 2017, 22, 353–358. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El–Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Sánchez, C.; Baranda, A.B.; De Marañón, I.M. The effect of High Pressure and High Temperature processing on carotenoids and chlorophylls content in some vegetables. Food Chem. 2014, 163, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Van Grondelle, R.; Boeker, E. Limits on Natural Photosynthesis. J. Phys. Chem. B 2017, 121, 7229–7234. [Google Scholar] [CrossRef] [PubMed]


| Proximate Composition | (g/100 g FW) |
|---|---|
| Moisture | 88.94 ± 0.55 |
| Proteins | 3.01 ± 0.04 |
| Lipid | 0.94 ± 0.5 |
| Available carbohydrate | 0.85 ± 0.05 |
| Dietary fiber | 3.39 ± 0.08 |
| Ash | 1.94 ± 0.03 |
| Energy (kcal/100 g) | 30.66 ± 0.21 |
| Soluble Sugars | (g/100 g FW) |
|---|---|
| Glucose | 0.44 ± 0.01 |
| Fructose | 0.18 ± 0.03 |
| Rhamnose | 0.08 ± 0.01 |
| Mineral Composition (mg/100 g FW) | |||||
|---|---|---|---|---|---|
| Ca | 333.09 ± 1.02 | Si | 1.53 ± 0.05 | B | 0.20 ± 0.01 |
| K | 215.53 ± 1.06 | Fe | 0.73 ± 0.02 | Ba | 0.08 ± 0.01 |
| S | 108.00 ± 0.19 | Sr | 0.62 ± 0.01 | Cu | 0.04 ± 0.01 |
| Na | 40.79 ± 0.01 | Zn | 0.32 ± 0.01 | ||
| P | 38.89 ± 0.02 | Mn | 0.30 ± 0.01 | ||
| Mg | 29.45 ± 0.07 | Al | 0.27 ± 0.01 | ||
| AAs Profile | (mg/100 g FW) |
|---|---|
| Glutamic acid | 470.25 ± 5.20 |
| Aspartic acid | 324.62 ± 0.53 |
| Lysine * | 263.31 ± 3.44 |
| Leucine * | 209.04 ± 1.16 |
| Arginine | 185.15 ± 1.69 |
| Alanine | 152.73 ± 0.62 |
| Valine * | 138.29 ± 0.85 |
| Threonine * | 133.82 ± 6.22 |
| Glycine | 132.08 ± 0.56 |
| Phenylalanine * | 129.90 ± 0.60 |
| Serine | 129.36 ± 0.34 |
| Proline | 125.32 ± 1.90 |
| Histidine * | 103.94 ± 2.49 |
| Isoleucine * | 96.43 ± 0.76 |
| Tyrosine | 48.09 ± 0.57 |
| Tryptophan * | 43.64 ± 0.16 |
| Cysteine | 31.36 ± 0.23 |
| Methionine * | 29.59 ± 5.47 |
| Total AAs | 2746.91 ± 18.96 |
| Total NEAAs | 1598.96 ± 0.34 |
| Total EAAs | 1147.95 ± 19.30 |
| EAAs/TAAs | 0.42 |
| EAAs/NEAAs | 0.72 |
| RDA * | CGLs 100 g ** | Soybeans 100 g | Beans 100 g | Wheat 100 g | Potato 100 g | Spinach 100 g | Cauliflower 100 g | |
|---|---|---|---|---|---|---|---|---|
| Protein content (g) | 3.01 | 36.9 | 10.2 | 13 | 2.1 | 3.4 | 3.2 | |
| Amino acid (mg) | ||||||||
| Histidine | 700 | 103.94 | 1170 | 303 | 228 | 28 | 83 | 37 |
| Isoleucine | 1400 | 96.43 | 2222 | 556 | 403 | 68 | 102 | 73 |
| Leucine | 2730 | 209.04 | 3689 | 885 | 741 | 96 | 196 | 126 |
| Lysine | 2100 | 263.31 | 3047 | 714 | 239 | 92 | 154 | 120 |
| Methionine + cysteine | 1050 | 60.95 | 1183 | 238 | 454 | 51 | 69 | 63 |
| Phenylalanine + tyrosine | 1750 | 177.99 | 3970 | 963 | 855 | 132 | 294 | 129 |
| Threonine | 1050 | 133.82 | 1843 | 428 | 310 | 59 | 113 | 74 |
| Tryptophan 1 | 280 | 43.64 | 618 | 113 | 116 | / | / | / |
| Valine | 1820 | 138.29 | 2176 | 616 | 452 | 99 | 139 | 104 |
| Total EAAs | 12,880 | 1227.40 | 19,918 | 4816 | 3798 | 625 | 1150 | 726 |
| Amino Acid Composition (mg/g Protein) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Amino Acid | WHO/FAO/UNU Requirement * | CGLs | Soybeans | Beans | Wheat | Potato | Spinach | Cauliflower |
| Histidine | 15.00 | 34.53 | 31.71 | 29.71 | 17.54 | 13.33 | 24.41 | 11.56 |
| Isoleucine | 30.00 | 32.04 | 60.22 | 54.51 | 31.00 | 32.38 | 30.00 | 22.81 |
| Leucine | 59.00 | 69.45 | 99.97 | 86.76 | 57.00 | 45.71 | 57.65 | 39.38 |
| Lysine | 45.00 | 87.48 | 82.57 | 70.00 | 18.38 | 43.81 | 45.29 | 37.50 |
| Methionine + Cysteine | 22.00 | 20.25 | 32.06 | 23.33 | 34.92 | 24.29 | 20.29 | 19.69 |
| Phenylalanine + Tyrosine | 38.00 | 59.13 | 107.59 | 94.41 | 65.77 | 62.86 | 86.47 | 40.31 |
| Threonine | 23.00 | 44.46 | 49.95 | 41.96 | 23.85 | 28.10 | 33.24 | 23.13 |
| Tryptophan 1 | 6.00 | 14.50 | 16.75 | 11.08 | 8.92 | / | / | / |
| Valine | 39.00 | 45.94 | 58.97 | 60.39 | 34.77 | 47.14 | 40.88 | 32.50 |
| Total EEAs | 277.00 | 407.77 | 19,918.00 | 472.16 | 292.15 | 297.62 | 338.24 | 226.88 |
| Fatty Acid | (% of the Total Fatty Acid) |
|---|---|
| C4:0: butyric acid | 0.02 ± 0.01 |
| C6:0: caproic acid | 0.14 ± 0.08 |
| C8:0: caprylic acid | 0.23 ± 0.01 |
| C10:0: capric acid | 0.19 ± 0.01 |
| C11:0: undecanoic acid | 0.03 ± 0.01 |
| C12:0: lauric acid | 0.11 ± 0.01 |
| C13:0: tridecanoic acid | 0.76 ± 0.02 |
| C14:0: myristic acid | 0.32 ± 0.04 |
| C15:0 anteiso: anteiso-pentadecanoic acid | 1.66 ± 0.05 |
| C15:0: pentadecanoic acid | 0.12 ± 0.01 |
| C16:0 iso: iso-palmitic acid | 0.15 ± 0.03 |
| C16:0: palmitic acid | 11.23 ± 0.57 |
| C17:0 iso: iso-margaric acid | 0.17 ± 0.02 |
| C17:0: margaric acid | 0.20 ± 0.01 |
| C18:0: stearic acid | 2.14 ± 0.45 |
| C20:0: arachidic acid | 0.19 ± 0.01 |
| C22:0: behenic acid | 0.18 ± 0.06 |
| C24:0: lignoceric acid | 0.51 ± 0.15 |
| C16:1 n9: palmitoleic acid | 4.76 ± 0.14 |
| C17:1 n7: heptadecenoic acid | 12.52 ± 0.33 |
| C18:1 n9: oleic acid | 3.89 ± 0.86 |
| C18:1 n7: vaccenic acid | 0.91 ± 0.08 |
| C18:1 n6: petroselinic acid | 0.04 ± 0.01 |
| C19:1: nonadecenoic acid | 0.11 ± 0.02 |
| C20:1 n9: eicosenoic acid | 0.07 ± 0.02 |
| C22:1 n9: erucic acid | 0.04 ± 0.02 |
| C24:1 n9: nervonic acid | 0.47 ± 0.08 |
| C18:3 n3: alpha-linolenic acid | 42.28 ± 1.82 |
| C20:3 n3: eicosatrienoic acid | 0.21 ± 0.01 |
| C20:5 n3 EPA: eicosapentaenoic acid | 0.27 ± 0.05 |
| C22:5 n3 DPA: docosapentaenoic acid (n–3) | 0.34 ± 0.01 |
| C22:6 n3 DHA: docosahexaenoic acid | 1.89 ± 0.10 |
| C18:2 n6: linoleic acid | 12.07 ± 0.21 |
| C18:3 n6: gamma-linolenic acid | 0.11 ± 0.01 |
| C20:2 n6: eicosadienoic acid | 0.34 ± 0.05 |
| C20:3 n6: dihomo-gamma-linolenic acid | 0.68 ± 0.01 |
| C20:4 n6: arachidonic acid | 0.44 ± 0.12 |
| C22:2 n6: docosadienoic acid | 0.13 ± 0.02 |
| C22:5 n6 DPA: docosapentaenoic acid (n–6) | ±0.02 |
| TSFA a | 18.34 |
| MUFAs b | 22.80 |
| PUFAs c | 58.87 |
| TUSFAs d | 81.66 |
| TUSFA/TSFA e | 4.45 |
| Fresh-cut CGLs | Fifth-Range CGLs | % Retention | Freeze-Dried CGLs | % Retention | |
|---|---|---|---|---|---|
| Chla (μg/g) | 275.64 ± 4.54 b | 218.69 ± 4.47 b | 83.50 | 2220.28 ± 46.56 a | 97.66 |
| Chlb (μg/g) | 152.30 ± 1.90 b | 138.64 ± 7.86 b | 79.34 | 1240.44 ± 63.63 a | 97.28 |
| Total Chl (μg/g) | 427.94 ± 2.64 b | 357.34 ± 12.33 c | 91.03 | 3460.72 ± 23.02 a | 98.36 |
| −a* value | –11.83 ± 0.19 b | –9.39 ± 0.23 c | –14.91 ± 0.03 a |
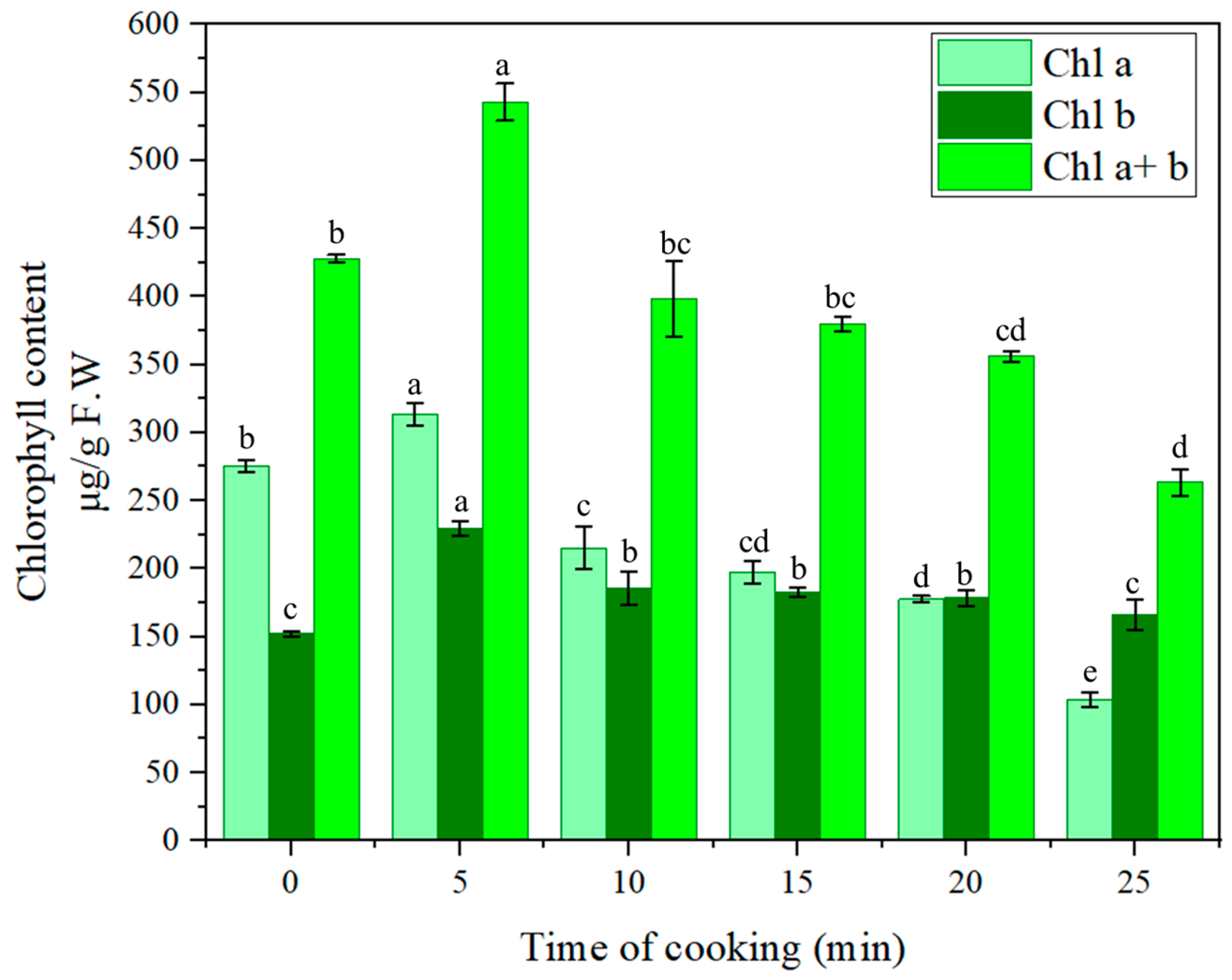
| 0 min | 5 min | 10 min | 15 min | 20 min | 25 min | |
|---|---|---|---|---|---|---|
| Chla | 275.64 ± 4.54 b | 313.69 ± 8.19 a | 215.20 ± 15.72 c | 197.38 ± 8.00 cd | 177.68 ± 2.37 d | 103.70 ± 5.46 e |
| Chlb | 152.30 ± 1.90 c | 229.32 ± 5.32 a | 185.59 ± 11.90 b | 182.67 ± 3.59 b | 178.46 ± 5.62 b | 166.37 ± 11.16 c |
| Chla/Chlb | 1.81 | 1.37 | 1.16 | 1.08 | 1 | 0.62 |
| Total Chl [Chl (a + b)] | 427.94 ± 2.64 b | 543.00 ± 13.51 a | 398.47 ± 27.62 bc | 380.05 ± 5.67 bc | 356.14 ± 3.65 cd | 270.08 ± 9.68 d |
| −a* value | –11.83 ± 0.19 a | –12.23 ± 0.53 a | –10.12 ± 0.46 b | –8.87 ± 0.10 c | –8.08 ± 0.32 c | –7.93 ± 0.26 c |
| pH | 5.972 ± 0.004 a | 5.853 ± 0.009 ab | 5.823 ± 0.015 ab | 5.737 ± 0.017 b | 5.630 ± 0.018 bc | 5.574 ± 0.013 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canazza, E.; Tessari, P.; Mayr Marangon, C.; Lante, A. Nutritional Profile and Chlorophyll Intake of Collard Green as a Convenience Food. Nutrients 2024, 16, 4015. https://doi.org/10.3390/nu16234015
Canazza E, Tessari P, Mayr Marangon C, Lante A. Nutritional Profile and Chlorophyll Intake of Collard Green as a Convenience Food. Nutrients. 2024; 16(23):4015. https://doi.org/10.3390/nu16234015
Chicago/Turabian StyleCanazza, Elisa, Paolo Tessari, Christine Mayr Marangon, and Anna Lante. 2024. "Nutritional Profile and Chlorophyll Intake of Collard Green as a Convenience Food" Nutrients 16, no. 23: 4015. https://doi.org/10.3390/nu16234015
APA StyleCanazza, E., Tessari, P., Mayr Marangon, C., & Lante, A. (2024). Nutritional Profile and Chlorophyll Intake of Collard Green as a Convenience Food. Nutrients, 16(23), 4015. https://doi.org/10.3390/nu16234015









