Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
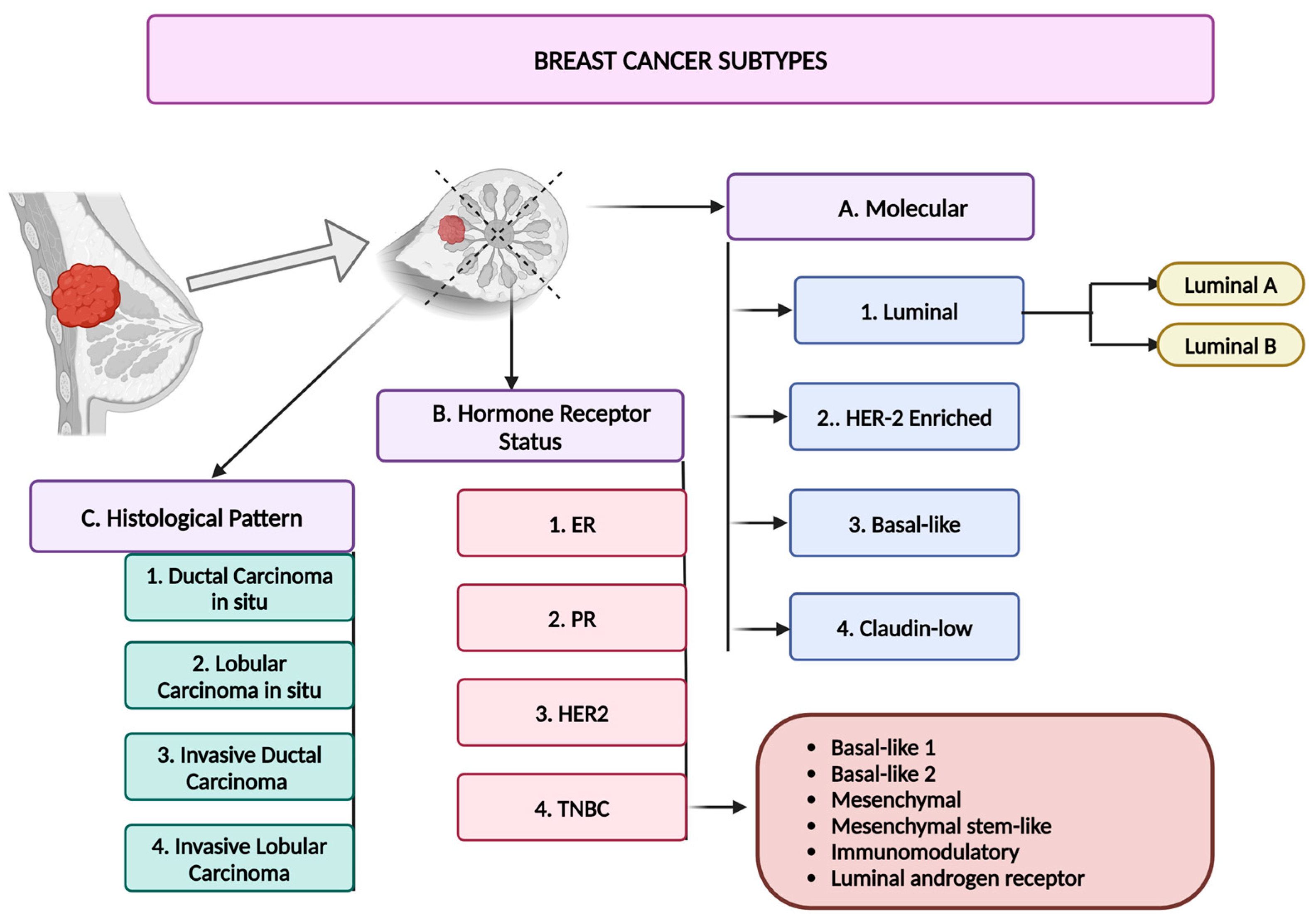
3. Breast Cancer
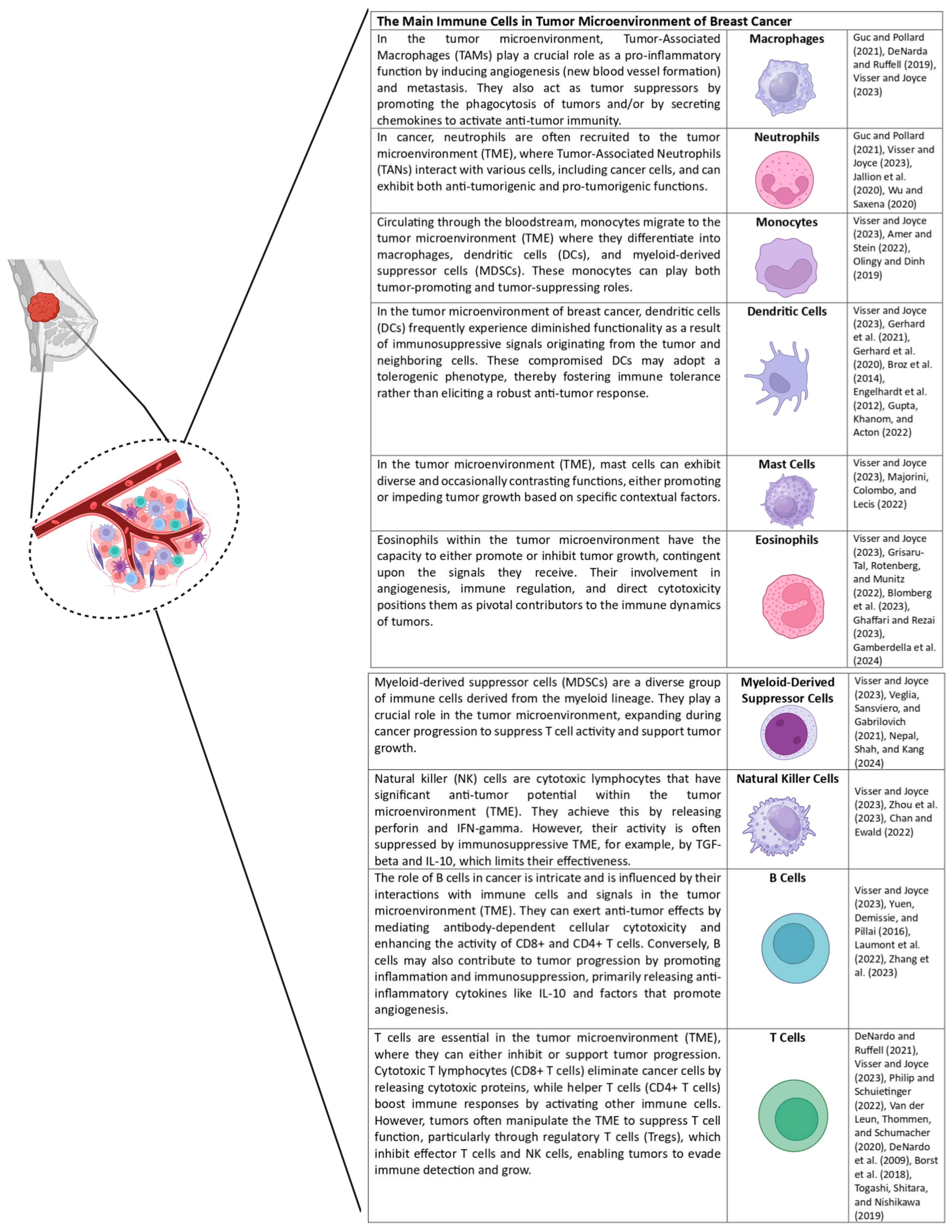
4. Immune System Regulation in Breast Cancer
5. Breast Cancer Treatment Types
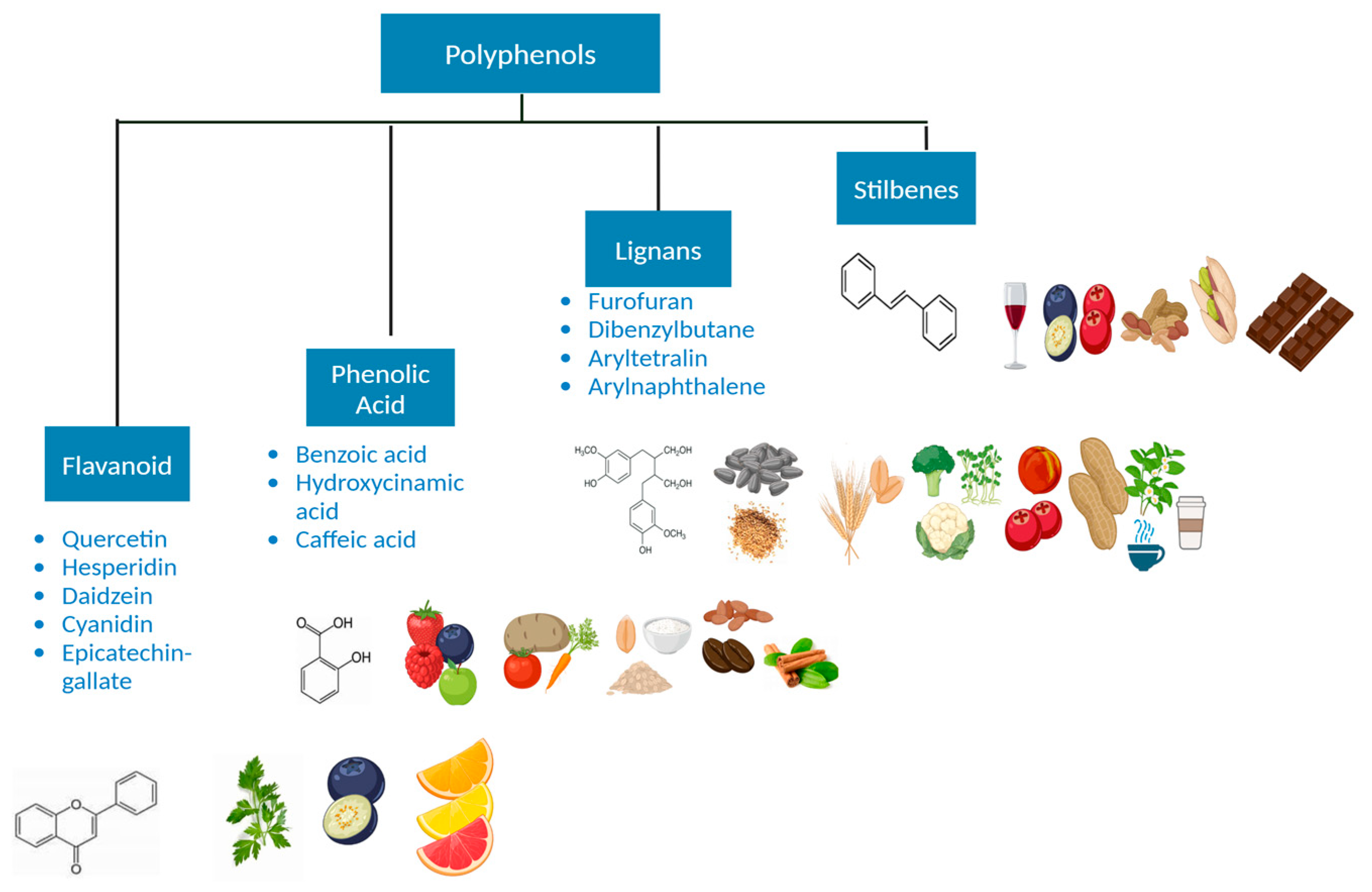
6. Polyphenols and Their Importance
7. Dietary Polyphenols and Their Impact on Immune System
8. Role of Polyphenols in Regulating the Immune System
8.1. Modulation of Immune Cells
8.2. Regulation of Inflammatory Responses
8.3. Immune Checkpoint Regulation
8.4. Regulation of T Cells and B Cells
9. Synergistic Potential with Conventional Therapies
10. Epigenetic Regulation by Polyphenols in Cancer Immunomodulation
11. Specific Polyphenols Used in Breast Cancer Treatment
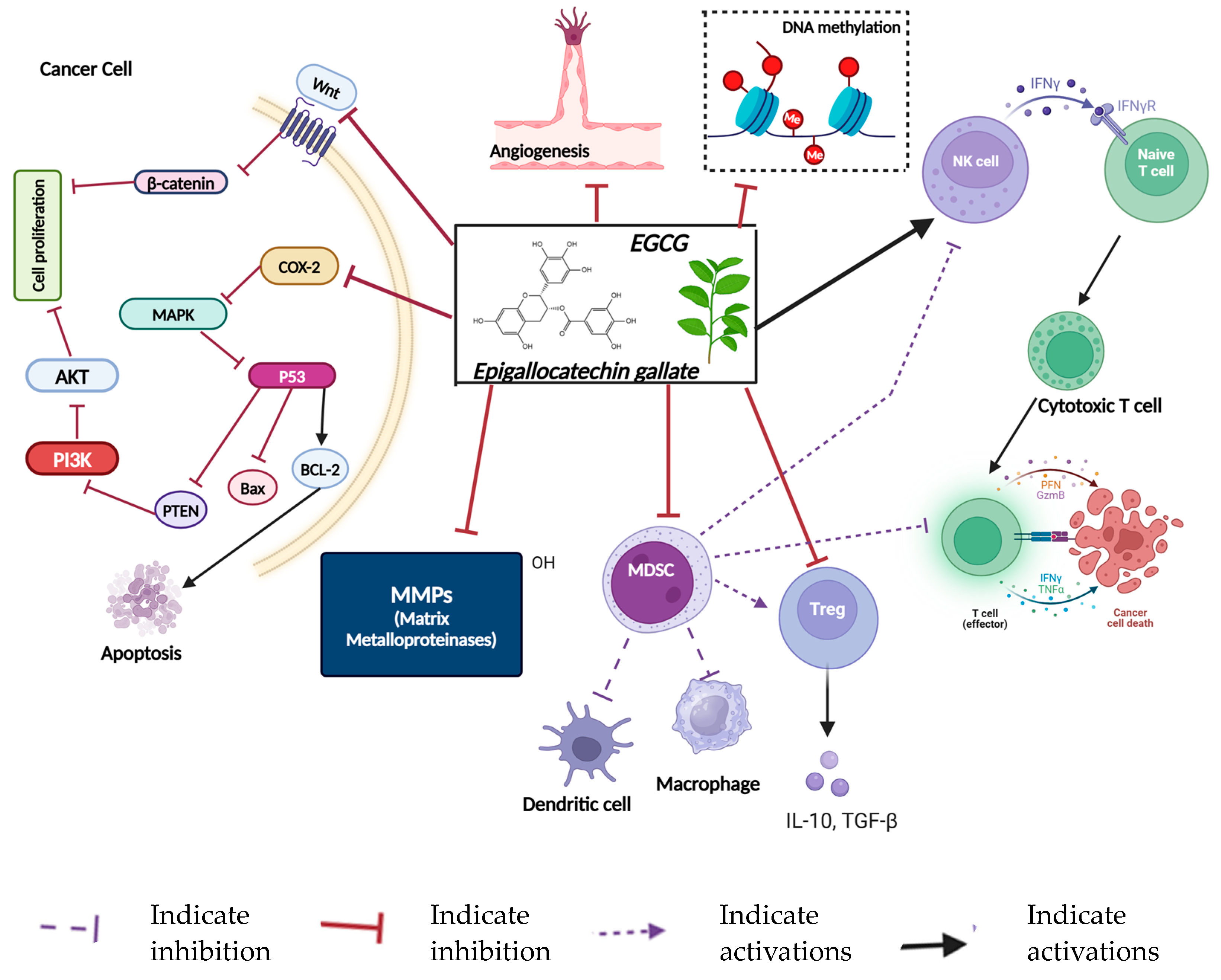
11.1. Epigallocatechin-3-Gallate (EGCG)
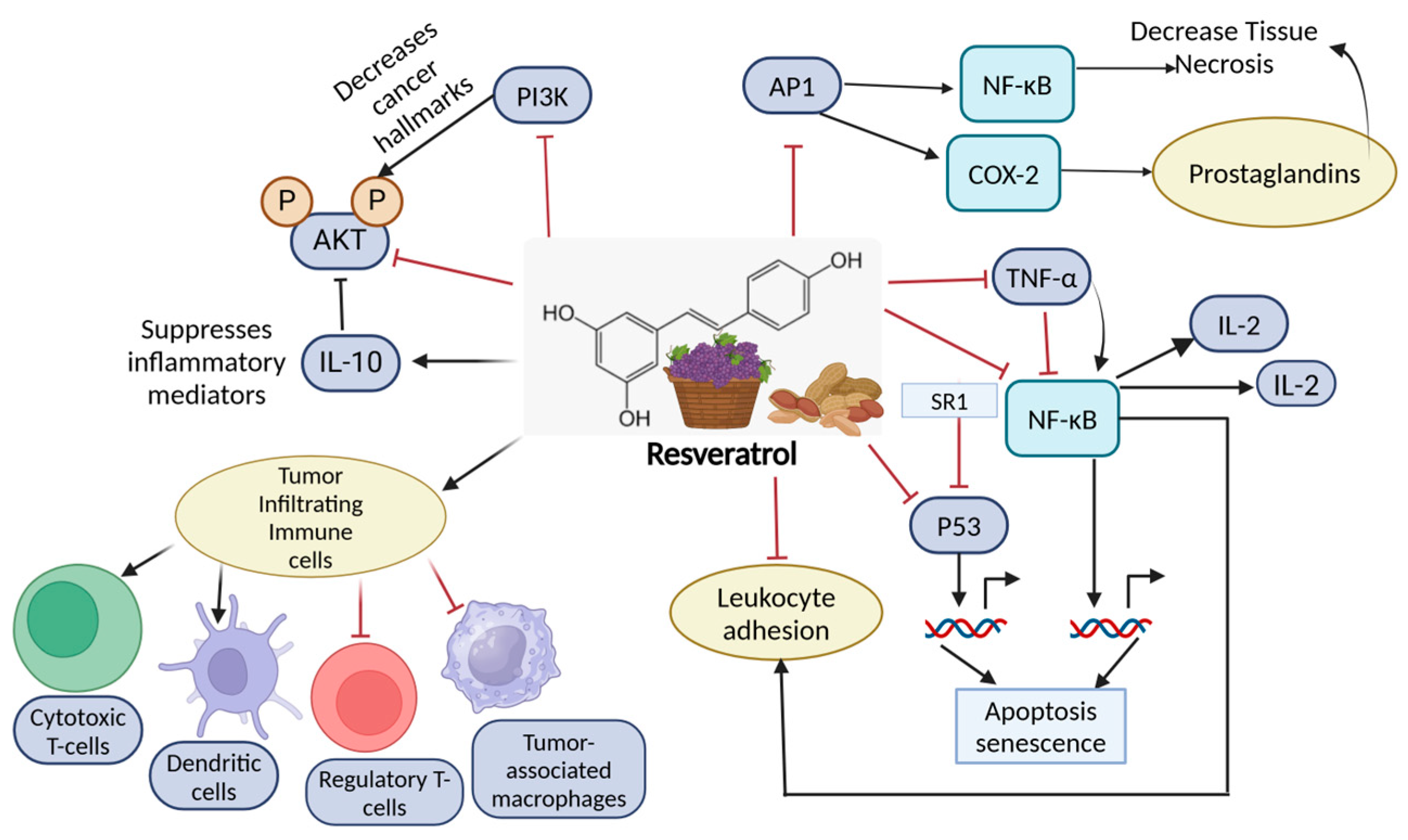
11.2. Resveratrol
11.3. Curcumin
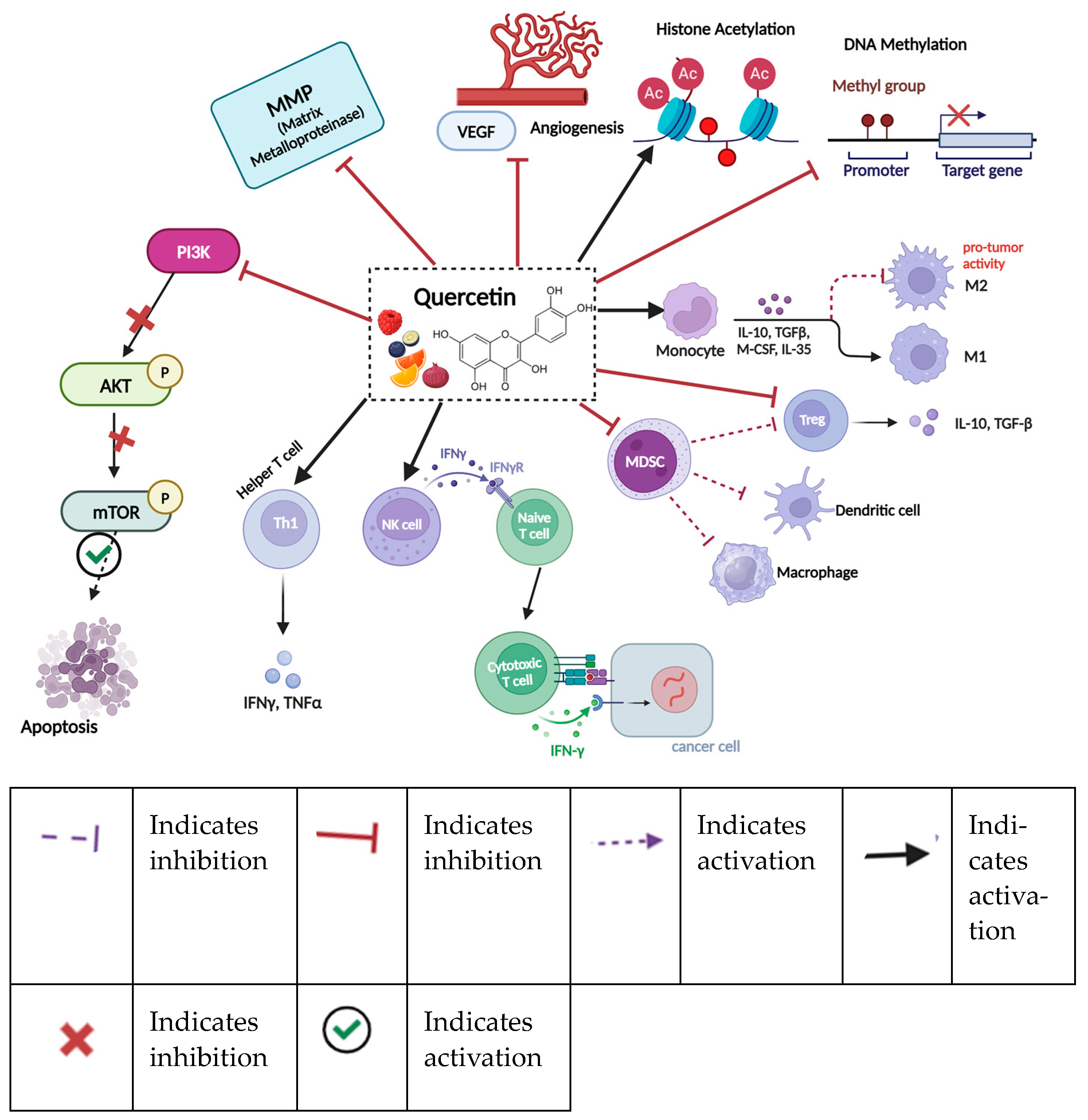
11.4. Quercetin
11.5. Genistein
11.6. Apigenin
11.7. Pterostilbene
12. Discussion
13. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| Bax | BCL2-Associated X (Apoptosis Regulator) |
| BC | Breast Cancer |
| Bcl-2 | B Cell Lymphoma Protein 2 |
| Bcl-xl | B Cell Lymphoma-Extra Large |
| BL1 | Basal-Like 1 |
| BL2 | Basal-Like 2 |
| CAFs | Cancer-Associated Fibroblasts |
| CAR-T | Chimeric Antigen Receptor T cells |
| CDK4 | Cyclin-Dependent Kinase 4 |
| CLBC | Claudin-Low Breast Cancer |
| CTLs | Cytotoxic T lymphocytes |
| CTLA4 | Cytotoxic T Lymphocyte-Associated Protein 4 |
| DC | Dendritic Cells |
| DCIS | Ductal Carcinoma In Situ |
| ECM | Extracellular Matrix |
| EGCG | Epigallocatechin Gallate |
| ER | Estrogen Receptor |
| ER+ | Estrogen Receptor-Positive |
| EMT | Epithelial–Mesenchymal Transition |
| HER2 | Human Epidermal Growth Factor 2 |
| HER2+ | Human Epidermal Growth Factor 2-Positive |
| HR | Hormone Receptor |
| HR+ | Hormone Receptor-Positive |
| HIF-1α | Hypoxia-Inducible Factor 1-Alpha |
| JAK/STAT | Janus Kinase–Signal Transducer and Activator of Transcription |
| IDC | Invasive Ductal Carcinoma |
| ILC | Invasive Lobular Carcinoma |
| IM | Immunomodulatory |
| LAR | Luminal Androgen Receptor |
| LCIS | Lobular Carcinoma In Situ |
| M | Mesenchymal |
| M1 | M1-Type Macrophage |
| M2 | M2-Type Macrophage |
| MAPK | Mitogen-Activated Protein Kinase |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MEK5 | Mitogen-Activated Protein Kinase 5 |
| MSL | Mesenchymal Stem-Like |
| MMP | Matrix Metalloproteinase |
| mTOR | Mammalian Target of Rapamycin |
| NFκB | Nuclear Factor Kappa B |
| NK Cells | Natural Killer Cells |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3K | Phosphoinositide 3-Kinase |
| PR | Progesterone Receptor |
| PR+ | Progesterone Receptor-Positive |
| Sp1 | Transcription Factor-Specific Protein 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAMs | Tumor-Associated Macrophages |
| Th1 | Type 1 T Helper |
| Th2 | Type 2 T Helper |
| Th9 | Type 9 T Helper |
| Th17 | Type 17 T Helper |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| Tregs | Regulatory T Cells |
| VEGF | Vascular Endothelial Growth Factor |
| ZAP-70 | Zeta Chain-Associated 70kDa Protein Receptor |
References
- Wu, H.; Van Der Pol, W.J.; Dubois, L.G.; Morrow, C.D.; Tollefsbol, T.O. Dietary Supplementation of Inulin Contributes to the Prevention of Estrogen Receptor-Negative Mammary Cancer by Alteration of Gut Microbial Communities and Epigenetic Regulations. Int. J. Mol. Sci. 2023, 24, 9015. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Wu, H.; Tollefsbol, T.O. A novel combinatorial approach using sulforaphane- and withaferin A-rich extracts for prevention of estrogen receptor-negative breast cancer through epigenetic and gut microbial mechanisms. Sci. Rep. 2024, 14, 12091. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Tabár, L.; Yen, A.M.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Törnberg, S.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Murakami, A.; Ohnishi, K. Target molecules of food phytochemicals: Food science bound for the next dimension. Food Funct. 2012, 3, 462–476. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef]
- Briguglio, G. Polyphenols in cancer prevention: New insights. Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sotiriou, C. Luminal breast cancer: From biology to treatment. Nat. Rev. Clin. Oncol. 2013, 10, 494–506. [Google Scholar] [CrossRef]
- Higgins, M.J.; Stearns, V. Understanding resistance to tamoxifen in hormone receptor-positive breast cancer. Clin. Chem. 2009, 55, 1453–1455. [Google Scholar] [CrossRef]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin. Med. Insights Oncol. 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishigami, S.; Kijima, Y.; Funasako, Y.; Hirata, M.; Okumura, H.; Shinchi, H.; Koriyama, C.; Ueno, S.; Yoshinaka, H.; et al. Clinical implication of HLA class I expression in breast cancer. BMC Cancer 2011, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef]
- Shaath, H.; Elango, R.; Alajez, N.M. Molecular Classification of Breast Cancer Utilizing Long Non-Coding RNA (lncRNA) Transcriptomes Identifies Novel Diagnostic lncRNA Panel for Triple-Negative Breast Cancer. Cancers 2021, 13, 5350. [Google Scholar] [CrossRef]
- Plasilova, M.L.; Hayse, B.; Killelea, B.K.; Horowitz, N.R.; Chagpar, A.B.; Lannin, D.R. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine 2016, 95, e4614. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon publications: Brisbane, Australia, 2022. [Google Scholar]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Wang, D.Y.; Jiang, Z.; Ben-David, Y.; Woodgett, J.R.; Zacksenhaus, E. Molecular stratification within triple-negative breast cancer subtypes. Sci. Rep. 2019, 9, 19107. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Xu, A.; Ma, X.; Yao, Y.; Zhao, Y.; Wang, C.; Chen, C. Research progress of Claudin-low breast cancer. Front. Oncol. 2023, 13, 1226118. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological types of breast cancer: How special are they? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef]
- Russnes, H.G.; Lingjærde, O.C.; Børresen-Dale, A.L.; Caldas, C. Breast Cancer Molecular Stratification: From Intrinsic Subtypes to Integrative Clusters. Am. J. Pathol. 2017, 187, 2152–2162. [Google Scholar] [CrossRef]
- Ruffell, B.; Au, A.; Rugo, H.S.; Esserman, L.J.; Hwang, E.S.; Coussens, L.M. Leukocyte composition of human breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 2796–2801. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Comba, A.; Faisal, S.M.; Varela, M.L.; Hollon, T.; Al-Holou, W.N.; Umemura, Y.; Nunez, F.J.; Motsch, S.; Castro, M.G.; Lowenstein, P.R. Uncovering Spatiotemporal Heterogeneity of High-Grade Gliomas: From Disease Biology to Therapeutic Implications. Front. Oncol. 2021, 11, 703764. [Google Scholar] [CrossRef]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [PubMed]
- Guc, E.; Pollard, J.W. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity 2021, 54, 885–902. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar]
- Amer, H.T.; Stein, U.; El Tayebi, H.M. The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers 2022, 14, 5460. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Gerhard, G.M.; Bill, R.; Messemaker, M.; Klein, A.M.; Pittet, M.J. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J. Exp. Med. 2021, 218, e20200264. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, J.J.; Boldajipour, B.; Beemiller, P.; Pandurangi, P.; Sorensen, C.; Werb, Z.; Egeblad, M.; Krummel, M.F. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 2012, 21, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.H.; Khanom, A.; Acton, S.E. Control of Dendritic Cell Function Within the Tumour Microenvironment. Front. Immunol. 2022, 13, 733800. [Google Scholar] [CrossRef] [PubMed]
- Majorini, M.T.; Colombo, M.P.; Lecis, D. Few, but Efficient: The Role of Mast Cells in Breast Cancer and Other Solid Tumors. Cancer Res. 2022, 82, 1439–1447. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Rothenberg, M.E.; Munitz, A. Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat. Immunol. 2022, 23, 1309–1316. [Google Scholar] [CrossRef]
- Blomberg, O.S.; Spagnuolo, L.; Garner, H.; Voorwerk, L.; Isaeva, O.I.; van Dyk, E.; Bakker, N.; Chalabi, M.; Klaver, C.; Duijst, M.; et al. IL-5-producing CD4(+) T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 2023, 41, 106–123.e10. [Google Scholar] [CrossRef]
- Ghaffari, S.; Rezaei, N. Eosinophils in the tumor microenvironment: Implications for cancer immunotherapy. J. Transl. Med. 2023, 21, 551. [Google Scholar] [CrossRef]
- Gambardella, A.R.; Antonucci, C.; Zanetti, C.; Noto, F.; Andreone, S.; Vacca, D.; Pellerito, V.; Sicignano, C.; Parrottino, G.; Tirelli, V.; et al. IL-33 stimulates the anticancer activities of eosinophils through extracellular vesicle-driven reprogramming of tumor cells. J. Exp. Clin. Cancer Res. 2024, 43, 209. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Nepal, M.R.; Shah, S.; Kang, K.T. Dual roles of myeloid-derived suppressor cells in various diseases: A review. Arch. Pharm. Res. 2024, 47, 597–616. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, L.; Liu, L.; Li, X. NK cells are never alone: Crosstalk and communication in tumour microenvironments. Mol. Cancer 2023, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.S.; Ewald, A.J. The changing role of natural killer cells in cancer metastasis. J. Clin. Investig. 2022, 132, e143762. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef]
- Laumont, C.M.; Banville, A.C.; Gilardi, M.; Hollern, D.P.; Nelson, B.H. Tumour-infiltrating B cells: Immunological mechanisms, clinical impact and therapeutic opportunities. Nat. Rev. Cancer 2022, 22, 414–430. [Google Scholar] [CrossRef]
- Zhang, E.; Ding, C.; Li, S.; Zhou, X.; Aikemu, B.; Fan, X.; Sun, J.; Zheng, M.; Yang, X. Roles and mechanisms of tumour-infiltrating B cells in human cancer: A new force in immunotherapy. Biomark. Res. 2023, 11, 28. [Google Scholar] [CrossRef]
- Philip, M.; Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer 2023, 15, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.E. Surgical management of locally advanced and recurrent breast cancer. Cancer 1984, 53 (Suppl. S3), 752–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, J.; Gu, P.; Wang, Z. Recent advances progress in radiotherapy for breast cancer after breast-conserving surgery: A review. Front. Oncol. 2023, 13, 1195266. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, I.; Melichar, B.; Sirák, I.; Vaňásek, J.; Petera, J.; Horáčková, K.; Pohanková, D.; Ďatelinka, F.; Šinkorová, Z.; Vošmik, M. The Role of Adjuvant Radiotherapy in the Treatment of Breast Cancer. Curr. Oncol. 2024, 31, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef]
- Mano, M.S.; Awada, A. Primary chemotherapy for breast cancer: The evidence and the future. Ann. Oncol. 2004, 15, 1161–1171. [Google Scholar] [CrossRef]
- Puhalla, S.; Bhattacharya, S.; Davidson, N.E. Hormonal therapy in breast cancer: A model disease for the personalization of cancer care. Mol. Oncol. 2012, 6, 222–236. [Google Scholar] [CrossRef]
- Ramadan, W.S.; Alseksek, R.K.; Mouffak, S.; Talaat, I.M.; Saber-Ayad, M.M.; Menon, V.; Ilce, B.Y.; El-Awady, R. Impact of HDAC6-mediated progesterone receptor expression on the response of breast cancer cells to hormonal therapy. Eur. J. Pharmacol. 2024, 983, 177001. [Google Scholar] [CrossRef]
- Du, L.; Yau, C.; Brown-Swigart, L.; Gould, R.; Krings, G.; Hirst, G.L.; Bedrosian, I.; Layman, R.M.; Carter, J.M.; Klein, M.; et al. Predicted sensitivity to endocrine therapy for stage II-III hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer before chemo-endocrine therapy. Ann. Oncol. 2021, 32, 642–651. [Google Scholar] [CrossRef]
- Mancuso, M.R.; Massarweh, S.A. Endocrine therapy and strategies to overcome therapeutic resistance in breast cancer. Curr. Probl. Cancer 2016, 40, 95–105. [Google Scholar] [CrossRef]
- Adams, S.; Gatti-Mays, M.E.; Kalinsky, K.; Korde, L.A.; Sharon, E.; Amiri-Kordestani, L.; Bear, H.; McArthur, H.L.; Frank, E.; Perlmutter, J.; et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol. 2019, 5, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Moradi-Kalbolandi, S.; Hosseinzadeh, A.; Kazemi, M.H.; Khorramdelazad, H.; Safari, E.; Farahmand, L. Breast cancer immunotherapy: Current and novel approaches. Int. Immunopharmacol. 2021, 98, 107886. [Google Scholar]
- Vasileiou, M. Current Advancements and Future Perspectives of Immunotherapy in Breast Cancer Treatment. Immuno 2023, 3, 195–216. [Google Scholar] [CrossRef]
- Vu, T.; Claret, F.X. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Capelan, M.; Pugliano, L.; De Azambuja, E.; Bozovic, I.; Saini, K.S.; Sotiriou, C.; Loi, S.; Piccart-Gebhart, M.J. Pertuzumab: New hope for patients with HER2-positive breast cancer. Ann. Oncol. 2013, 24, 273–282. [Google Scholar] [CrossRef]
- June, C.H.; Riddell, S.R.; Schumacher, T.N. Adoptive cellular therapy: A race to the finish line. Sci. Transl. Med. 2015, 7, 280ps7. [Google Scholar] [CrossRef]
- Alaluf, E.; Shalamov, M.M.; Sonnenblick, A. Update on current and new potential immunotherapies in breast cancer, from bench to bedside. Front. Immunol. 2024, 15, 1287824. [Google Scholar] [CrossRef]
- Semiglazov, V.; Tseluiko, A.; Kudaybergenova, A.; Artemyeva, A.; Krivorotko, P.; Donskih, R. Immunology and immunotherapy in breast cancer. Cancer Biol. Med. 2022, 19, 609–618. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef]
- Li, S.; Wu, H.; Chen, M.; Tollefsbol, T.O. Paternal Combined Botanicals Contribute to the Prevention of Estrogen Receptor-Negative Mammary Cancer in Transgenic Mice. J. Nutr. 2023, 153, 1959–1973. [Google Scholar] [CrossRef]
- Putti, T.C.; El-Rehim, D.M.; Rakha, E.A.; Paish, C.E.; Lee, A.H.; Pinder, S.E.; Ellis, I.O. Estrogen receptor-negative breast carcinomas: A review of morphology and immunophenotypical analysis. Mod. Pathol. 2005, 18, 26–35. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Braakhuis, A.J.; Bishop, K.S. The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare 2019, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Arora, I.; Stoll, M.L.; Li, Y.; Morrow, C.D.; Barnes, S.; Berryhill, T.F.; Li, S.; Tollefsbol, T.O. Nutritional combinatorial impact on the gut microbiota and plasma short-chain fatty acids levels in the prevention of mammary cancer in Her2/neu estrogen receptor-negative transgenic mice. PLoS ONE 2020, 15, e0234893. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Geelen, A.; Kromhout, D. Dietary flavonol intake may lower stroke risk in men and women. J. Nutr. 2010, 140, 600–604. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 215S–217S. [Google Scholar] [CrossRef]
- Bolca, S.; Urpi-Sarda, M.; Blondeel, P.; Roche, N.; Vanhaecke, L.; Possemiers, S.; Al-Maharik, N.; Botting, N.; De Keukeleire, D.; Bracke, M.; et al. Disposition of soy isoflavones in normal human breast tissue. Am. J. Clin. Nutr. 2010, 91, 976–984. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Arora, I.; Tollefsbol, T.O. Impact of Stilbenes as Epigenetic Modulators of Breast Cancer Risk and Associated Biomarkers. Int. J. Mol. Sci. 2021, 22, 10033. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fei, Y.J.; Cao, X.H.; Xu, D.; Tang, W.J.; Yang, K.; Xu, W.X.; Tang, J.H. Lignans intake and enterolactone concentration and prognosis of breast cancer: A systematic review and meta-analysis. J. Cancer 2021, 12, 2787–2796. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nistico, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Szliszka, E.; Krol, W. The role of dietary polyphenols in tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur. J. Cancer Prev. 2011, 20, 63–69. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Yang, C.C.; Wu, C.J.; Chien, C.Y.; Chien, C.T. Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice. Antioxidants 2021, 10, 928. [Google Scholar] [CrossRef]
- Ferreira, C.; Vieira, P.; Sá, H.; Malva, J.; Castelo-Branco, M.; Reis, F.; Viana, S. Polyphenols: Immunonutrients tipping the balance of immunometabolism in chronic diseases. Front. Immunol. 2024, 15, 1360065. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Yang, W.; Dong, J.; Li, L. Regulation of dietary polyphenols on cancer cell pyroptosis and the tumor immune microenvironment. Front. Nutr. 2022, 9, 974896. [Google Scholar] [CrossRef]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green Tea Polyphenol EGCG Attenuates MDSCs-mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.; Riesco-Llach, G.; Polonio-Alcalá, E.; Morla-Barcelo, P.M.; Ruiz-Martínez, S.; Feliu, L.; Planas, M.; Puig, T. Inhibition of Cancer Stem-like Cells by Curcumin and Other Polyphenol Derivatives in MDA-MB-231 TNBC Cells. Int. J. Mol. Sci. 2024, 25, 7446. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Mari, L.; van de Luijtgaarden, W.; Parikh, K.; Calpe, S.; Krishnadath, K.K. Nano-curcumin inhibits proliferation of esophageal adenocarcinoma cells and enhances the T cell mediated immune response. Front. Oncol. 2013, 3, 137. [Google Scholar] [CrossRef]
- Hong, E.H.; Heo, E.Y.; Song, J.H.; Kwon, B.E.; Lee, J.Y.; Park, Y.; Kim, J.; Chang, S.Y.; Chin, Y.W.; Jeon, S.M.; et al. Trans-scirpusin A showed antitumor effects via autophagy activation and apoptosis induction of colorectal cancer cells. Oncotarget 2017, 8, 41401–41411. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Pourhanifeh, M.H.; Mirzaei, H.R.; Sahebkar, A.; Asemi, Z.; Mirzaei, H. Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy. Pharmacol. Res. 2019, 147, 104353. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Z.; Tao, J.; Hu, H.; Li, Z.; Zhang, Z.; Cheng, F.; Sun, Y.; Zhang, Y.; Yang, J.; et al. Resveratrol induces PD-L1 expression through snail-driven activation of Wnt pathway in lung cancer cells. J. Cancer Res. Clin. Oncol. 2021, 147, 1101–1113. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Zheng, F.; Liu, H.; Qiu, F.; Chen, Y.; Liang, C.L.; Dai, Z. Resveratrol exerts antitumor effects by downregulating CD8(+)CD122(+) Tregs in murine hepatocellular carcinoma. Oncoimmunology 2020, 9, 1829346. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Sato, M.; Miyazaki, T.; Kambe, F.; Maeda, K.; Seo, H. Quercetin, a bioflavonoid, inhibits the induction of interleukin 8 and monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha in cultured human synovial cells. J. Rheumatol. 1997, 24, 1680–1684. [Google Scholar] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef]
- Garcia-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef]
- Pan, P.; Huang, Y.W.; Oshima, K.; Yearsley, M.; Zhang, J.; Arnold, M.; Yu, J.; Wang, L.S. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit. Rev. Food Sci. Nutr. 2019, 59, 992–1007. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.K.; Jeon, Y.K.; Kim, C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Sahebkar, A.; Cicero, A.F.G.; Simental-Mendía, L.E.; Aggarwal, B.B.; Gupta, S.C. Curcumin downregulates human tumor necrosis factor-alpha levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacol. Res. 2016, 107, 234–242. [Google Scholar] [CrossRef]
- Cheng, S.C.; Huang, W.C.; S Pang, J.H.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1beta-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, B.; Wang, N.; Gu, J. Tumor immunomodulatory effects of polyphenols. Front. Immunol. 2022, 13, 1041138. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain-Liver-Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Soliman, K.F.A. Polyphenols Modulating Effects of PD-L1/PD-1 Checkpoint and EMT-Mediated PD-L1 Overexpression in Breast Cancer. Nutrients 2021, 13, 1718. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Frebel, H.; Nindl, V.; Schuepbach, R.A.; Braunschweiler, T.; Richter, K.; Vogel, J.; Wagner, C.A.; Loffing-Cueni, D.; Kurrer, M.; Ludewig, B.; et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 2012, 209, 2485–2499. [Google Scholar] [CrossRef]
- Delmas, D.; Hermetet, F.; Aires, V. PD-1/PD-L1 Checkpoints and Resveratrol: A Controversial New Way for a Therapeutic Strategy. Cancers 2021, 13, 4509. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Lin, D.Y.; Tanaka, Y.; Iwasaki, M.; Gittis, A.G.; Su, H.P.; Mikami, B.; Okazaki, T.; Honjo, T.; Minato, N.; Garboczi, D.N. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 3011–3016. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Al-Massarani, G.; Aljapawe, A.; Ekhtiar, A.; Bakir, M.A. Resveratrol Modulates the Inflammatory Profile of Immune Responses and Circulating Endothelial Cells’ (CECs’) Population During Acute Whole Body Gamma Irradiation. Front. Pharmacol. 2020, 11, 528400. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. Biofactors 2006, 26, 105–121. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; A Alsahli, M.; S M Aljohani, A.; Alhumaydhi, F.A.; Babiker, A.Y.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Resveratrol, a Plant Polyphenol, and Its Role in the Therapy of Various Types of Cancer. Molecules 2022, 27, 2665. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 2665. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Salazar, L.A.; Abdalla, D.S. Modulation of immune function by polyphenols: Possible contribution of epigenetic factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef]
- Ciz, M.; Dvořáková, A.; Skočková, V.; Kubala, L. The Role of Dietary Phenolic Compounds in Epigenetic Modulation Involved in Inflammatory Processes. Antioxidants 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Borsoi, F.T.; Neri-Numa, I.A.; de Oliveira, W.Q.; de Araújo, F.F.; Pastore, G.M. Dietary polyphenols and their relationship to the modulation of non-communicable chronic diseases and epigenetic mechanisms: A mini-review. Food Chem. 2023, 6, 100155. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Keller, M.; Hoffmann, A.; Rinott, E.; Tsaban, G.; Kaplan, A.; Zelicha, H.; Hagemann, T.; Ceglarek, U.; Isermann, B.; et al. The effect of polyphenols on DNA methylation-assessed biological age attenuation: The DIRECT PLUS randomized controlled trial. BMC Med. 2023, 21, 364. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Epigenetic and disease targets by polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, Y.Y.; Meeran, S.M.; Tollefsbol, T.O. Synergistic epigenetic reactivation of estrogen receptor-alpha (ERalpha) by combined green tea polyphenol and histone deacetylase inhibitor in ERalpha-negative breast cancer cells. Mol. Cancer 2010, 9, 274. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741. [Google Scholar] [CrossRef]
- Akhavan-Niaki, H.; Samadani, A.A. DNA methylation and cancer development: Molecular mechanism. Cell Biochem. Biophys. 2013, 67, 501–513. [Google Scholar] [CrossRef]
- Yuan, Z.; Syed, M.A.; Panchal, D.; Rogers, D.; Joo, M.; Sadikot, R.T. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int. J. Biochem. Cell Biol. 2012, 44, 2032–2043. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Cho, S.G. The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Wahyudi, S.; Sargowo, D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med. Indones. 2007, 39, 66–70. [Google Scholar]
- Yang, L.; Zhang, W.; Chopra, S.; Kaur, D.; Wang, H.; Li, M.; Chen, P.; Zhang, W. The Epigenetic Modification of Epigallocatechin Gallate (EGCG) on Cancer. Curr. Drug Targets 2020, 21, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chen, T.S.; Liou, S.Y.; Hsieh, C.C. Immunomodulatory effects of EGCG fraction of green tea extract in innate and adaptive immunity via T regulatory cells in murine model. Immunopharmacol. Immunotoxicol. 2014, 36, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Amini, S.; Hejazi, M.; Hosseinpanah, F.; Zarghi, A.; Abbaspour, F.; Valizadeh, M. Tea’s anti-obesity properties, cardiometabolic health-promoting potentials, bioactive compounds, and adverse effects: A review focusing on white and green teas. Food Sci. Nutr. 2023, 11, 5818–5836. [Google Scholar] [CrossRef] [PubMed]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Zhang, S.; Kiarasi, F. Therapeutic effects of resveratrol on epigenetic mechanisms in age-related diseases: A comprehensive review. Phytother. Res. 2024, 38, 2347–2360. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Silva GD, B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 1201. [Google Scholar] [CrossRef]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Stanevičienė, I.; Mongirdienė, A.; Bernatonienė, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes. Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Shabir, I.; Kumar Pandey, V.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, X.; Naumovski, N.; Hu, L.; Wang, K. Epigenetic regulation by quercetin: A comprehensive review focused on its biological mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]
- Manjunath, S.H.; Thimmulappa, R.K. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: Potential role in prevention and management of COVID-19. J. Pharm. Anal. 2022, 12, 29–34. [Google Scholar] [CrossRef]
- Sutrisno, S.; Aprina, H.; Simanungkalit, H.M.; Andriyani, A.; Barlianto, W.; Sujuti, H.; Santoso, S.; Dwijayasa, P.M.; Wahyuni, E.S.; Mustofa, E. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J. Tradit. Complement. Med. 2018, 8, 278–281. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Unni, S.; Somvanshi, P.; Bhardwaj, T.; Mandal, R.K.; Hussain, A.; Haque, S. Genistein Modulates Signaling Pathways and Targets Several Epigenetic Markers in HeLa Cells. Genes 2019, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; McCay, J.A.; Zhang, L.X.; Brown, R.D.; You, L.; Karrow, N.A.; Germolec, D.R.; White, K.L., Jr. Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J. Nutr. 2001, 131, 3251–3258. [Google Scholar] [CrossRef]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual Role in Women’s Health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Su, Z.Y.; Kong, A.N. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P + cells through epigenetics modifications. AAPS J. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Feng, Y.B.; Chen, L.; Chen, F.X.; Yang, Y.; Chen, G.H.; Zhou, Z.H.; Xu, C.F. Immunopotentiation effects of apigenin on NK cell proliferation and killing pancreatic cancer cells. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231161174. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene induces apoptosis and cell cycle arrest in diffuse large B-cell lymphoma cells. Sci. Rep. 2016, 6, 37417. [Google Scholar] [CrossRef] [PubMed]
- Harandi-Zadeh, S.; Boycott, C.; Beetch, M.; Yang, T.; Martin, B.J.E.; Ren, K.; Kwasniak, A.; Dupuis, J.H.; Lubecka, K.; Yada, R.Y.; et al. Pterostilbene Changes Epigenetic Marks at Enhancer Regions of Oncogenes in Breast Cancer Cells. Antioxidants 2021, 10, 1232. [Google Scholar] [CrossRef]
- Liu, P.; Tang, W.; Xiang, K.; Li, G. Pterostilbene in the treatment of inflammatory and oncological diseases. Front. Pharmacol. 2023, 14, 1323377. [Google Scholar] [CrossRef]
- Joo, S.Y.; Song, Y.A.; Park, Y.L.; Myung, E.; Chung, C.Y.; Park, K.J.; Cho, S.B.; Lee, W.S.; Kim, H.S.; Rew, J.S.; et al. Epigallocatechin-3-gallate Inhibits LPS-Induced NF-kappaB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages. Gut Liver 2012, 6, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.K.; Guo, W.; Liu, L.; Band, M.A.; Paulson, E.K.; Meydani, M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int. J. Cancer 2006, 118, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.F.; Kane, S.E.; Sonenshein, G.E. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007, 67, 9018–9023. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.R.; Pietras, R.; Marquez-Garban, D.C.; Chen, H.W.; Heber, D.; Henning, S.M.; Sartippour, G.; Zhang, L.; Lu, M.; Weinberg, O.; et al. The combination of green tea and tamoxifen is effective against breast cancer. Carcinogenesis 2006, 27, 2424–2433. [Google Scholar] [CrossRef]
- Delmas, D.; Lançon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Teng, M.; Jiao, K.; Zhen, J.; Wu, M.; Li, Z. Resveratrol inhibits the proliferation of estrogen receptor-positive breast cancer cells by suppressing EZH2 through the modulation of ERK1/2 signaling. Cell Biol. Toxicol. 2019, 35, 445–456. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar]
- Baek, S.H.; Ko, J.H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.W.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef]
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget 2018, 9, 24718–24730. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/beta-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Kyritsis, A.P.; Galani, V.; Lazari, D.; Sioka, C.; Voulgaris, S.; Alexiou, G.A. The Role of Curcumin in Cancer: A Focus on the PI3K/Akt Pathway. Cancers 2024, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barnes, C.A.; D’Souza, J.S.; Hosur, R.V.; Mishra, P. Curcumin, a potential initiator of apoptosis via direct interactions with Bcl-xL and Bid. Proteins 2022, 90, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, Z.; Fang, L. Curcumin inhibits LPS-induced EMT through downregulation of NF-kappaB-Snail signaling in breast cancer cells. Oncol. Rep. 2013, 29, 117–124. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2144. [Google Scholar] [CrossRef]
- Kasiri, N.; Rahmati, M.; Ahmadi, L.; Eskandari, N.; Motedayyen, H. Therapeutic potential of quercetin on human breast cancer in different dimensions. Inflammopharmacology 2020, 28, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Kiyga, E.; Şengelen, A.; Adıgüzel, Z.; Önay Uçar, E. Investigation of the role of quercetin as a heat shock protein inhibitor on apoptosis in human breast cancer cells. Mol. Biol. Rep. 2020, 47, 4957–4967. [Google Scholar] [CrossRef]
- Michalski, J.; Deinzer, A.; Stich, L.; Zinser, E.; Steinkasserer, A.; Knippertz, I. Quercetin induces an immunoregulatory phenotype in maturing human dendritic cells. Immunobiology 2020, 225, 151929. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 300–312. [Google Scholar] [CrossRef]
- Ge, Y.W.; Feng, K.; Liu, X.L.; Zhu, Z.A.; Chen, H.F.; Chang, Y.Y.; Sun, Z.Y.; Wang, H.W.; Zhang, J.W.; Yu, D.G.; et al. Quercetin inhibits macrophage polarization through the p-38alpha/beta signalling pathway and regulates OPG/RANKL balance in a mouse skull model. J. Cell Mol. Med. 2020, 24, 3203–3216. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Shen, H.; He, M.; Yang, S.; Gong, X.; Huang, C.; Guo, L.; Wang, H.; Feng, S.; Mi, T.; et al. Quercetin promotes the proportion and maturation of NK cells by binding to MYH9 and improves cognitive functions in aged mice. Immun. Ageing 2024, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14. [Google Scholar] [CrossRef]
- Wu, A.H.; Koh, W.P.; Wang, R.; Lee, H.P.; Yu, M.C. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br. J. Cancer 2008, 99, 196–200. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ahmed, F.; Ali, S.; Philip, P.A.; Kucuk, O.; Sarkar, F.H. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005, 65, 6934–6942. [Google Scholar] [CrossRef]
- Prietsch, R.F.; Monte, L.G.; da Silva, F.A.; Beira, F.T.; Del Pino, F.A.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–342. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef]
- Konstantinou, E.K.; Gioxari, A.; Dimitriou, M.; Panoutsopoulos, G.I.; Panagiotopoulos, A.A. Molecular Pathways of Genistein Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 5556. [Google Scholar] [CrossRef]
- Sohel, M. Genistein, a Potential Phytochemical against Breast Cancer Treatment-Insight into the Molecular Mechanisms. Processes 2022, 10, 415. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, J.; Mi, M.; Chen, W.; Pan, Q.; Wei, M. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med. Oncol. 2012, 29, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Blackburn, G.L.; Zhou, J.R. Genistein sensitizes inhibitory effect of tamoxifen on the growth of estrogen receptor-positive and HER2-overexpressing human breast cancer cells. Mol. Carcinog. 2007, 46, 534–542. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef]
- Liang, Y.-C. Apigenin inhibits migration and induces apoptosis of human endometrial carcinoma Ishikawa cells via PI3K-AKT-GSK-3β pathway and endoplasmic reticulum stress. J. Funct. Foods 2022, 94, 105116. [Google Scholar] [CrossRef]
- Tong, J.; Shen, Y.; Zhang, Z.; Hu, Y.; Zhang, X.; Han, L. Apigenin inhibits epithelial-mesenchymal transition of human colon cancer cells through NF-kappaB/Snail signaling pathway. Biosci. Rep. 2019, 39, BSR20190452. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.G. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFkappaB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.; Gupta, S. Suppression of NF-kappaB and NF-kappaB-Regulated Gene Expression by Apigenin through IkappaBalpha and IKK Pathway in TRAMP Mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, A.A.; Le Maitre, C.L.; Cross, N.A.; Jordan-Mahy, N. The effect of apigenin and chemotherapy combination treatments on apoptosis-related genes and proteins in acute leukaemia cell lines. Sci. Rep. 2022, 12, 8858. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef]
- Elsherbini, A.M.; Sheweita, S.A.; Sultan, A.S. Pterostilbene as a Phytochemical Compound Induces Signaling Pathways Involved in the Apoptosis and Death of Mutant P53-Breast Cancer Cell Lines. Nutr. Cancer 2021, 73, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Surien, O.; Masre, S.F.; Basri, D.F.; Ghazali, A.R. Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway. Int. J. Mol. Sci. 2023, 24, 9707. [Google Scholar] [CrossRef] [PubMed]
- Ma, K. Pterostilbene inhibits the metastasis of TNBC via suppression of β-catenin-mediated epithelial to mesenchymal transition and stemness. J. Funct. Foods 2022, 96, 105219. [Google Scholar] [CrossRef]
- Su, C.M.; Lee, W.H.; Wu, A.T.; Lin, Y.K.; Wang, L.S.; Wu, C.H.; Yeh, C.T. Pterostilbene inhibits triple-negative breast cancer metastasis via inducing microRNA-205 expression and negatively modulates epithelial-to-mesenchymal transition. J. Nutr. Biochem. 2015, 26, 675–685. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Li, M.J.; Lin, Y.C.; Wang, J.; Jiang, Y.F. Green tea compounds in breast cancer prevention and treatment. World J. Clin. Oncol. 2014, 5, 520–528. [Google Scholar] [CrossRef]
- Asensi, M.; Mena, S.; Feddi, F.; Estrela, J.M. Natural polyphenols in cancer therapy. Crit. Rev. Clin. Lab. Sci. 2011, 48, 197–216. [Google Scholar] [CrossRef]
- Pan, M.H.; Ho, C.T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008, 37, 2558–2574. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Nagy, P.; Szollosi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134 (Suppl. S12), 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar]
- V Ganesh, G.; Ramkumar, K.M. Pterostilbene attenuates hemin-induced dysregulation of macrophage M2 polarization via Nrf2 activation in experimental hyperglycemia. Inflammopharmacology 2023, 31, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti- inflammatory agents. Recent. Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Ostios-Garcia, L.; Villamayor, J.; Garcia-Lorenzo, E.; Vinal, D.; Feliu, J. Understanding the immune response and the current landscape of immunotherapy in pancreatic cancer. World J. Gastroenterol. 2021, 27, 6775–6793. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Cimino, S.; Sortino, G.; Favilla, V.; Castelli, T.; Madonia, M.; Sansalone, S.; Russo, G.I.; Morgia, G. Polyphenols: Key issues involved in chemoprevention of prostate cancer. Oxid. Med. Cell Longev. 2012, 2012, 632959. [Google Scholar] [CrossRef]

| Name | Their Importance | Epigenetic Effects | Immunomodulatory Effects | Found In |
|---|---|---|---|---|
| Epigallocatechin gallate (EGCG) | Strong antioxidant and anti-inflammatory properties and potential anti-cancer properties; inhibition of tumor cell proliferation [163,164] | DNA methylation inhibition, histone modification, miRNA expression changes [165] | It enhances cytotoxic T cell function, inhibits Tregs, and increases macrophages. It also reduces inflammation and suppresses NF-kB signaling [166]. | Green tea, white tea [167] |
| Resveratrol | Cardioprotective and anti-inflammatory properties, potential cancer prevention; promotes cell death in cancer cells [168,169] | Promotes histone acetylation, inhibits DNA methyltransferases, modifies miRNA [170,171] | It modulates T helper cell activity, particularly by promoting a shift from a pro-inflammatory Th1 response (which could contribute to chronic inflammation) to a more balanced Th2 or regulatory T cell (Treg) response, reducing inflammation while maintaining immune surveillance against cancer [172]. | Grapes, red wine, berries, peanuts [173] |
| Curcumin | Anti-inflammatory and antioxidant properties; modulates multiple cell signaling pathways; potential anti-cancer agent [174] | Histone acetylation inhibition [175,176] | It modulates the differentiation of T helper cells, reduces the expression of immune suppressive Tregs, and modulates macrophages and NK cells [177]. | Turmeric (Curcuma longa) [178]
 |
| Quercetin | Anti-inflammatory, antioxidant, and antiviral properties; may enhance immunity and reduce cancer risk [179] | Demethylation of tumor suppressor genes and histone acetylation, modifies miRNA [180] | Monocyte activation leads to the synthesis of interleukin-10 and TGF-β, inducing M1 macrophages while inhibiting M2 macrophages, which are associated with pro-tumor activity [181]. | Apples, onions, berries, leafy greens [179] |
| Genistein | Modulates estrogen receptors; potentially prevents breast cancer; antioxidant properties [182] | Inhibits DNA methylation, induces histone acetylation, and alters miRNA expression [183] | It enhances the activity of cytotoxic T cells. It modulates the function of T helper cells and leads to the activation of B cells and macrophages [184]. | Soybeans, tofu, soy products [185] |
| Apigenin | Anti-inflammatory and antioxidant properties; may induce apoptosis in cancer cells; neuroprotective effects [186] | Inhibits histone deacetylases, promotes DNA demethylation [187] | Inducing NK cell activity, boosting their ability to destroy tumor cells [188]. | Parsley, chamomile, celery, citrus fruits [186,189] |
| Pterostilbene | Antioxidant and anti-inflammatory properties; potential anti-cancer properties; modulates lipid metabolism [190] | Modulates histone acetylation and DNA methylation, affects miRNA profiles [191] | It suppresses macrophage activity and promotes dendritic cells’ maturation and has an antigen-presenting function [192]. | Blueberries, grapes, heartwood of Pterocarpus marsupium [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eren, E.; Das, J.; Tollefsbol, T.O. Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer. Nutrients 2024, 16, 4143. https://doi.org/10.3390/nu16234143
Eren E, Das J, Tollefsbol TO. Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer. Nutrients. 2024; 16(23):4143. https://doi.org/10.3390/nu16234143
Chicago/Turabian StyleEren, Esmanur, Jyotirmoyee Das, and Trygve O. Tollefsbol. 2024. "Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer" Nutrients 16, no. 23: 4143. https://doi.org/10.3390/nu16234143
APA StyleEren, E., Das, J., & Tollefsbol, T. O. (2024). Polyphenols as Immunomodulators and Epigenetic Modulators: An Analysis of Their Role in the Treatment and Prevention of Breast Cancer. Nutrients, 16(23), 4143. https://doi.org/10.3390/nu16234143






