Nutrient–Nutrient Interactions Among Broccoli Glucosinolates and Their Implications for Breeding Cruciferous Crops to Enhance Human Health
Abstract
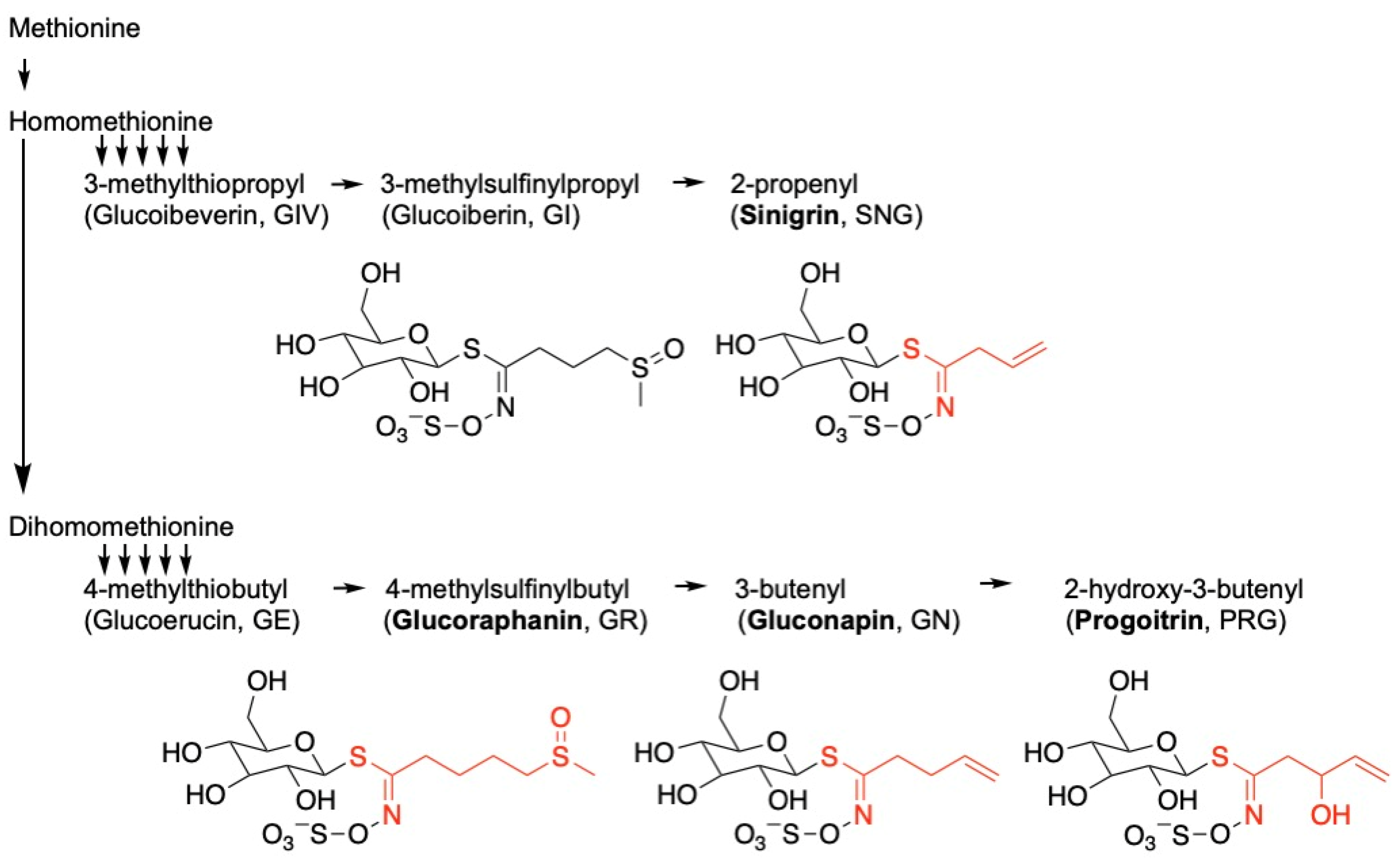
:1. Introduction
2. Materials and Methods
2.1. Broccoli Mapping Population
2.2. Chemicals and Reagents
2.3. Myrosinase Treatments
2.4. Cell Culture
2.5. Antiproliferative Cell Assay
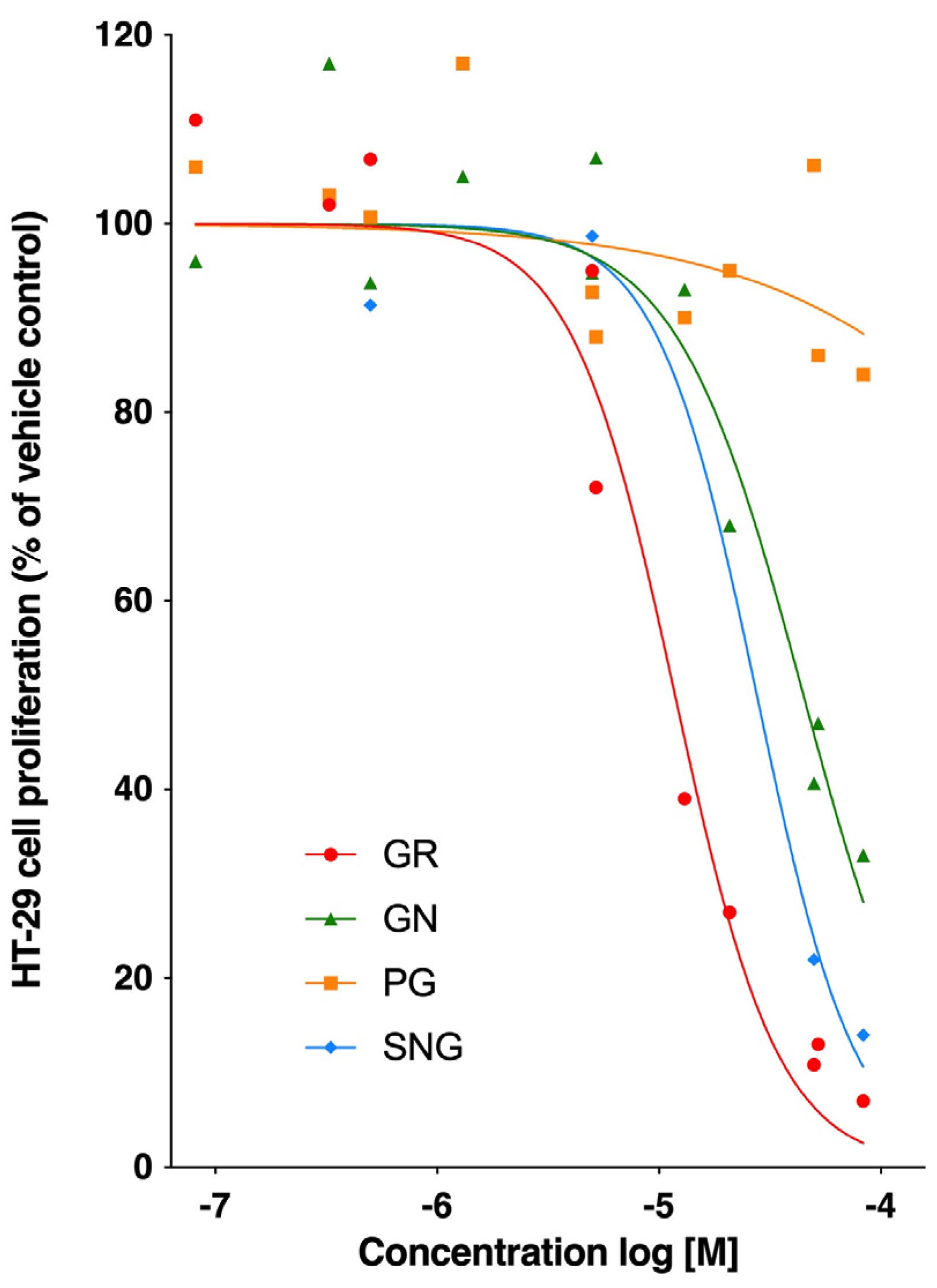
2.6. Determination of IC50 Values
2.7. Determination of Combination Indexes (CI)
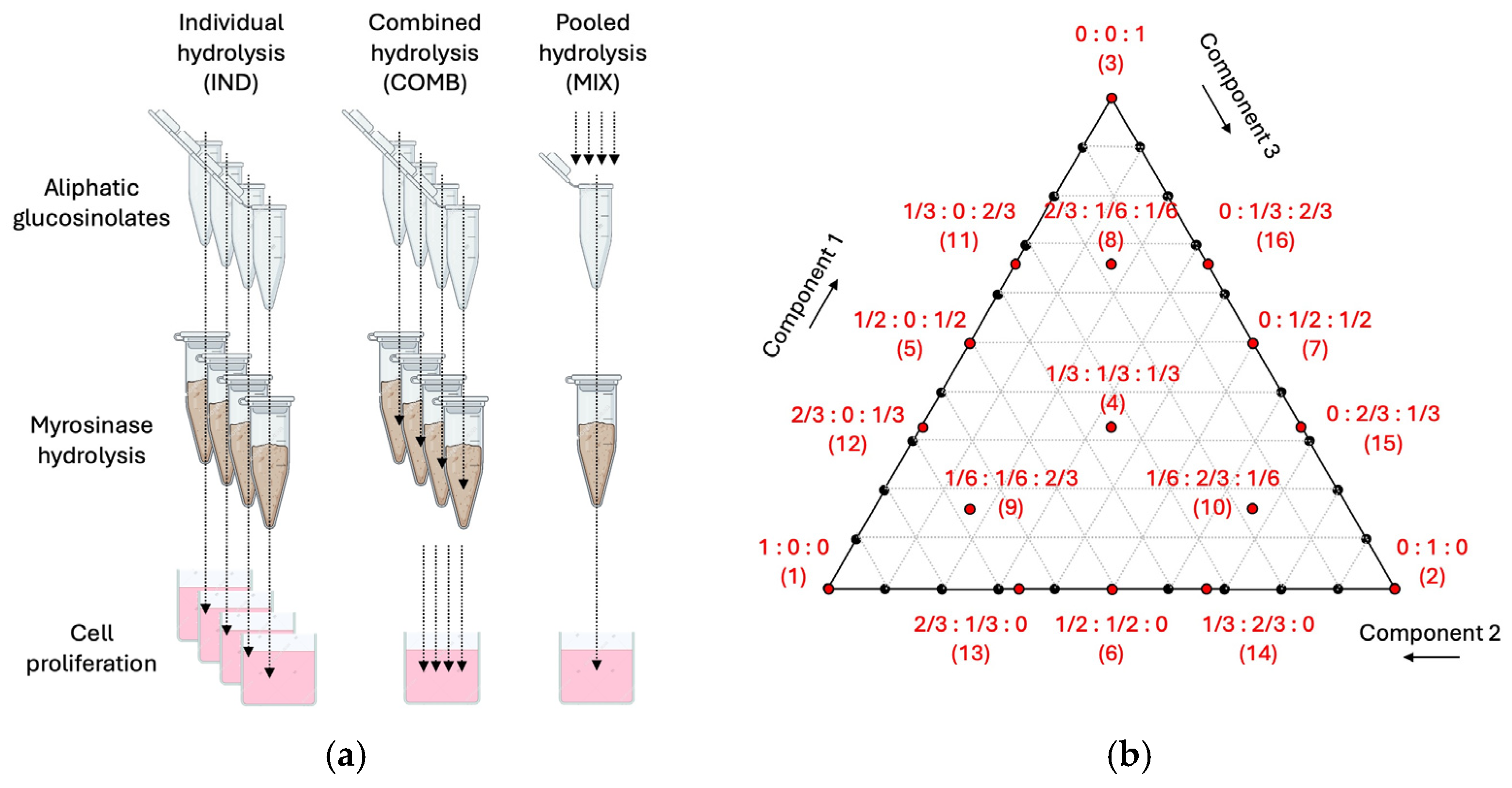
2.8. Mixture Design Model
2.9. Statistical Analysis
3. Results
3.1. Comparative Antiproliferative Potencies of Aliphatic Glucosinolates
3.2. Combination Index of Aliphatic Glucosinolates
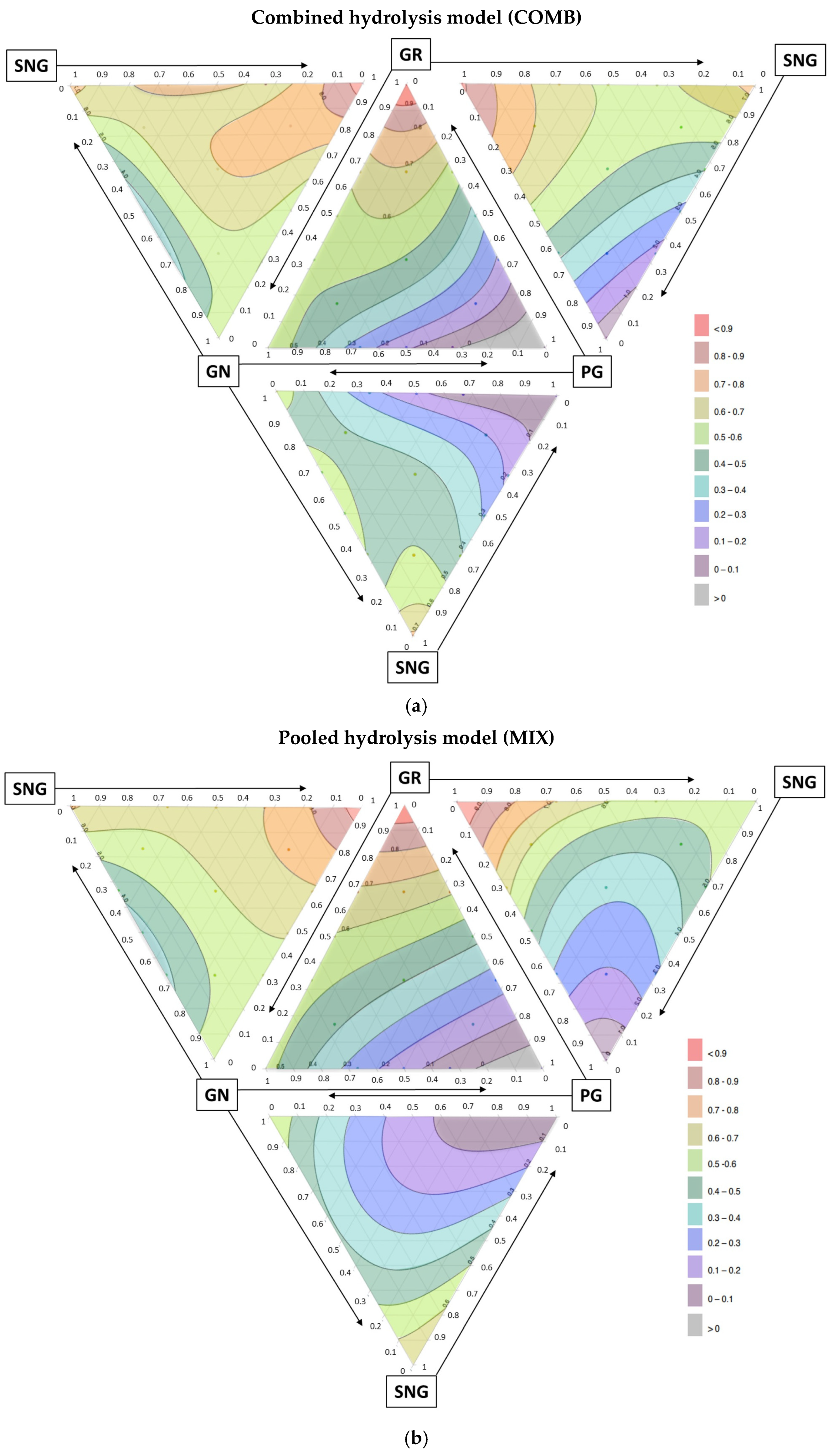
3.3. Mixture Design Analysis for Nutrient Interactions
3.4. Significant Interactions Among Aliphatic Glucosinolates
3.5. Predicted Optimal Aliphatic Glucosinolate Ratio in Broccoli Population
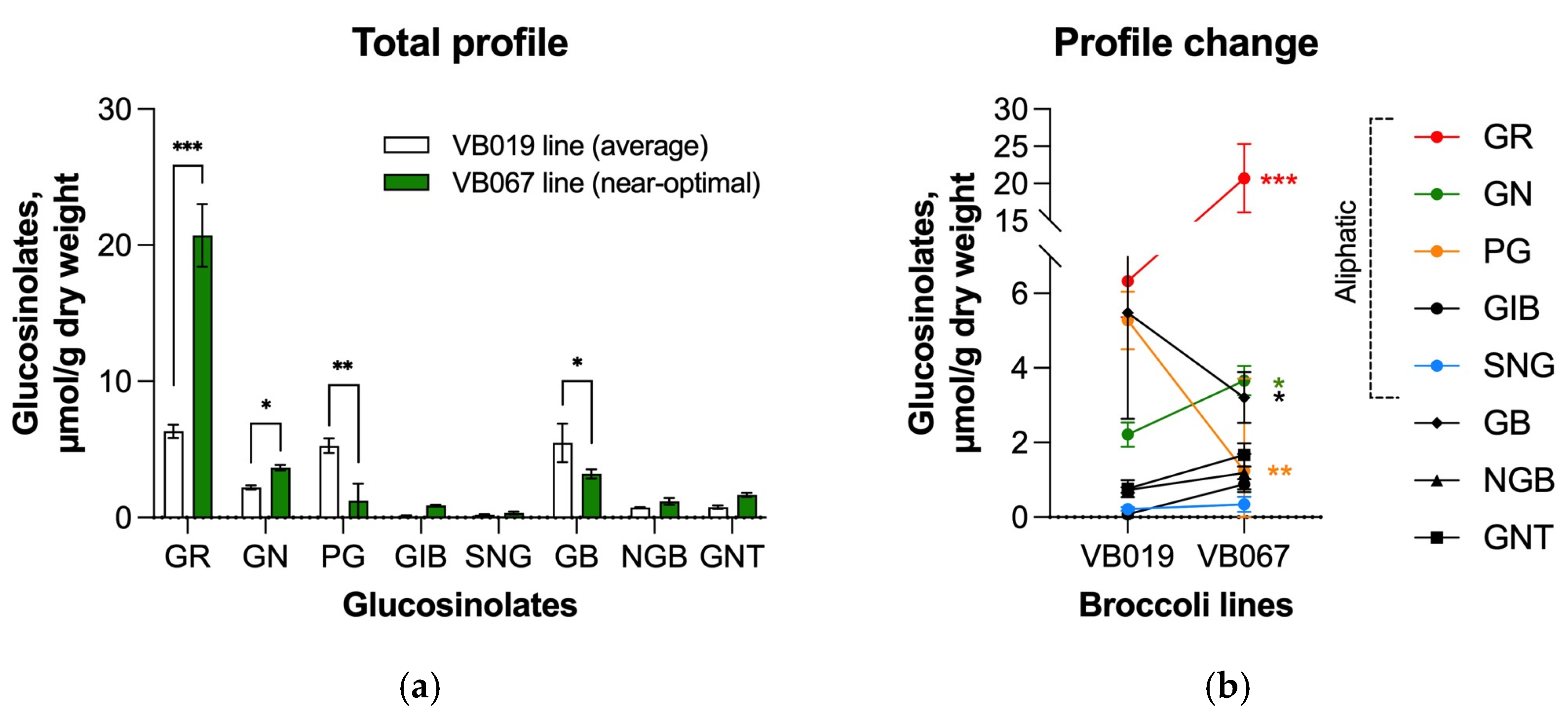
3.6. Antiproliferative Activity of the Selected Broccoli Isogenic Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esquivel, M.K. Nutrition Benefits and Considerations for Whole Foods Plant-Based Eating Patterns. Am. J. Lifestyle Med. 2022, 16, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Komarnytsky, S.; Wagner, C.; Gutierrez, J.; Shaw, O.M. Berries in Microbiome-Mediated Gastrointestinal, Metabolic, and Immune Health. Curr. Nutr. Rep. 2023, 12, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli Consumption Affects the Human Gastrointestinal Microbiota. J. Nutr. Biochem. 2018, 63, 27. [Google Scholar] [CrossRef]
- Rattan, S.I.S.; Kaur, G. Nutrition, Food and Diet in Health and Longevity: We Eat What We Are. Nutrients 2022, 14, 5376. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, Fruit, and Cancer. I. Epidemiology. Cancer Causes Control. 1991, 2, 325–357. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous Vegetables Intake and the Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate Metabolism, Functionality and Breeding for the Improvement of Brassicaceae Vegetables. Breed. Sci. 2014, 64, 48. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Olsen, C.E. Glucosinolate Structures in Evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel Sources and Biological Potential. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–58. ISBN 978-3-319-26479-0. [Google Scholar]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in Food. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–132. ISBN 978-3-319-25462-3. [Google Scholar]
- Nastruzzi, C.; Cortesi, R.; Esposito, E.; Menegatti, E.; Leoni, O.; Iori, R.; Palmieri, S. In Vitro Cytotoxic Activity of Some Glucosinolate-Derived Products Generated by Myrosinase Hydrolysis. J. Agric. Food Chem. 1996, 44, 1014–1021. [Google Scholar] [CrossRef]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The Cancer Chemopreventive Actions of Phytochemicals Derived from Glucosinolates. Eur. J. Nutr. 2008, 47 (Suppl. S2), 73–88. [Google Scholar] [CrossRef] [PubMed]
- Björkman, R.; Janson, J.-C. Studies on Myrosinases: I. Purification and Characterization of a Myrosinase from White Mustard Seed (Sinapis alba, L.). Biochim. Biophys. Acta BBA-Enzymol. 1972, 276, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Daxenbichler, M.E.; Van Etten, C.H.; Spencer, G.F. Glucosinolates and Derived Products in Cruciferous Vegetables. Identification of Organic Nitriles from Cabbage. J. Agric. Food Chem. 1977, 25, 121–124. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, Isothiocyanates and Human Health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Morimitsu, Y.; Nakagawa, Y.; Hayashi, K.; Fujii, H.; Kumagai, T.; Nakamura, Y.; Osawa, T.; Horio, F.; Itoh, K.; Iida, K.; et al. A Sulforaphane Analogue That Potently Activates the Nrf2-Dependent Detoxification Pathway. J. Biol. Chem. 2002, 277, 3456–3463. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Li, P.; Lumeau, S.; Cassar, G.; Dupont, M.A.; Chevolleau, S.; Gasc, N.; Tulliez, J.; Tercé, F. Sulforaphane, a Naturally Occurring Isothiocyanate, Induces Cell Cycle Arrest and Apoptosis in HT29 Human Colon Cancer Cells. Cancer Res. 2000, 60, 1426–1433. [Google Scholar]
- Myzak, M.C.; Karplus, P.A.; Chung, F.-L.; Dashwood, R.H. A Novel Mechanism of Chemoprotection by Sulforaphane: Inhibition of Histone Deacetylase. Cancer Res. 2004, 64, 5767–5774. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic Variation of Glucosinolates in Broccoli (Brassica oleracea var. italica) Florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Genotypic Effects on the Phytochemical Quality of Seeds and Sprouts from Commercial Broccoli Cultivars. Food Chem. 2011, 125, 348–354. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Jeffery, E.H.; Klein, B.P.; Wallig, M.A.; Kushad, M.M.; Juvik, J.A. Glucosinolate Profiles in Broccoli: Variation in Levels and Implications in Breeding for Cancer Chemoprotection. J. Am. Soc. Hortic. Sci. 2002, 127, 807–813. [Google Scholar] [CrossRef]
- Kestwal, R.M.; Lin, J.C.; Bagal-Kestwal, D.; Chiang, B.H. Glucosinolates Fortification of Cruciferous Sprouts by Sulphur Supplementation during Cultivation to Enhance Anti-Cancer Activity. Food Chem. 2011, 126, 1164–1171. [Google Scholar] [CrossRef]
- Traka, M.H.; Saha, S.; Huseby, S.; Kopriva, S.; Walley, P.G.; Barker, G.C.; Moore, J.; Mero, G.; van den Bosch, F.; Constant, H.; et al. Genetic Regulation of Glucoraphanin Accumulation in Beneforté Broccoli. New Phytol. 2013, 198, 1085–1095. [Google Scholar] [CrossRef]
- Mithen, R.; Faulkner, K.; Magrath, R.; Rose, P.; Williamson, G.; Marquez, J. Development of Isothiocyanate-Enriched Broccoli, and Its Enhanced Ability to Induce Phase 2 Detoxification Enzymes in Mammalian Cells. TAG Theor. Appl. Genet. Theor. Angew. Genet. 2003, 106, 727–734. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Reid, R.W.; Chebrolu, K.K.; Thomas, A.; Krueger, C.; Jeffery, E.; Jackson, E.; Juvik, J.A. Genetic Analysis of Glucosinolate Variability in Broccoli Florets Using Genome-Anchored Single Nucleotide Polymorphisms. Theor. Appl. Genet. 2015, 128, 1431–1447. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter Taste, Phytonutrients, and the Consumer: A Review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Retchin, S.; Vong, C.I.; Lila, M.A. Gains and Losses of Agricultural Food Production: Implications for the Twenty-First Century. Annu. Rev. Food Sci. Technol. 2022, 13, 239–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Discovery and Development of Sulforaphane as a Cancer Chemopreventive Phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Lawless, L.; Threlfall, R.; Howard, L. Applying a Mixture Design for Consumer Optimization of Black Cherry, Concord Grape and Pomegranate Juice Blends. J. Sens. Stud. 2013, 28, 102–112. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yildiz, Ö.; Yurt, B.; Toker, O.S.; Karaman, S.; Baştürk, A. A Mixture Design Study to Determine Interaction Effects of Wheat, Buckwheat, and Rice Flours in an Aqueous Model System. LWT-Food Sci. Technol. 2015, 61, 583–589. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; De Nicola, G.R.; Pagnotta, E.; Iori, R.; Ioannides, C. 4-Methylsulfanyl-3-Butenyl Isothiocyanate Derived from Glucoraphasatin Is a Potent Inducer of Rat Hepatic Phase II Enzymes and a Potential Chemopreventive Agent. Arch. Toxicol. 2012, 86, 183–194. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A Novel Investigational Antimicrotubule Agent. J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef]
- Rampal, G.; Thind, T.S.; Arora, R.; Vig, A.P.; Arora, S. Synergistic Antimutagenic Effect of Isothiocyanates against Varied Mutagens. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109, 879–887. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-118-15049-8. [Google Scholar]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Park, K. The Role of Dietary Phytochemicals: Evidence from Epidemiological Studies. Nutrients 2023, 15, 1371. [Google Scholar] [CrossRef] [PubMed]
- Vong, C.I.; Rathinasabapathy, T.; Moncada, M.; Komarnytsky, S. All Polyphenols Are Not Created Equal: Exploring the Diversity of Phenolic Metabolites. J. Agric. Food Chem. 2022, 70, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a Natural Chemical Arsenal: More to Tell than the Myrosinase Story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates From Cruciferous Vegetables and Their Potential Role in Chronic Disease: Investigating the Preclinical and Clinical Evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Granato, D.; Grevink, R.; Zielinski, A.A.F.; Nunes, D.S.; van Ruth, S.M. Analytical Strategy Coupled with Response Surface Methodology to Maximize the Extraction of Antioxidants from Ternary Mixtures of Green, Yellow, and Red Teas (Camellia sinensis var. sinensis). J. Agric. Food Chem. 2014, 62, 10283–10296. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.H.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of Glucoraphanin and Sulforaphane from High-Glucoraphanin Broccoli. Mol. Nutr. Food Res. 2018, 62, e1700911. [Google Scholar] [CrossRef]
- Veeranki, O.L.; Bhattacharya, A.; Marshall, J.R.; Zhang, Y. Organ-Specific Exposure and Response to Sulforaphane, a Key Chemopreventive Ingredient in Broccoli: Implications for Cancer Prevention. Br. J. Nutr. 2013, 109, 25–32. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Zimny, K.; Beneduce, L. The Metabolism of Glucosinolates by Gut Microbiota. Nutrients 2021, 13, 2750. [Google Scholar] [CrossRef] [PubMed]
- Pappa, G.; Strathmann, J.; Löwinger, M.; Bartsch, H.; Gerhäuser, C. Quantitative Combination Effects between Sulforaphane and 3,3’-Diindolylmethane on Proliferation of Human Colon Cancer Cells In Vitro. Carcinogenesis 2007, 28, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.A.; Saxon, A.; Diaz-Sanchez, D. Oral Sulforaphane Increases Phase II Antioxidant Enzymes in the Human Upper Airway. Clin. Immunol. Orlando Fla 2009, 130, 244–251. [Google Scholar] [CrossRef]
- Graczyk, F.; Gębalski, J.; Makuch-Kocka, A.; Gawenda-Kempczyńska, D.; Ptaszyńska, A.A.; Grzyb, S.; Bogucka-Kocka, A.; Załuski, D. Phenolic Profile, Antioxidant, Anti-Enzymatic and Cytotoxic Activity of the Fruits and Roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Molecules 2022, 27, 5579. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Chen, J.; Xu, Y. Development and Validation of High-Glucoraphanin Broccoli F1 Hybrids and Parental Lines. J. Am. Soc. Hortic. Sci. 2014, 139, 460–468. [Google Scholar] [CrossRef]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A Mechanistic Perspective on Process-Induced Changes in Glucosinolate Content in Brassica Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef]

| Interactions | Proportion Ratios | IC50 Value | Combination Index | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GR | GN | PG | SNG | IND | COMB | MIX | COMB | MIX | ||
| 1 | Singular | 1 | – | – | – | 11.80 | – | – | – | – |
| 2 | Singular | – | 1 | – | – | 44.76 | – | – | – | – |
| 3 | Singular | – | – | 1 | – | 2100.62 | – | – | – | – |
| 4 | Singular | – | – | – | 1 | 29.51 | – | – | – | – |
| 5 | Tertiary | 1/3 | 1/3 | 1/3 | – | – | 64.19 | 52.25 | 2.31 | 1.88 |
| 6 | Tertiary | – | 1/3 | 1/3 | 1/3 | – | 54.23 | 62.06 | 1.03 | 1.18 |
| 7 | Tertiary | 1/3 | – | 1/3 | 1/3 | – | 28.76 | 50.16 | 1.41 | 2.66 |
| 8 | Tertiary | 1/3 | 1/3 | – | 1/3 | – | 27.69 | 46.18 | 1.35 | 2.40 |
| 9 | Tertiary | 2/3 | 1/6 | 1/6 | – | – | 24.66 | 45.24 | 1.49 | 2.73 |
| 10 | Tertiary | 2/3 | – | 1/6 | 1/6 | – | 25.94 | 35.03 | 1.61 | 2.18 |
| 11 | Tertiary | 2/3 | 1/6 | – | 1/6 | – | 29.84 | 43.07 | 2.00 | 2.93 |
| 12 | Tertiary | 1/6 | 2/3 | 1/6 | – | – | 48.01 | 56.32 | 0.99 | 1.16 |
| 13 | Tertiary | 1/6 | 2/3 | – | 1/6 | – | 47.74 | 50.23 | 1.78 | 1.89 |
| 14 | Tertiary | 1/6 | 1/6 | 2/3 | – | – | 205.83 | 62.74 | 3.88 | 1.14 |
| 15 | Tertiary | 1/6 | 1/6 | – | 2/3 | – | 48.18 | 50.00 | 2.08 | 2.17 |
| 16 | Tertiary | 1/6 | – | 2/3 | 1/6 | – | 58.31 | 59.65 | 1.18 | 1.20 |
| 17 | Tertiary | 1/6 | – | 1/6 | 2/3 | – | 47.09 | 48.24 | 1.73 | 1.78 |
| 18 | Tertiary | – | 2/3 | 1/6 | 1/6 | – | 66.16 | 62.67 | 1.37 | 1.29 |
| 19 | Tertiary | – | 1/6 | 2/3 | 1/6 | – | 57.15 | >2,500 | 0.55 | nd |
| 20 | Tertiary | – | 1/6 | 1/6 | 2/3 | – | 47.82 | 51.77 | 1.26 | 1.37 |
| Interactions | Panel 1 | Panel 2 | Panel 3 | Panel 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GR | GN | PG | GR | GN | SNG | GR | PG | SNG | GN | PG | SNG | ||
| 1 | Singular | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – | – |
| 2 | Singular | – | 1 | – | – | 1 | – | – | 1 | – | – | 1 | – |
| 3 | Singular | – | – | 1 | – | – | 1 | – | – | 1 | – | – | 1 |
| 4 | Tertiary | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 | 1/3 |
| 5 | Binary | 1/2 | – | 1/2 | 1/2 | – | 1/2 | 1/2 | – | 1/2 | 1/2 | – | 1/2 |
| 6 | Binary | 1/2 | 1/2 | – | 1/2 | 1/2 | – | 1/2 | 1/2 | – | 1/2 | 1/2 | – |
| 7 | Binary | – | 1/2 | 1/2 | – | 1/2 | 1/2 | – | 1/2 | 1/2 | – | 1/2 | 1/2 |
| 8 | Tertiary | 2/3 | 1/6 | 1/6 | 2/3 | 1/6 | 1/6 | 2/3 | 1/6 | 1/6 | 2/3 | 1/6 | 1/6 |
| 9 | Tertiary | 1/6 | 1/6 | 2/3 | 1/6 | 1/6 | 2/3 | 1/6 | 1/6 | 2/3 | 1/6 | 1/6 | 2/3 |
| 10 | Tertiary | 1/6 | 2/3 | 1.6 | 1/6 | 2/3 | 1.6 | 1/6 | 2/3 | 1.6 | 1/6 | 2/3 | 1.6 |
| 11 | Binary | 1/3 | – | 2/3 | 1/3 | – | 2/3 | 1/3 | – | 2/3 | 1/3 | – | 2/3 |
| 12 | Binary | 2/3 | – | 1/3 | 2/3 | – | 1/3 | 2/3 | – | 1/3 | 2/3 | – | 1/3 |
| 13 | Binary | 2/3 | 1/3 | – | 2/3 | 1/3 | – | 2/3 | 1/3 | – | 2/3 | 1/3 | – |
| 14 | Binary | 1/3 | 2/3 | – | 1/3 | 2/3 | – | 1/3 | 2/3 | – | 1/3 | 2/3 | – |
| 15 | Binary | – | 2/3 | 1/3 | – | 2/3 | 1/3 | – | 2/3 | 1/3 | – | 2/3 | 1/3 |
| 16 | Binary | – | 1/3 | 2/3 | – | 1/3 | 2/3 | – | 1/3 | 2/3 | – | 1/3 | 2/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bussler, W.; DeZego, K.; Chandler, H.; Reid, R.W.; Komarnytsky, S. Nutrient–Nutrient Interactions Among Broccoli Glucosinolates and Their Implications for Breeding Cruciferous Crops to Enhance Human Health. Nutrients 2025, 17, 344. https://doi.org/10.3390/nu17020344
Bussler W, DeZego K, Chandler H, Reid RW, Komarnytsky S. Nutrient–Nutrient Interactions Among Broccoli Glucosinolates and Their Implications for Breeding Cruciferous Crops to Enhance Human Health. Nutrients. 2025; 17(2):344. https://doi.org/10.3390/nu17020344
Chicago/Turabian StyleBussler, Weston, Katelyn DeZego, Holli Chandler, Robert W. Reid, and Slavko Komarnytsky. 2025. "Nutrient–Nutrient Interactions Among Broccoli Glucosinolates and Their Implications for Breeding Cruciferous Crops to Enhance Human Health" Nutrients 17, no. 2: 344. https://doi.org/10.3390/nu17020344
APA StyleBussler, W., DeZego, K., Chandler, H., Reid, R. W., & Komarnytsky, S. (2025). Nutrient–Nutrient Interactions Among Broccoli Glucosinolates and Their Implications for Breeding Cruciferous Crops to Enhance Human Health. Nutrients, 17(2), 344. https://doi.org/10.3390/nu17020344








