Association Between the EAT-Lancet Reference Diet and Gestational Diabetes Mellitus: A Mini-Review
Abstract
1. Introduction
2. The EAT-Lancet Reference Diet Components and GDM
2.1. Whole Grains
2.2. Tubers or Starchy Vegetables
2.3. Fruits
2.4. Vegetables
2.5. Dairy Foods
2.6. Red Meat
2.7. Fish
2.8. Poultry
2.9. Eggs
2.10. Legumes
2.11. Nuts
2.12. Added Fats
2.13. Added Sugars
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mack, L.R.; Tomich, P.G. Gestational Diabetes: Diagnosis, Classification, and Clinical Care. Obstet. Gynecol. Clin. N. Am. 2017, 44, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.-F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and management of gestational diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Li, X.; Wang, Y.; Liu, H.; Chen, K.; Quan, H.; Zhang, H.; Ran, J. Periostin Acts as a Bridge between Gestational Diabetes Mellitus (GDM) and Chronic Inflammation to Modulate Insulin Resistance by Modulating PPARα/NF-κB/TNF-α Signaling Pathway. Endocr. Metab. Immune Disord.—Drug Targets 2023, 23, 1649–1659. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Di Renzo, G.C.; Meyyazhagan, A.; Tersigni, C.; Scambia, G.; Di Simone, N.; Marzioni, D. HtrA1 in Gestational Diabetes Mellitus: A Possible Biomarker? Diagnostics 2022, 12, 2705. [Google Scholar] [CrossRef]
- Heitritter, S.M.; Solomon, C.G.; Mitchell, G.F.; Skali-Ounis, N.; Seely, E.W. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2005, 90, 3983–3988. [Google Scholar] [CrossRef]
- Thilak, S.; Rajendra, A.; Ganesh, V. Association of obesity and insulin resistance to gestational diabetes mellitus. Bioinformation 2023, 19, 211–214. [Google Scholar] [CrossRef]
- Perugini, J.; Di Mercurio, E.; Tossetta, G.; Severi, I.; Monaco, F.; Reguzzoni, M.; Tomasetti, M.; Dani, C.; Cinti, S.; Giordano, A. Biological Effects of Ciliary Neurotrophic Factor on hMADS Adipocytes. Front. Endocrinol. 2019, 10, 768. [Google Scholar] [CrossRef]
- Daly, B.; Toulis, K.; Thomas, N.; Gokhale, K.; Martin, J.; Webber, J.; Keerthy, D.; Jolly, K.; Saravanan, P.; Nirantharakumar, K. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med. 2019, 16, e1002881. [Google Scholar] [CrossRef]
- Bianco, M.E.; Josefson, J.L. Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes. Curr. Diab. Rep. 2019, 19, 143. [Google Scholar] [CrossRef]
- Desoye, G.; Carter, A. Fetoplacental oxygen homeostasis in pregnancies with maternal diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2022, 18, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Nilsson, I.A.K.; Brismar, K.; Gissler, M.; Lavebratt, C. Associations of Different Types of Maternal Diabetes and Body Mass Index with Offspring Psychiatric Disorders. JAMA Netw. Open 2020, 3, e1920787. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dainelli, L.; Yu, K.; Ma, L.; Silva Zolezzi, I.; Detzel, P.; Fang, H. The short-term health and economic burden of gestational diabetes mellitus in China: A modelling study. BMJ Open 2017, 7, e018893. [Google Scholar] [CrossRef] [PubMed]
- Dall, T.M.; Yang, W.; Gillespie, K.; Mocarski, M.; Byrne, E.; Cintina, I.; Beronja, K.; Semilla, A.P.; Iacobucci, W.; Hogan, P.F. The Economic Burden of Elevated Blood Glucose Levels in 2017: Diagnosed and Undiagnosed Diabetes, Gestational Diabetes Mellitus, and Prediabetes. Diabetes Care 2019, 42, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Rubi, S.G.; Dainelli, L.; Silva-Zolezzi, I.; Detzel, P.; Espino, Y.S.S.; Reyes-Muñoz, E.; Chivardi, C.; Ortiz-Panozo, E.; Lopez-Ridaura, R. Short-term health and economic burden of gestational diabetes mellitus in Mexico: A modeling study. Diabetes Res. Clin. Pract. 2019, 153, 114–124. [Google Scholar] [CrossRef]
- Garnweidner-Holme, L.; Torheim, L.; Henriksen, L.; Borgen, I.; Holmelid, S.; Lukasse, M. Adherence to the Norwegian dietary recommendations in a multi-ethnic pregnant population prior to being diagnosed with gestational diabetes mellitus. Food Sci. Nutr. 2020, 8, 3031–3040. [Google Scholar] [CrossRef]
- Xu, S.; Yu, Q.; Mi, J.; Li, H. Clinical efficacy of nutritional diet therapy on gestational diabetes mellitus. Am. J. Transl. Res. 2022, 14, 3488. [Google Scholar]
- Kasuga, Y.; Takahashi, M.; Kajikawa, K.; Akita, K.; Otani, T.; Ikenoue, S.; Tanaka, M. Perinatal Outcomes of Diet Therapy in Gestational Diabetes Mellitus Diagnosed before 24 Gestational Weeks. Nutrients 2024, 16, 1553. [Google Scholar] [CrossRef]
- Amati, F.; Hassounah, S.; Swaka, A. The Impact of Mediterranean Dietary Patterns During Pregnancy on Maternal and Offspring Health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef]
- Du, H.Y.; Jiang, H.; Karmin, O.; Chen, B.; Xu, L.J.; Liu, S.P.; Yi, J.P.; He, G.S.; Qian, X. Association of Dietary Pattern during Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study in Northern China. Biomed. Environ. Sci. 2017, 30, 887–897. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2017, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Stubbendorff, A.; Olsson, K.; Ericson, U.; Niu, K.; Qi, L.; Borné, Y.; Sonestedt, E. Adherence to the EAT-Lancet diet, genetic susceptibility, and risk of type 2 diabetes in Swedish adults. Metabolism 2023, 141, 155401. [Google Scholar] [CrossRef]
- Klapp, R.; Laxamana, J.A.; Shvetsov, Y.B.; Park, S.Y.; Kanehara, R.; Setiawan, V.W.; Danquah, I.; Le Marchand, L.; Maskarinec, G. The EAT-Lancet Diet Index Is Associated with Lower Obesity and Incidence of Type 2 Diabetes in the Multiethnic Cohort. J. Nutr. 2024, 154, 3407–3415. [Google Scholar] [CrossRef] [PubMed]
- Hassani Zadeh, S.; Boffetta, P.; Hosseinzadeh, M. Dietary patterns and risk of gestational diabetes mellitus: A systematic review and meta-analysis of cohort studies. Clin. Nutr. ESPEN 2020, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tobias, D.K.; Hu, F.B.; Chavarro, J.E.; Zhang, C. Pre-pregnancy potato consumption and risk of gestational diabetes mellitus: Prospective cohort study. BMJ 2016, 352, h6898. [Google Scholar] [CrossRef]
- Tryggvadottir, E.A.; Halldorsson, T.I.; Landberg, R.; Hrolfsdottir, L.; Birgisdottir, B.E.; Magnusdottir, O.K.; Hreidarsdottir, I.T.; Hardardottir, H.; Gunnarsdottir, I. Higher Alkylresorcinol Concentrations, a Consequence of Whole-Grain Intake, are Inversely Associated with Gestational Diabetes Mellitus in Iceland. J. Nutr. 2021, 151, 1159–1166. [Google Scholar] [CrossRef]
- Chehab, R.F.; Ferrara, A.; Zheng, S.; Barupal, D.K.; Ngo, A.L.; Chen, L.; Fiehn, O.; Zhu, Y. In utero metabolomic signatures of refined grain intake and risk of gestational diabetes: A metabolome-wide association study. Am. J. Clin. Nutr. 2023, 117, 731–740. [Google Scholar] [CrossRef]
- Özer, Y.; Cengiz, H.; Demirci, T.; Kızılgül, M.; Varim, C.; Tamer, A. Glycemic responses to whole grain sourdough bread versus refined white bread in patients with gestational diabetes. Wien. Klin. Wochenschr. 2023, 135, 349–357. [Google Scholar] [CrossRef]
- Dong, H.L.; Cai, C.J.; Bai, D.; Pang, X.X.; Lan, X.; Zhang, Y.Q.; Zhang, J.; Zhou, F.M.; Sun, H.; Zeng, G. Association between dietary glycemic load during first trimester and the risk of gestational diabetes mellitus: A prospective study. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2020, 41, 1352–1358. [Google Scholar]
- Li, F.; Sun, H.; Dong, H.L.; Zhang, Y.Q.; Pang, X.X.; Cai, C.J.; Bai, D.; Wang, P.P.; Yang, M.Y.; Zeng, G. Starchy vegetable intake in the first trimester is associated with a higher risk of gestational diabetes mellitus: A prospective population-based study. J. Matern.—Fetal Neonatal. Med. 2022, 35, 6794–6801. [Google Scholar] [CrossRef]
- Goshtasebi, A.; Hosseinpour-Niazi, S.; Mirmiran, P.; Lamyian, M.; Moghaddam Banaem, L.; Azizi, F. Pre-pregnancy consumption of starchy vegetables and legumes and risk of gestational diabetes mellitus among Tehranian women. Diabetes Res. Clin. Pract. 2018, 139, 131–138. [Google Scholar] [CrossRef]
- Sahariah, S.A.; Potdar, R.D.; Gandhi, M.; Kehoe, S.H.; Brown, N.; Sane, H.; Coakley, P.J.; Marley-Zagar, E.; Chopra, H.; Shivshankaran, D.; et al. A Daily Snack Containing Leafy Green Vegetables, Fruit, and Milk before and during Pregnancy Prevents Gestational Diabetes in a Randomized, Controlled Trial in Mumbai, India. J. Nutr. 2016, 146, 1453S–1460S. [Google Scholar] [CrossRef] [PubMed]
- Jaworsky, K.; DeVillez, P.; Alexander, J.M.; Basu, A. Effects of an Eating Pattern Including Colorful Fruits and Vegetables on Management of Gestational Diabetes: A Randomized Controlled Trial. Nutrients 2023, 15, 3624. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Peng, C.; Zou, H.; Pan, Y.; Wu, M.; Xie, Q.; Lin, Q. Association of Vegetables-Fruits Dietary Patterns with Gestational Diabetes Mellitus: Mediating Effects of Gut Microbiota. Nutrients 2024, 16, 2300. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, S.; Pang, X.; Dong, H.; Cai, C.; Bai, D.; Wang, P.; Yang, M.; Li, F.; Zeng, G. Association between fruit intake during pregnancy and blood glucose metabolism. J. Hyg. Res. 2022, 51, 550–555. [Google Scholar]
- Jia, X.; Xuan, L.; Dai, H.; Zhu, W.; Deng, C.; Wang, T.; Li, M.; Zhao, Z.; Xu, Y.; Lu, J.; et al. Fruit intake, genetic risk and type 2 diabetes: A population-based gene-diet interaction analysis. Eur. J. Nutr. 2021, 60, 2769–2779. [Google Scholar] [CrossRef]
- Wu, L.; Ouyang, J.; Lai, Y.; Wu, P.; Wang, Y.; Ye, Y.; Wang, J.; Hu, M.; Zhang, J.; Xu, J.; et al. Combined healthy lifestyle in early pregnancy and risk of gestational diabetes mellitus: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1611–1619. [Google Scholar] [CrossRef]
- Liao, Y.P.; Zheng, Q.X.; Jiang, X.M.; Chen, X.Q.; Gao, X.X.; Pan, Y.Q. Fruit, vegetable, and fruit juice consumption and risk of gestational diabetes mellitus: A systematic review and meta-analysis. Nutr. J. 2023, 22, 27. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Zhang, X.; Xia, Y.; Zhang, Y.; Wang, L. Mid-pregnancy consumption of fruit, vegetable and fruit juice and the risk of gestational diabetes mellitus: A correlation study. Clin. Nutr. ESPEN 2021, 46, 505–509. [Google Scholar] [CrossRef]
- Sewor, C.; Obeng, A.A.; Eliason, S.; Agbeno, E.K.; Amegah, A.K. Fruits and vegetables intake improves birth outcomes of women with gestational diabetes mellitus and hypertensive disorders of pregnancy. BMC Nutr. 2024, 10, 2. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Yang, H.; Zhang, P.; Feng, Y.; Wang, K.; Wang, Y.; Wang, S.; Zhang, Y. A Vegetable Dietary Pattern Is Associated with Lowered Risk of Gestational Diabetes Mellitus in Chinese Women. Diabetes Metab. J. 2020, 44, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.J.; Harding, M.C.; Conley, A.T. Dietary Guidelines for Americans 2020–2025: Recommendations from the U.S. Departments of Agriculture and Health and Human Services. Am. Fam. Physician 2021, 104, 533–536. [Google Scholar] [PubMed]
- Wang, S.S.; Lay, S.; Yu, H.N.; Shen, S.R. Dietary Guidelines for Chinese Residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 2016, 17, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Pouladi, F.; Nozari, E.; Hosseinzadeh, F.; Hashemi, S. The protective association of dairy intake and the adverse impact of iron on gestational diabetes risk. Int. J. Vitam. Nutr. Res. 2024, 94, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.; Jensen, P.N.; Kratz, M.; Lemaitre, R.N.; Howard, B.V.; Cole, S.A.; Fretts, A.M. Full-Fat Dairy Food Intake is Associated with a Lower Risk of Incident Diabetes Among American Indians with Low Total Dairy Food Intake. J. Nutr. 2019, 149, 1238–1244. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Garcia de la Torre, N.; Bordiu, E.; Del Valle, L.; Valerio, J.; Jimenez, I.; Duran, A.; Fuentes, M.; Herraiz, M.A.; Izquierdo, N.; et al. Consumption of fat-free dairy products is not associated with a lower risk of maternofetal adverse events. BMJ Open Diabetes Res. Care 2020, 8, e001145. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.A.; Tafazoli, N. Effect of probiotic yogurt on gestational diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102758. [Google Scholar] [CrossRef]
- Zhong, T.; Huang, Y.Q.; Wang, G.M. Causal relationship association of cheese intake with gestational hypertension and diabetes result from a Mendelian randomization study. World J. Clin. Cases 2023, 11, 7318. [Google Scholar] [CrossRef]
- Li, Q.; Xing, B. Vitamin D3-Supplemented Yogurt Drink Improves Insulin Resistance and Lipid Profiles in Women with Gestational Diabetes Mellitus: A Randomized Double Blinded Clinical Trial. Ann. Nutr. Metab. 2016, 68, 285–290. [Google Scholar] [CrossRef]
- Marí-Sanchis, A.; Díaz-Jurado, G.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Martínez-González, M.A.; Bes-Rastrollo, M. Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: The SUN project. Eur. J. Nutr. 2018, 57, 939–949. [Google Scholar] [CrossRef]
- Bai, D.; Pang, X.; Dong, H.; Cai, C.; Lan, X.; Bao, Y.; Zhang, Y.; Gao, Y.; Li, F.; Zeng, G. Prospective study of red meat intake in the first and second trimesters and the risk of gestational diabetes mellitus in Chengdu in 2017. J. Hyg. Res. 2021, 50, 63–68. [Google Scholar]
- Norouziasl, R.; Jayedi, A.; Mirmohammadkhani, M.; Emadi, A.; Aghaamo, S.; Shab-Bidar, S. Consumption of red and processed meat during early pregnancy and risk of gestational diabetes: A prospective birth cohort study. Sci. Rep. 2024, 14, 5209. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Ghoreishy, S.; Ghavami, A.; Sikaroudi, M.K.; Nielsen, S.M.; Talebi, A.; Mohammadi, H. Dose-response association between animal protein sources and risk of gestational diabetes mellitus: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 1460–1472. [Google Scholar] [CrossRef]
- Stennett, R.; Gerstein, H.C.; Bangdiwala, S.I.; Rafiq, T.; Teo, K.K.; Morrison, K.M.; Atkinson, S.A.; Anand, S.S.; de Souza, R. The association of red and processed meat with gestational diabetes mellitus: Results from 2 Canadian birth cohort studies. PLoS ONE 2024, 19, e0302208. [Google Scholar] [CrossRef] [PubMed]
- Lamri, A.; Limbachia, J.; Schulze, K.M.; Desai, D.; Kelly, B.; de Souza, R.J.; Paré, G.; Lawlor, D.A.; Wright, J.; Anand, S.S. The genetic risk of gestational diabetes in South Asian women. eLife 2022, 11, e81498. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tang, N.; Zeng, J.; Jing, J.; Cai, L. Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women. Nutrients 2022, 14, 1623. [Google Scholar] [CrossRef]
- Quan, W.; Zeng, M.; Jiao, Y.; Li, Y.; Xue, C.; Liu, G.; Wang, Z.; Qin, F.; He, Z.; Chen, J. Western Dietary Patterns, Foods, and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2021, 12, 1353–1364. [Google Scholar] [CrossRef]
- Alibrandi, A.; Zirilli, A.; Le Donne, M.; Giannetto, C.; Lanfranchi, M.; De Pascale, A.; Politi, C.; Incognito, G.G.; Ercoli, A.; Granese, R. Association between Fish Consumption during Pregnancy and Maternal and Neonatal Outcomes: A Statistical Study in Southern Italy. J. Clin. Med. 2024, 13, 2131. [Google Scholar] [CrossRef]
- Mesa, M.D.; Gil, F.; Olmedo, P.; Gil, A. Nutritional Importance of Selected Fresh Fishes, Shrimps and Mollusks to Meet Compliance with Nutritional Guidelines of n-3 LC-PUFA Intake in Spain. Nutrients 2021, 13, 465. [Google Scholar] [CrossRef]
- Wadhwani, N.; Patil, V.; Joshi, S. Maternal long chain polyunsaturated fatty acid status and pregnancy complications. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 143–152. [Google Scholar] [CrossRef]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Vahlberg, T.; Houttu, N.; Koivuniemi, E.; Lahti, L.; Laitinen, K. Impact of combined consumption of fish oil and probiotics on the serum metabolome in pregnant women with overweight or obesity. eBioMedicine 2021, 73, 103655. [Google Scholar] [CrossRef] [PubMed]
- Pellonperä, O.; Vahlberg, T.; Mokkala, K.; Houttu, N.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Weight gain and body composition during pregnancy: A randomised pilot trial with probiotics and/or fish oil. Br. J. Nutr. 2021, 126, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Houttu, N.; Vahlberg, T.; Miles, E.; Calder, P.; Laitinen, K. The impact of fish oil and/or probiotics on serum fatty acids and the interaction with low-grade inflammation in pregnant women with overweight and obesity: Secondary analysis of a randomised controlled trial. Br. J. Nutr. 2024, 131, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Bartáková, V.; Kuricová, K.; Zlámal, F.; Bělobrádková, J.; Kaňková, K. Differences in food intake and genetic variability in taste receptors between Czech pregnant women with and without gestational diabetes mellitus. Eur. J. Nutr. 2018, 57, 513–521. [Google Scholar] [CrossRef]
- Wen, L.; Ge, H.; Qiao, J.; Zhang, L.; Chen, X.; Kilby, M.D.; Zhou, Y.; Gan, J.; Saffery, R.; Yan, J.; et al. Maternal dietary patterns and risk of gestational diabetes mellitus in twin pregnancies: A longitudinal twin pregnancies birth cohort study. Nutr. J. 2020, 19, 13. [Google Scholar] [CrossRef]
- Blesso, C.N.; Fernandez, M.L. Dietary Cholesterol, Serum Lipids, and Heart Disease: Are Eggs Working for or Against You? Nutrients 2018, 10, 426. [Google Scholar] [CrossRef]
- Milajerdi, A.; Tehrani, H.; Haghighatdoost, F.; Larijani, B.; Surkan, P.J.; Azadbakht, L. Associations between higher egg consumption during pregnancy with lowered risks of high blood pressure and gestational diabetes mellitus. Int. J. Vitam. Nutr. Res. 2019, 88, 166–175. [Google Scholar] [CrossRef]
- Qiu, C.; Frederick, I.O.; Zhang, C.; Sorensen, T.K.; Enquobahrie, D.A.; Williams, M.A. Risk of gestational diabetes mellitus in relation to maternal egg and cholesterol intake. Am. J. Epidemiol. 2011, 173, 649–658. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, G.; Zhou, X.; Zhong, C.; Chen, R.; Xiong, T.; Li, Q.; Yi, N.; Xiong, G.; Hao, L.; et al. Pregnancy dietary cholesterol intake, major dietary cholesterol sources, and the risk of gestational diabetes mellitus: A prospective cohort study. cohort study. Clin. Nutr. 2020, 39, 1525–1534. [Google Scholar] [CrossRef]
- Zhang, Y.; Lan, X.; Li, F.; Sun, H.; Zhang, J.; Li, R.; Gao, Y.; Dong, H.; Cai, C.; Zeng, G. Dietary cholesterol and egg intake are associated with the risk of gestational diabetes: A prospective study from Southwest China. BMC Pregnancy Childb. 2022, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Hezaveh, Z.S.; Feizy, Z.; Dehghani, F.; Sarbakhsh, P.; Moini, A.; Vafa, M. The Association between Maternal Dietary Protein Intake and Risk of Gestational Diabetes Mellitus. Int. J. Prev. Med. 2019, 10, 197. [Google Scholar]
- Dong, J.Y.; Kimura, T.; Ikehara, S.; Cui, M.; Kawanishi, Y.; Kimura, T.; Ueda, K.; Iso, H. Soy consumption and incidence of gestational diabetes mellitus: The Japan Environment and Children’s Study. Eur. J. Nutr. 2021, 60, 897–904. [Google Scholar] [CrossRef]
- Pang, X.; Cai, C.; Dong, H.; Lan, X.; Zhang, Y.; Bai, D.; Hao, L.; Sun, H.; Li, F.; Zeng, G. Soy foods and nuts consumption during early pregnancy are associated with decreased risk of gestational diabetes mellitus: A prospective cohort study. J. Matern.—Fetal Neonatal Med. 2022, 35, 9122–9130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, B.; Xiang, J. The association between soy intake and risk of gestational diabetes mellitus: A prospective cohort study. BMC Pregnancy Childb. 2021, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Asemi, Z. The Effect of Soy Intake on Metabolic Profiles of Women with Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- Tsarna, E.; Eleftheriades, A.; Tsomi, E.; Ziogou, G.; Vakas, P.; Panoskaltsis, T.; Christopoulos, P. The Role of Diet during Pregnancy in Protecting against Gestational Diabetes Mellitus in a Population with Mediterranean Dietary Habits: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 1857. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, P.; Zheng, Q.; Deka, A.; Choudhury, R.; Rastogi, S. Does a MediDiet with Additional Extra Virgin Olive Oil and Pistachios Reduce the Incidence of Gestational Diabetes? Endocr. Pract. 2022, 28, 135–141. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; Li, Z.; Carughi, A.; Ge, S. Acute Effect of Pistachio Intake on Postprandial Glycemic and Gut Hormone Responses in Women with Gestational Diabetes or Gestational Impaired Glucose Tolerance: A Randomized, Controlled, Crossover Study. Front. Nutr. 2019, 6, 186. [Google Scholar] [CrossRef]
- Brawerman, G.M.; Kereliuk, S.M.; Brar, N.; Cole, L.K.; Seshadri, N.; Pereira, T.J.; Xiang, B.; Hunt, K.L.; Fonseca, M.A.; Hatch, G.M.; et al. Maternal resveratrol administration protects against gestational diabetes-induced glucose intolerance and islet dysfunction in the rat offspring. J. Physiol. 2019, 597, 4175–4192. [Google Scholar] [CrossRef]
- Ajjarapu, A.S.; Hinkle, S.N.; Wu, J.; Li, M.; Rawal, S.; Francis, E.C.; Chen, L.; Pitsava, G.; Bjerregaard, A.A.; Grunnet, L.G.; et al. Nut Consumption and Renal Function Among Women with a History of Gestational Diabetes. J. Ren. Nutr. 2020, 30, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Gomez Ribot, D.; Diaz, E.; Fazio, M.V.; Gómez, H.L.; Careaga, V.; Maier, M.; Macchi, S.B.; Gresta, C.A.; Capobianco, E.; Jawerbaum, A. Metabolic and molecular effects of dietary extra virgin olive oil in blood and placenta of women with GDM. Front. Endocrinol. 2023, 14, 1219276. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; García de la Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef] [PubMed]
- Gomez Ribot, D.; Diaz, E.; Fazio, M.V.; Gómez, H.L.; Fornes, D.; Macchi, S.B.; Gresta, C.A.; Capobianco, E.; Jawerbaum, A. An extra virgin olive oil-enriched diet improves maternal, placental, and cord blood parameters in GDM pregnancies. Diabetes/Metab. Res. Rev. 2020, 36, e3349. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.E.; Figueroa, J.; Welsh, J.A. Added Sugar Intake among Pregnant Women in the United States: National Health and Nutrition Examination Survey 2003–2012. J. Acad. Nutr. Diet. 2018, 118, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Yuste Gómez, A.; Ramos Álvarez, M.d.P.; Bartha, J.L. Influence of Diet and Lifestyle on the Development of Gestational Diabetes Mellitus and on Perinatal Results. Nutrients 2022, 14, 2954. [Google Scholar] [CrossRef]
- Shin, D.; Lee, K.W.; Song, W.O. Dietary Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus. Nutrients 2015, 7, 9369–9382. [Google Scholar] [CrossRef]
- Eng, J.M.; Estall, J.L. Diet-Induced Models of Non-Alcoholic Fatty Liver Disease: Food for Thought on Sugar, Fat, and Cholesterol. Cells 2021, 10, 1805. [Google Scholar] [CrossRef]
- Ker, C.R.; Yang, H.C.; Wang, S.H.; Chan, T.F. Assessing sugar-sweetened beverage consumption in early pregnancy using a substance abuse framework. Sci. Rep. 2023, 13, 18979. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, X.; Yu, H.; Chan, E.-M.; Shi, Z.; Shi, S.; Shen, L.; Sun, Z.; Song, Q.; Lu, W.; et al. Association of Beverage Consumption during Pregnancy with Adverse Maternal and Offspring Outcomes. Nutrients 2024, 16, 2412. [Google Scholar] [CrossRef]
- Chen, L.; Hu, F.B.; Yeung, E.; Willett, W.; Zhang, C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care 2009, 32, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Lundeen, E.A.; Park, S.; Woo Baidal, J.A.; Sharma, A.J.; Blanck, H.M. Sugar-Sweetened Beverage Intake Among Pregnant and Non-pregnant Women of Reproductive Age. Matern. Child Health J. 2020, 24, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, A.; Hantunen, S.; Voutilainen, A.; Ruusunen, A.; Uusitalo, L.; Backman, K.; Voutilainen, R.; Pasanen, M.; Kirjavainen, P.V.; Keski-Nisula, L. Maternal caffeine, coffee and cola drink intake and the risk of gestational diabetes—Kuopio Birth Cohort. Prim. Care Diabetes 2024, 18, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Vandyousefi, S.; Whaley, S.E.; Widen, E.M.; Asigbee, F.M.; Landry, M.; Ghaddar, R.; Davis, J. Association of breastfeeding and early exposure to sugar-sweetened beverages with obesity prevalence in offspring born to mothers with and without gestational diabetes mellitus. Pediatr. Obes. 2019, 14, e12569. [Google Scholar] [CrossRef]
- Grundt, J.H.; Eide, G.E.; Brantsaeter, A.L.; Haugen, M.; Markestad, T. Is consumption of sugar-sweetened soft drinks during pregnancy associated with birth weight? Matern. Child Nutr. 2017, 13, e12405. [Google Scholar] [CrossRef]
- Zhang, S.; Stubbendorff, A.; Ericson, U.; Wändell, P.; Niu, K.; Qi, L.; Borné, Y.; Sonestedt, E. The EAT-Lancet diet, genetic susceptibility and risk of atrial fibrillation in a population-based cohort. BMC Med. 2023, 21, 280. [Google Scholar] [CrossRef]
- Berthy, F.; Brunin, J.; Allès, B.; Fezeu, L.K.; Touvier, M.; Hercberg, S.; Galan, P.; Pointereau, P.; Lairon, D.; Baudry, J.; et al. Association between adherence to the EAT-Lancet diet and risk of cancer and cardiovascular outcomes in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2022, 116, 980–991. [Google Scholar] [CrossRef]
- Hanley-Cook, G.; Argaw, A.; de Kok, B.P.; Vanslambrouck, K.W.; Toe, L.C.; Kolsteren, P.W.; Jones, A.D.; Lachat, C.K. EAT-Lancet diet score requires minimum intake values to predict higher micronutrient adequacy of diets in rural women of reproductive age from five low- and middle-income countries. Br. J. Nutr. 2021, 126, 92–100. [Google Scholar] [CrossRef]
- Stubbendorff, A.; Stern, D.; Ericson, U.; Sonestedt, E.; Hallström, E.; Borné, Y.; Lajous, M.; Forouhi, N.G.; Olsen, A.; Dahm, C.C.; et al. A systematic evaluation of seven different scores representing the EAT-Lancet reference diet and mortality, stroke, and greenhouse gas emissions in three cohorts. Lancet Planet. Health 2024, 8, e391–e401. [Google Scholar] [CrossRef]
- Ravelli, M.N.; Schoeller, D.A. Traditional Self-Reported Dietary Instruments Are Prone to Inaccuracies and New Approaches Are Needed. Front. Nutr. 2020, 7, 90. [Google Scholar] [CrossRef]
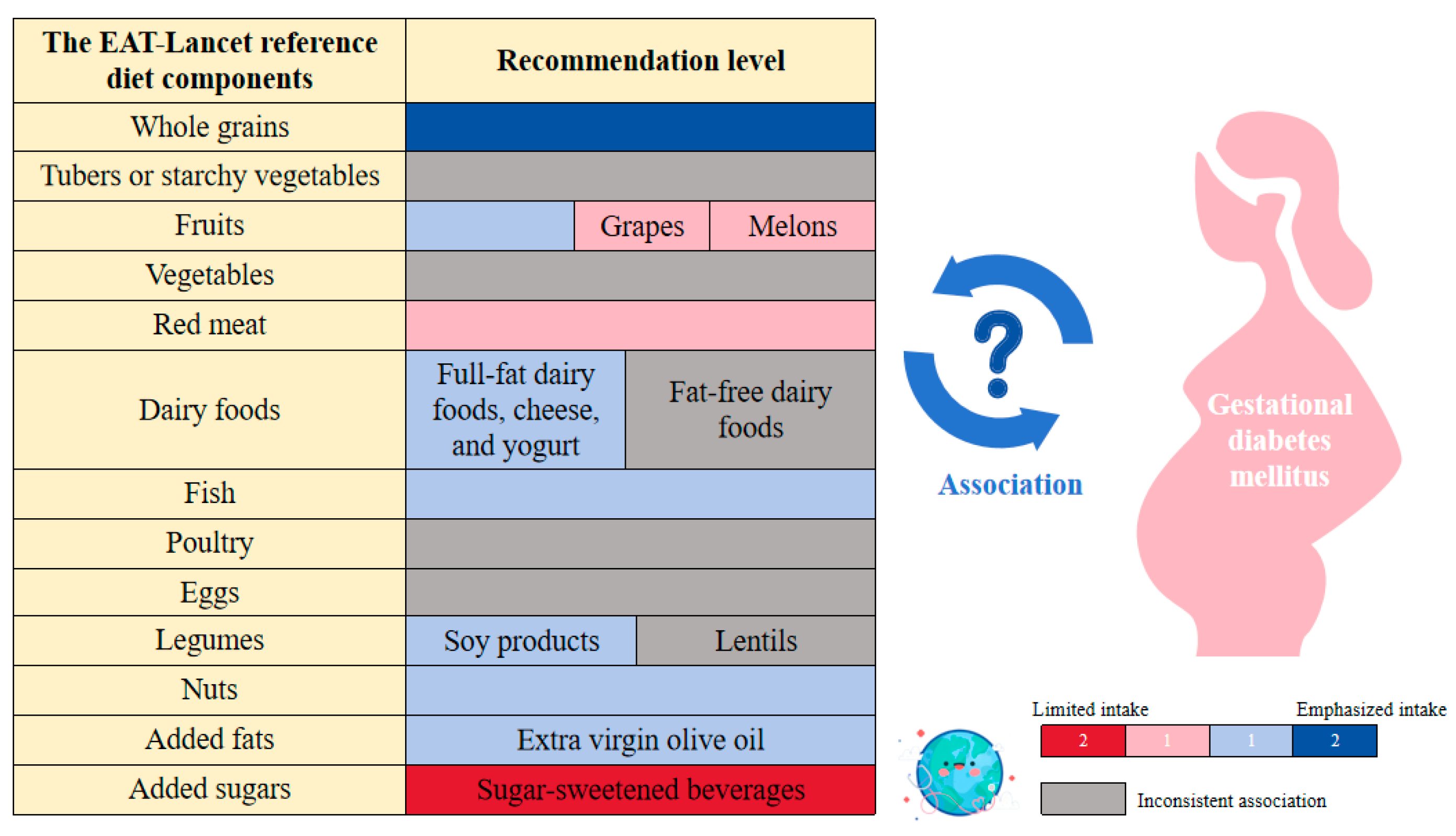
| The EAT-Lancet Reference Diet Components | Conclusions from Available Evidence |
|---|---|
| Whole grains | Cohort studies indicate that whole grain consumption is inversely associated with the risk of GDM, with different processing methods potentially affecting this association. |
| Tubers or starchy vegetables | Inconsistencies among cohort studies hinder establishing a definitive association between tuber or starchy vegetable intake and GDM. |
| Fruits | Both RCTs and cohort studies indicate that moderate fruit intake can reduce the incidence of GDM. Furthermore, cohort studies suggest that fruit consumption is associated with improved outcomes for pregnant women with GDM. |
| vegetables | Cohort studies suggest that combined fruit and vegetable intake may reduce GDM risk, but few have investigated vegetable intake alone, leading to inconsistent evidence regarding its association with GDM. |
| Dairy foods | Cohort studies suggest that while full-fat dairy products may reduce GDM risk and cheese and yogurt are associated with a lower risk, the relationship between fat-free dairy products and GDM remains uncertain. RCT studies prove that vitamin D3-enriched yogurt is beneficial for insulin sensitivity in pregnant women with GDM. |
| Red meat | Cohort studies indicate a positive association between red meat intake and an increased risk of GDM, although this association may be masked by confounding factors. |
| Fish | Cohort studies and RCT evidence suggest that fish consumption is associated with a reduced risk of GDM, but the effects of fish oil may vary, potentially increasing GDM risk in overweight expectant mothers. |
| Poultry | Current evidence does not establish a clear association between poultry consumption and GDM. |
| Eggs | Cohort and case-control studies suggest that excessive egg consumption should be avoided. As a protein source, eggs show no association with GDM, but as a source of cholesterol, higher egg intake is positively associated with an increased risk of GDM. |
| Legumes | Cohort study results indicate that consuming at least 40 g of soybeans daily is associated with a reduced risk of GDM, while the relationship between other legumes, such as lentils, remains unclear. |
| Nuts | Cohort studies and RCTs suggest that a higher nut intake is associated with a lower risk of GDM, while consuming pistachios may benefit blood glucose management and improve kidney health in pregnant women with GDM. |
| Added fats | Cohort studies and RCTs suggest that a diet rich in extra virgin olive oil may protect against GDM and benefit the health of pregnant women with GDM. |
| Added sugars | Cohort studies indicate that SSBs are linked to an increased risk of GDM, with pregnant women consuming more than the median cola level (33.3 mL/day) showing a significantly higher risk. Additionally, SSB intake is associated with the weight of offspring from mothers with GDM. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Wen, S.; Huo, Z.; He, Z.; Sun, T.; Hu, J.; Sonestedt, E.; Borné, Y.; Zhang, S. Association Between the EAT-Lancet Reference Diet and Gestational Diabetes Mellitus: A Mini-Review. Nutrients 2024, 16, 4073. https://doi.org/10.3390/nu16234073
Sun N, Wen S, Huo Z, He Z, Sun T, Hu J, Sonestedt E, Borné Y, Zhang S. Association Between the EAT-Lancet Reference Diet and Gestational Diabetes Mellitus: A Mini-Review. Nutrients. 2024; 16(23):4073. https://doi.org/10.3390/nu16234073
Chicago/Turabian StyleSun, Niuniu, Shubo Wen, Zhenyu Huo, Zitong He, Tongyao Sun, Jingxi Hu, Emily Sonestedt, Yan Borné, and Shunming Zhang. 2024. "Association Between the EAT-Lancet Reference Diet and Gestational Diabetes Mellitus: A Mini-Review" Nutrients 16, no. 23: 4073. https://doi.org/10.3390/nu16234073
APA StyleSun, N., Wen, S., Huo, Z., He, Z., Sun, T., Hu, J., Sonestedt, E., Borné, Y., & Zhang, S. (2024). Association Between the EAT-Lancet Reference Diet and Gestational Diabetes Mellitus: A Mini-Review. Nutrients, 16(23), 4073. https://doi.org/10.3390/nu16234073







